Published online Jan 7, 2008. doi: 10.3748/wjg.14.95
Revised: September 17, 2007
Published online: January 7, 2008
AIM: To confirm whether insulin regulates resistin expression and secretion during differentiation of 3T3-L1 preadipocytes and the relationship of resistin with insulin resistance both in vivo and in vitro.
METHODS: Supernatant resistin was measured during differentiation of 3T3-L1 preadipocytes. L6 rat myoblasts and hepatoma cell line H4IIE were used to confirm the cellular function of resistin. Diet-induced obese rats were used as an insulin resistance model to study the relationship of resistin with insulin resistance.
RESULTS: Resistin expression and secretion were enhanced during differentiation 3T3-L1 preadipocytes. This cellular differentiation stimulated resistin expression and secretion, but was suppressed by insulin. Resistin also induced insulin resistance in H4IIE hepatocytes and L6 myoblasts. In diet-induced obese rats, serum resistin levels were negatively correlated with insulin sensitivity, but not with serum insulin.
CONCLUSION: Insulin can inhibit resistin expression and secretion in vitro, but insulin is not a major regulator of resistin in vivo. Fat tissue mass affects insulin sensitivity by altering the expression and secretion of resistin.
- Citation: Liu F, Fan HQ, Qiu J, Wang B, Zhang M, Gu N, Zhang CM, Fei L, Pan XQ, Guo M, Chen RH, Guo XR. A paradox: Insulin inhibits expression and secretion of resistin which induces insulin resistance. World J Gastroenterol 2008; 14(1): 95-100
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/95.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.95
Obesity is a worldwide health problem directly linked to several disease processes such as hypertension and type 2 diabetes mellitus[1]. Adipose tissue is not only an organ for passive energy reserve, but also an active endocrine organ secreting a wide range of hormones and other protein factors called adipokines[12]. Among the adipokines, resistin is involved in insulin sensitivity and glucose tolerance[34] while others are involved in hemostasis, inflammatory and stress responses, and energy balance[56].
Resistin, initially identified in screening for adipocyte-specific transcripts down-regulated by treatment with thiozolidinedione (TZDs), belongs to a novel family of cysteine-rich proteins, each with a unique tissue distribution[34]. In rodents, resistin predominantly expressed in white adipose tissue[4] reduces insulin sensitivity in adipocytes and skeletal muscles by impairing insulin-mediated glucose transport and inducing the expression of suppressor of cytokine signaling 3 (SOCS3)[7–9], and regulates fasting blood glucose by increasing hepatic glucose release[10]. Therefore, resistin might provide a link between obesity and diabetes mellitus.
Initial studies on the regulation of resistin indicate that resistin expression is reduced by fasting and increases rapidly on refeeding[3]. Circulating resistin levels are elevated in genetically obese (ob/ob, db/db) mice, and obese is induced by a high-fat diet[3]. Insulin inhibits resistin mRNA expression in 3T3-L1 preadipocytes[1112]. However, these data do not support a role of resistin in insulin resistance[11]. If resistin is mainly regulated by insulin, the major function of resistin is to induce insulin resistance, forming an insulin-resistin-insulin sensitivity positive feedback loop that cannot exist in vivo.
In the present study, resistin expression and secretion were elevated during 3T3-differentiation of L1 preadipocytes. This cellular differentiation-stimulated resistin expression and secretion were suppressed by insulin. Resistin also induced insulin resistance in H4IIE hepatocytes and L6 myoblasts. In diet-induced obese rats, serum resistin levels were negatively correlated with the insulin sensitivity index (ISI). No negative correlation was found between the levels of fasting serum insulin and resistin, suggesting that insulin is not the major regulator of resistin in rodents.
3T3-L1 preadipocytes were cultured at 37°C in an atmosphere containing 50 mL/L CO2 and 950 mL/L air. The cells were maintained in growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, USA), 45 mmol/L glucose, 10% heat-inactivated fetal bovine serum (FBS, Gibco BRL, USA), 2 mmol/L L-glutamine, and 50 U/mL penicillin and 50 ng/mL streptomycin (Sigma, USA). Induction of adipocytic differentiation of 3T3-L1 cells was performed as described elsewhere[13]. Briefly, 3T3-L1 cells were grown in DMEM supplemented with 10% FBS until confluence. Two days after complete confluence (d 0), cells were cultured in DMEM supplemented with 10% FBS and 0.5 mmol/L 1-methyl-3-isobutylxanthine (Sigma, USA), 0.25 &mgr;mol/L dexamethasone (Sigma, USA) and 100 nmol/L insulin (Sigma, USA) for 48 h. From d 2 to 4, the full medium was supplemented with 100 nmol/L insulin only. The cells were then switched back to DMEM containing only 10% FBS for the remaining days. Cultures were replenished every 2 d.
