Published online Jan 7, 2008. doi: 10.3748/wjg.14.101
Revised: September 3, 2007
Published online: January 7, 2008
AIM: To investigate the distribution pattern of lymphatic vessels and microvessels in sporadic colorectal carcinoma (SCRC) and their relationship to metastasis and prognosis.
METHODS: The lymphatic vessel density (LVD) and microvessel density (MVD) in tumor tissue obtained from 132 patients with primary SCRC, including 74 with metastases and 58 without metastases, were evaluated by immunohistochemistry using antibodies directed against D2-40 and von Willebrand factor (vWF).
RESULTS: (1) The lymphatic vessels and microvessels at central portions of SCRC often had a reticular architecture with numerous tiny and ill-defined lumina, while those at tumor borders had large and open lumina. The LVD and MVD were both obviously higher in colorectal cancer patients with metastases than in those without (P < 0.001). (2) For each one lymphatic vessel increased, there was a 1.45-fold increase in the risk of metastasis in SCRC. The specificity and sensitivity of LVD in predicting metastasis or non-metastasis in SCRC were 71.62% and 56.90%, respectively, and the corresponding LVD was 5. For each one microvessel increased, there was a 1.11-fold increase in the risk of metastasis in SCRC. The specificity and sensitivity of MVD were 66.22% and 51.72%, respectively. (3) Double labeling immunohistochemistry showed D2-40 immunoreactivity to be specific for lymphatic vessels. (4) Univariate analysis indicated that high LVD, high MVD, as well as co-accounting of high LVD and high MVD were associated with patient’s poor disease-free survival (Puni < 0.05); multivariate analysis indicated that co-accounting of LVD and MVD was an independent prognostic factor of colorectal cancer.
CONCLUSION: D2-40 is a new specific antibody for lymphatic endothelial cells. Lymphogenesis and angiogenesis are commonly seen in SCRC, especially at tumor borders. The detection of LVD and MVD at tumor borders may be useful in predicting metastasis and prognosis in patients with SCRC, and, in particular, co-accounting of LVD and MVD might be a useful prognostic factor in SCRC.
- Citation: Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ, Du X. Lymphangiogenic and angiogenic microvessel density in human primary sporadic colorectal carcinoma. World J Gastroenterol 2008; 14(1): 101-107
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/101.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.101
Lymphangiogenesis (lymph vessel growth) and angiogenesis (blood vessel growth) are critical processes for tumor growth, invasion, and metastasis. Angiogenesis has established its role in the development and progression of a variety of malignancies, playing a crucial role in the dissemination of tumor cells[12]. However, lymphatic spread of cancer cells to lymph nodes is an important early event in the metastasis of carcinoma[3]. Previous studies have been limited by the lack of specific lymphatic endothelial makers that allow to discriminate between lymphatics and blood vessels. Recently, the monoclonal antibody D2-40, which is directed against the oncofetal membrane antigen M2A that has been identified in ovarian carcinoma cell lines and germ-cell tumors[4], was reported to be a specific marker for lymphatic endothelium in normal and neoplastic tissue[56].
In this study, using the antibody D2-40 and von Willebrand factor (vWF), we investigated the distribution patterns of microvessles and lymphatic vessels and calculated the lymphatic vessel density (LVD) and microvessel denisty (MVD) in sporadic colorectal carcinoma (SCRC). Moreover, we analyzed their relationship with tumor metastasis and disease prognosis. By double labeling immunohistochemistry we were able to confirm D2-40 specificity.
A total of 132 colorectal carcinoma samples were obtained from the archives of the Department of Pathology, Cancer Hospital, Fudan University, from July 2004 to May 2005. Samples were derived from patients who were solely surgically treated without prior chemo- or radiotherapy. There were 77 men (58.3%) and 55 women (41.7%) with a median age of 57 years (range, 22-82 years), 46 patients with colon and 86 patients with rectum tumors. 74 patients (56.1%) had histologically confirmed lymph node metastases, whereas the remaining 58 patients (43.9%) were found to have no clinical or histopathologic evidence of lymph node involvement. According to the current World Health Organization classification, tumors of 14 (10.6%) patients were well differentiated, that of 96 patients (72.7%) were moderately differentiated and that of 22 (16.7%) patients were poorly differentiated. Follow-up for this cohort was updated to September 2006. Complete data were available for 127 (96.2%) patients, with a median follow-up time of 22 mo (range, 2-26 mo). At the end of follow-up, 18 (14.17%) patients had died of disease and 9 (7.09%) had developed distant metastases. The study was approved by the local ethical committee in Cancer Hospital of Fudan University.