The rat hepatoma cell line H4IIE was cultured at 37°C in an atmosphere containing 50 mL/L CO2 and 950 mL/L air, and maintained in DMEM containing 1 g/L glucose and 10% FBS. The cells were incubated in serum-free DMEM (1 g/L glucose) overnight before assay. Glucose levels were adjusted to 4.5 g/L and H4IIE cells were treated with resistin (50 ng/mL) (Alexis, USA) for 2 h prior to insulin (100 nmol/L) stimulation for 2 h. Glycogen synthesis was then assayed as previously described[14].
L6 rat myoblasts were maintained in DMEM supplemented with 10% FBS and differentiated into myotubes by exposure to DMEM supplemented with 2% FBS. Myogenic differentiation to myotubes was confirmed morphologically and biochemically as previously described[15].
The supernatants of 3T3-L1 preadipocytes were collected on d 0, 4, 6, and 8 after differentiation and centrifuged to remove cells that might have detached from the culture flasks. The supernatants were kept at -20°C until assayed for resistin content by enzyme immunoassay (ADL, USA).
Total RNA was isolated from cultured 3T3-L1 cells using the TRIZOL method (Invitrogen, USA). Single strand cDNA synthesis was performed. In brief, the reverse transcription mixture contained 1 &mgr;g total RNA, 0.5 &mgr;g of oligo d(T) primer, 4 &mgr;L of 5 × RT buffer, 0.5 mmol/L deoxynucleotides, 50 U of RNase inhibitor, and 200 U of reverse transcriptase (Promega, USA) in a total volume of 20 &mgr;L, the reaction was carried out at 42°C for 1 h followed by heat inactivation at 95°C for 5 min. The number of cycles and reaction temperatures used in the PCR assay were optimized to provide a linear relationship between the amount of input template and the amount of PCR product[16]. The primers used for amplification, together with their specific optimum cycling conditions, were as follows:
Mouse resistin (a 415 bp product): [sense primer: 5’-CAA ACAAGACTTCAACTCCC-3’, antisense primer: 5’-ACA CACACCCTTCTCCACTA-3’, annealing temperature (TA) 58°C, 33 cycles].
β-actin (a 240 bp product): [sense primer: 5’-TAA AGA CCTCTATGCCAACACAGT-3’, antisense primer: 5’-CAC GATGGAGGGGCCGGACTCATC-3’ annealing temperature (TA) 57°C, 25 cycles].
H4IIE cells were serum starved overnight in DMEM containing 0.2% FBS prior to resistin and/or insulin treatment in all experiments. Cells were lysed with 30% KOH and the vials were kept at 100°C for 20 min. After addition of anhydrous ethanol, the vials were centrifuged at 4000 ×g for 15 min with the supernatants discarded. Distilled water (0.5 mL) and 1 mL of 0.2% anthrone [0.2 g of anthrone in 100 mL of 98% H2SO4 (g/mL), prepared freshly within 1 h] were added, and the vials were placed into boiling water for 20 min. The optical density at 620 nm of the solution in vials was determined by photometry. This method could detect 1.6 &mgr;g of glucose per mL, which is equivalent to 1.44 &mgr;g of glycogen per mL[17].
Myotubes were serum starved overnight in DMEM containing 0.2% FBS prior to resistin and/or insulin (10 nmol/L 15 min) treatment in all experiments. Uptake of 2-deoxy-D-[3H] glucose (CIC, China) was assayed for 10 min as previously described[18]. Briefly, the cells were washed with ice-cold phosphate-buffered saline, and then 200&mgr;L NaOH (1 mol/L) was added to each well. Aliquots of the cell lysate were transferred to the scintillation vials for radioactivity counting and the remainder were used for protein assay. Non-specific uptake was determined in the presence of cytochalasin B (10&mgr;mol/L) and subtracted from all values.
Forty-eight weaned male Sprague-Dawley rats, supplied by the Animal Center of Jiangsu Province, were acclimated to 22°C in a 12 h light/12 h dark cycle with free access to a standard chow diet for at least a week before grouping. High energy diet contained 10% milk powder, 10% glucose, 10% egg, 10% oil, and 60% standard feed[19]. Animals received this diet for 7 wk. Insulin sensitivity was defined by a value of ISI {ISI = Ln [1/(fasting plasma insulin*glucose)]}[20].
The data were presented as mean ± SE. Statistical analysis was undertaken using one-way ANOVA or the paired Student’s t-test where appropriate. Serum resistin levels in diet-induced obese rats were compared with the insulin sensitivity index and levels using the Bivariate correlation. Differences between groups were considered statistically significant when P < 0.05.
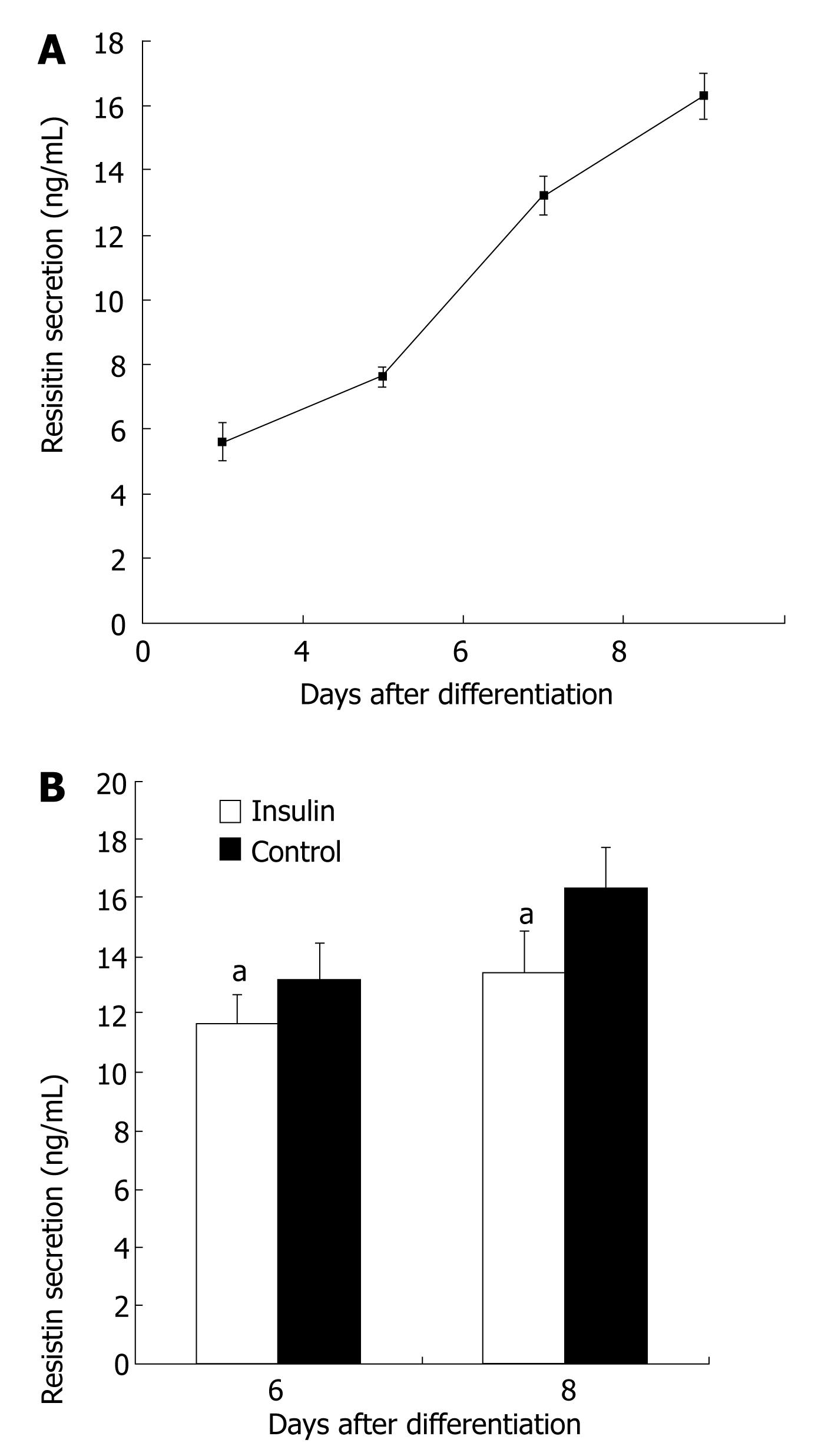
Resistin secretion was enhanced during 3T3-L1 preadipocyte differentiation (P < 0.05, Figure 1A). Resistin secrtion was about 3-fold higher in matured 3T3-L1 adipoctyes (d 8) than in 3T3-L1 preadipocyte (d 0) (P < 0.05). The effect of inslulin (100 nmol/L) on resistin secretion was then assessed in cultured 3T3-L1 adipocytes on d 6 and d 8. Six days after induction of differentiation, 100 nmol/L insulin reduced secretion of resistin by 13% (P < 0.05, Figure 1B). Eight days after induction of differentiation, 100 nmol/L insulin reduced secretion of resistin by 20% (P < 0.05, Figure 1B).