Tissues were fixed in 10% buffered formalin, processed, and stained with hematoxylin and eosin (H&E). H&E-stained slides of all samples were reviewed to confirm the diagnosis. One paraffin block with the bulk of tumor tissue was used for immunohistochemical studies. All slides showed the nonneoplastic colorectal tissue-carcinoma junction. Sections, 3 mm-thick, of formalin-fixed paraffin embedded tissues were cut and mounted on coated slides. The sections were deparaffinized in xylene and rehydrated in a descending ethanol series. Heat induced epitope retrieval techniques were used for antigen retrieval as follows: citrate buffer (pH 6.0) and a water bath at 95°C-98°C for 30 min for D2-40, Tris-EDTA buffer (pH 8.0) and a water bath at 95°C-98°C for 30 min for vWF. Sections were incubated for 10 min in 3% hydrogen peroxide to quench endogenous tissue peroxidase. The sections were immunostained with a monoclonal antibody (Clone D2-40, m3619; Dako Corp., Carpinteria, CA, USA) at a 1:100 dilution directed against D2-40 and a rabbit polyclonal antibody (A0082; Dako) at a dilution of 1:200 directed against vWF. Tissue sections were incubated with the primary antibody for 12 h at 4°C.After washing with phosphate-buffered saline, a Super picture secondary antibody (Zymed Lab Inc, CA, USA) and 3-3' diaminobenzidine detection kit (Dako) were used. A lymphangioma tissue sample served as a positive control and replacement of the primary antibody by PBS as a negative control. Thirty samples were picked randomly for double labeling immunohistochemistry (HistostainTM-DS double labeling immunohistochemistry kit, Zymed). The sections were first subjected to D2-40 staining using BCIP/NBT as chromogenic agent, followed by a vWF staining using AEC as chromogenic agent.
Immunohistochemical reactions for D2-40 and vWF were interpreted independently by two authors (Y.G. and Z.GH.) using a two-headed microscope. After scanning the immunostained section at low magnification (× 100), five areas of carcinoma with the greatest number of distinctly highlighted intratumoral lymphatic/vascular foci (hot spots) were selected and vessels were counted in a representative high magnification (× 200) field in each of these five areas. Single immunoreactive endothelial cells, or endothelial cell clusters separate from other microvessels, were counted as individual microvessels. Endothelial staining in large vessels with tunica media, and non-specific staining of nonendothelial structures, were disregarded in microvessel counts. Mean visual microvessel density for D2-40 and vWF was calculated as the average of five counts[7]. In double labeling immunohistochemistry, lymphatic vessels were amethyst and blood vessels were bright red.
Mean differences in microvessel counts were compared with the use of “t” tests. The probability of differences between the high-vessel group and the low-vessel group in disease-free survival (DFS) was determined as a function of time by the use of Kaplan-Meier test, with significance probing by applying the log-rank test. We used multivariate regression analysis based on the Cox proportional hazard model to test the independence of these parameters to predict overall survival. Logistic regression analysis and ROC curve were used to determine specificity and sensitivity of LVD and MVD in assessment of metastasis in SCRC. Generally, P value < 0.05 was regarded as significant. For all statistical procedures, SPSS v12.0 and Stata v7.0 software were used.