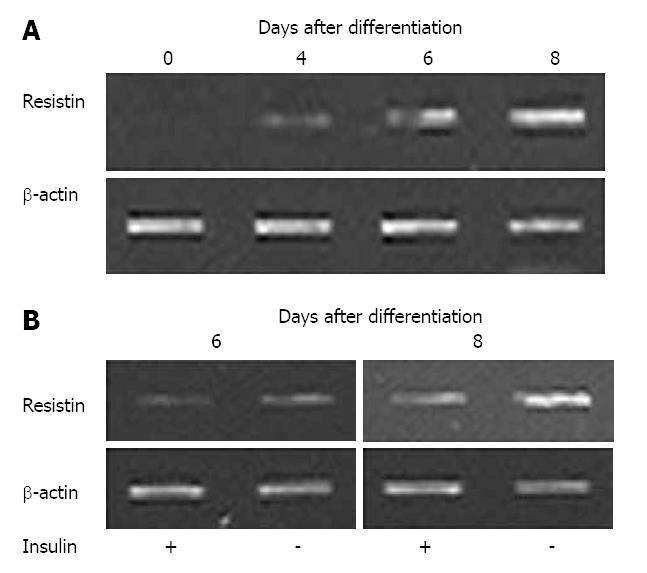
Resistin mRNA was not detectable in undifferentiated 3T3-L1 cells, but was evident by d 4 after the induction of differentiation into adipocytes (Figure 2A). Resistin mRNA was up-regulated during 3T3-L1 preadipocyte differentiation (Figure 2A), and 100 nmol/L insulin decreased resistin mRNA 6 and 8 d after differentiation (Figure 2B).
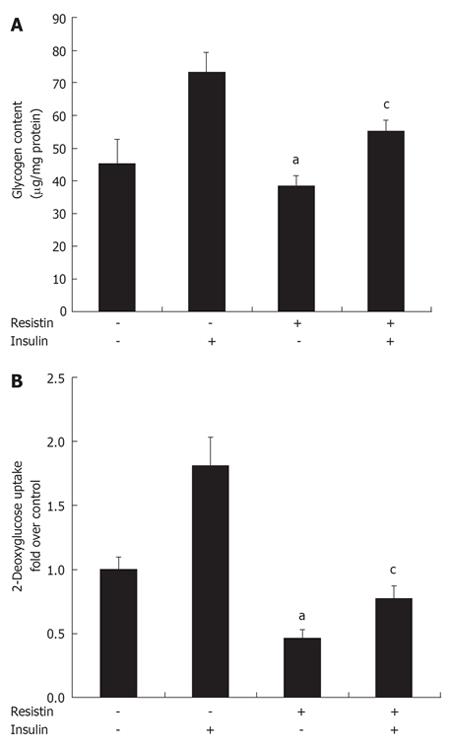
Since insulin could inhibit resistin expression and secretion in vitro, additional studies were initiated to determine whether resistin plays a role in insulin resistance. Hepatocytes and myotubes are two important targets of insulin[21]. Glycogen synthesis in insulin-stimulated hepatocytes is the most important marker of hepatocyte insulin sensitivity[22]. After treatment with resistin for 2 h, basal glycogen synthesis decreased about 15% and insulin-stimulated glycogen synthesis decreased about 25% in H4IIE cells (P < 0.05, Figure 3A). After treatment with resistin for 2 h, basal 2-deoxyglucose uptake decreased about 50% and insulin-stimulated 2-deoxyglucose uptake decreased about 60% in myotubes (P < 0.05, Figure 3B), suggesting that resistin could induce cellular insulin resistance, and both hepatocytes and myotubes are important targets of resistin.
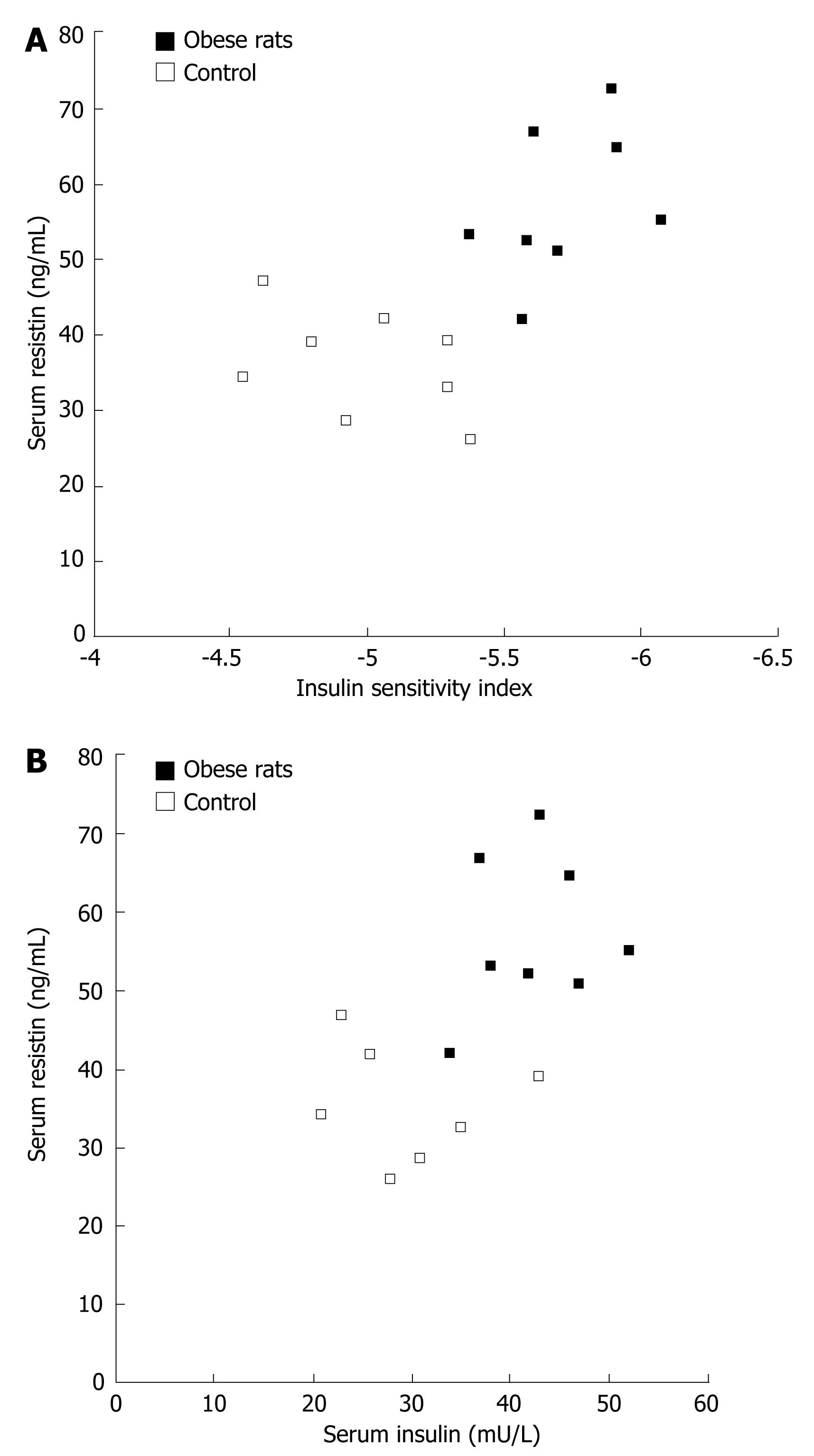
Serum resistin levels correlated with rat ISI (r = -0.662, P = 0.005) in both control and diet-induced obese rats (Figure 4A). Resistin was positively correlated with insulin (r = 0.592, P = 0.016, Figure 4B), suggesting that insulin could not inhibit resistin secretion in vivo.
Obesity is associated with insulin resistance and type 2 diabetes, implying that adipose tissue plays a role in the development of such disorders[23]. Besides storing fat, adipose tissue is also an active regulation centre, providing signals to guide metabolism by secreting adipokines[12]. Resistin is a newly discovered adipokine that is believed to provide a link between obesity and diabetes[34]. Under conditions of insulin resistance and type 2 diabetes, fat tissue is subjected to increased levels of insulin, which may have a major impact on adipokine levels[2425]. Studies have verified that circulating levels of insulin are correlated with specific adipokines in rodents and humans, with interleukin-6 and leptin levels showing a consistently positive association with insulin levels[24–26]. However, the association of resistin with insulin remains contradictory[27].
Resistin is one of the adipocytokines secreted by adipose tissue and has been shown to modulate both glucose and lipid metabolism in vivo and in vitro[34]. In L6 rat skeletal muscle cells, it has been shown that resistin does not alter insulin receptor signaling but affects insulin-stimulated glucose uptake, presumably by decreasing the intrinsic activity of cell surface glucose transporters[78]. In mature 3T3-L1 adipocytes, resistin reduces insulin-stimulated glucose uptake by activating SOCS3, which is an inhibitor of insulin signaling[9]. In addition, it was reported that resistin takes part in insulin resistance in resistin fat-specific transgenic rats by releasing free fatty acids (FFA) from adipose tissue[28].
In the present study, resistin expression and secretion were increased during 3T3-L1 preadipocyte differentiation and resistin mRNA was undetectable in undifferentiated 3T3-L1 cells but was evident by d 4 after the induction of differentiation into adipocytes. The highest expression of resistin mRNA was detected on d 8 after induction of differentiation. Insulin had a marked suppressive effect on resistin mRNA levels in 3T3-L1 adipocytes and inhibited resistin secretion 6 and 8 d after induction of differentiation, suggesting that resistin does not play a role in insulin resistance.
Then, we investigated whether resistin impairs insulin sensitivity in vitro, showing that resistin could induce cellular insulin resistance in hepatocytes and myotubes. That is a paradox, because resistin is not regulated by insulin but induces insulin resistance[11]. If both are correct, they will form a deadly insulin-resistin-insulin sensitivity positive feedback loop.
To confirm which one plays the primary role in vivo, we analyzed the relationship between serum resistin and insulin and their sensitivity in diet-induced obese rats. Diet-induced insulin resistance is a relevant model for the most common form of insulin resistance in humans[29]. In our study, serum resistin strongly correlated with rat insulin sensitivity and resistin was positively correlated with insulin, suggesting that insulin could not inhibit resistin secretion in vivo. A number of factors can regulate resistin secretion, such as glucose, epinephrine, and somatropin[2730]. Therefore, insulin may regulate resistin although it is not the major regulator.
In summary, insulin inhibits resistin secretion while resistin induces insulin resistance. Serum resistin correlates with rat insulin sensitivity, meaning that insulin is not the major regulator of resistin. Resistin may play a role in diet-induced insulin resistance by inducing insulin resistance in hepatocytes and myotubes.
Type 2 diabetes mellitus is closely associated with obesity. Resistin is a recently identified adipokine involved in insulin sensitivity and glucose tolerance. So the investigation of insulin and resistin interaction seems to be important.
In this article, resistin biological function and interaction of resistin to insulin were studied. We also demonstrated the secretion levels of resistin in vivo and in vitro.
Resistin induces insulin resistance in hepatocytes, but insulin inhibits resistin secretion in vitro. Since serum resistin correlates with rat insulin sensitivity, insulin is not the major regulator of resistin and resistin may play a role in diet-induced insulin resistance by inducing insulin resistance in hepatocytes and myotubes.
Resistin is a newly identified hormone secreted by adipocytes. Resistin induces insulin resistance both in vivo and in vitro. This establishes a new field in the pathogenesis of type 2 diabetes.
This paper discusses the regulatory mechanisms of resistin and insulin. The study is well designed and the paper is well written. The topic is of scientific value.
| 1. | Kaplan NM. Hypertension and diabetes. J Hum Hypertens. 2002;16 Suppl 1:S56-S60. |
| 2. | Mackall JC, Student AK, Polakis SE, Lane MD. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976;251:6462-6464. |
| 3. | Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502-506. |
| 4. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. |
| 5. | Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13-19. |
| 6. | Zhang L, Jin M, Hu XS, Zhu JH. Homocysteine stimulates nuclear factor kappaB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int. 2006;30:592-597. |
| 7. | Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia. 2006;49:183-190. |
| 8. | Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579:5049-5054. |
| 9. | Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569-1575. |
| 10. | Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195-1198. |
| 11. | Haugen F, Jorgensen A, Drevon CA, Trayhurn P. Inhibition by insulin of resistin gene expression in 3T3-L1 adipocytes. FEBS Lett. 2001;507:105-108. |
| 12. | Shojima N, Sakoda H, Ogihara T, Fujishiro M, Katagiri H, Anai M, Onishi Y, Ono H, Inukai K, Abe M. Humoral regulation of resistin expression in 3T3-L1 and mouse adipose cells. Diabetes. 2002;51:1737-1744. |
| 13. | Dowell P, Flexner C, Kwiterovich PO, Lane MD. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem. 2000;275:41325-41332. |
| 14. | Cheng A, Zhang M, Crosson SM, Bao ZQ, Saltiel AR. Regulation of the mouse protein targeting to glycogen (PTG) promoter by the FoxA2 forkhead protein and by 3’,5’-cyclic adenosine 5’-monophosphate in H4IIE hepatoma cells. Endocrinology. 2006;147:3606-3612. |
| 15. | el-Naggar EA, Kanda F, Okuda S, Maeda N, Nishimoto K, Ishihara H, Chihara K. Direct effects of tumor necrosis factor alpha (TNF-alpha) on L6 myotubes. Kobe J Med Sci. 2004;50:39-46. |
| 16. | Wang B, Zhang M, Ni YH, Liu F, Fan HQ, Fei L, Pan XQ, Guo M, Chen RH, Guo XR. Identification and characterization of NYGGF4, a novel gene containing a phosphotyrosine-binding (PTB) domain that stimulates 3T3-L1 preadipocytes proliferation. Gene. 2006;379:132-140. |
| 17. | Chun Y, Yin ZD. Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J Clin Microbiol. 1998;36:1081-1082. |
| 18. | Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132-139. |
| 19. | Gong HX, Guo XR, Fei L, Guo M, Liu QQ, Chen RH. Lipolysis and apoptosis of adipocytes induced by neuropeptide Y-Y5 receptor antisense oligodeoxynucleotides in obese rats. Acta Pharmacol Sin. 2003;24:569-575. |
| 20. | Liu ML, Xu X, Rang WQ, Li YJ, Song HP. Influence of ovariectomy and 17beta-estradiol treatment on insulin sensitivity, lipid metabolism and post-ischemic cardiac function. Int J Cardiol. 2004;97:485-493. |
| 21. | Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem. 2004;279:23942-23952. |
| 22. | Gao Y, Walder K, Sunderland T, Kantham L, Feng HC, Quick M, Bishara N, de Silva A, Augert G, Tenne-Brown J. Elevation in Tanis expression alters glucose metabolism and insulin sensitivity in H4IIE cells. Diabetes. 2003;52:929-934. |
| 23. | Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9:1-10. |
| 24. | Wang P, Keijer J, Bunschoten A, Bouwman F, Renes J, Mariman E. Insulin modulates the secretion of proteins from mature 3T3-L1 adipocytes: a role for transcriptional regulation of processing. Diabetologia. 2006;49:2453-2462. |
| 25. | Faraj M, Lu HL, Cianflone K. Diabetes, lipids, and adipocyte secretagogues. Biochem Cell Biol. 2004;82:170-190. |
| 26. | Cammisotto PG, Gelinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white adipocytes by insulin, glycolytic substrates, and amino acids. Am J Physiol Endocrinol Metab. 2005;289:E166-E171. |
| 27. | Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671-1679. |
| 28. | Pravenec M, Kazdova L, Cahova M, Landa V, Zidek V, Mlejnek P, Simakova M, Wang J, Qi N, Kurtz TW. Fat-specific transgenic expression of resistin in the spontaneously hypertensive rat impairs fatty acid re-esterification. Int J Obes (Lond). 2006;30:1157-1159. |
| 29. | Clore JN, Helm ST, Blackard WG. Loss of hepatic autoregulation after carbohydrate overfeeding in normal man. J Clin Invest. 1995;96:1967-1972. |
| 30. | Lu HL, Wang HW, Wen Y, Zhang MX, Lin HH. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J Gastroenterol. 2006;12:1747-1751. |