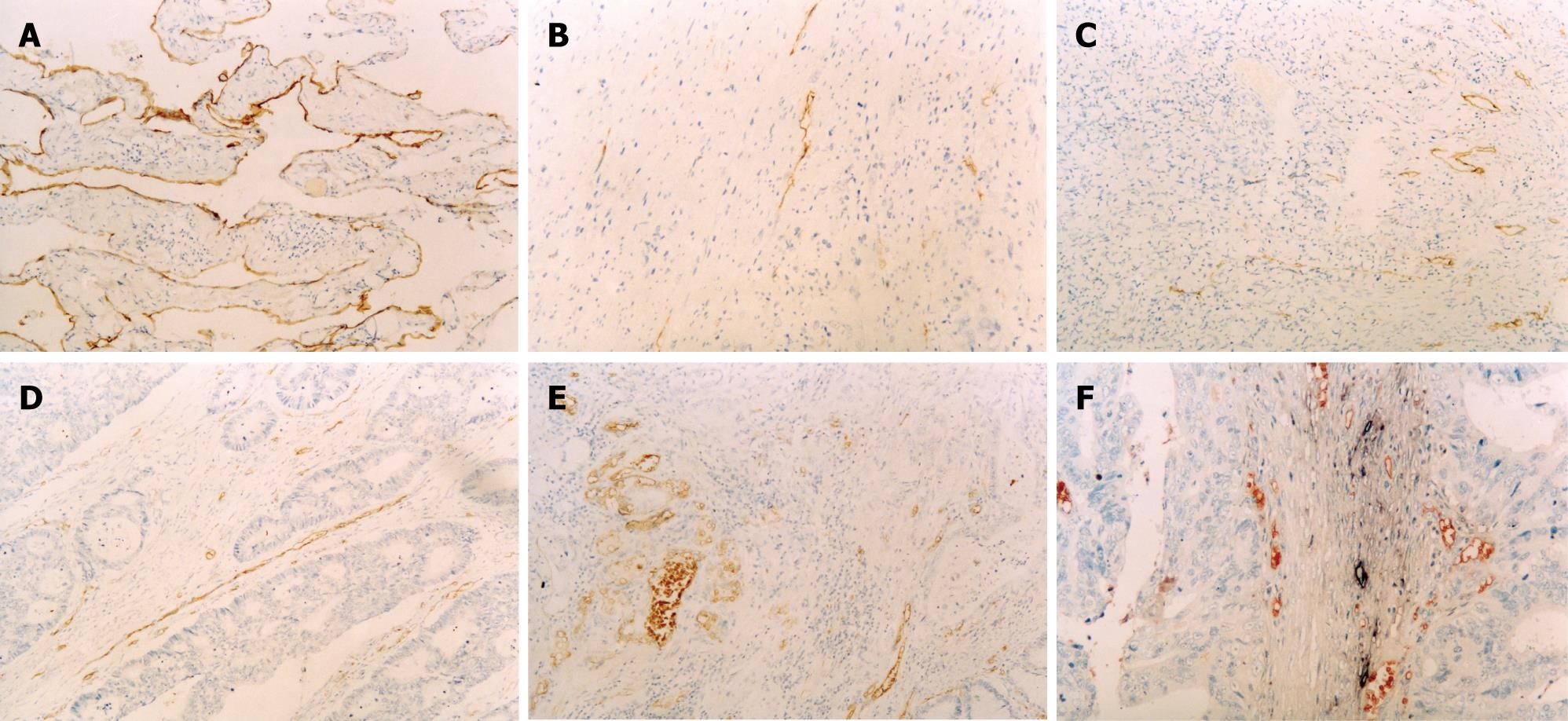
A tissue sample of lymphangioma was chosen as a positive control for D2-40 staining. As shown in Figure 1A, endothelial cells in this sample were found to be positive. In SCRC samples, definite lymphatic vessels were stained by D2-40. Lymphatic vessels at central portions were rare, even absent in some case, and often had a reticular architecture with numerous tiny and ill-defined lumina (Figure 1B). The LVD was higher at tumor borders (10.32 ± 4.94) than at central portions and had large and open lumina (Figure 1C). The LVD was obviously higher in the CRC samples with metastases (12.08 ± 4.96) than in those without (8.26 ± 4.08) (P < 0.001). There was no significant correlation between LVD with age, gender, tumor size, location, degree of differentiation, or invasive depth (P > 0.05) (Table 1).
| Clinicopathologic features | LVD | MVD | |||
| n | mean ± SD | P | mean ± SD | P | |
| Age (yr) | |||||
| <50 | 28 | 11.03 ± 5.71 | 0.396 | 19.58 ± 9.56 | 0.332 |
| ≥ 50 | 104 | 10.14 ± 4.73 | 21.92 ± 11.76 | ||
| Gender | |||||
| Male | 77 | 10.50 ± 4.73 | 0.641 | 21.00 ± 11.74 | 0.609 |
| Female | 55 | 10.09 ± 5.27 | 22.02 ± 10.82 | ||
| Tumor size | |||||
| < 5 cm | 77 | 10.37 ± 4.84 | 0.904 | 22.26 ± 11.92 | 0.313 |
| ≥ 5 cm | 55 | 10.26 ± 5.13 | 20.24 ± 10.46 | ||
| Location | |||||
| Right colon | 23 | 9.36 ± 3.95 | 0.323 | 23.36 ± 10.33 | 0.248 |
| Left colon | 23 | 9.52 ± 4.65 | 18.06 ± 11.25 | ||
| Rectum | 86 | 10.80 ± 5.23 | 21.81 ± 11.55 | ||
| Invasive depth | |||||
| Intra-deep muscular | 36 | 9.31 ± 5.82 | 0.15 | 21.74 ± 11.02 | 0.845 |
| Whole layer | 96 | 10.71 ± 5.82 | 21.30 ± 5.76 | ||
| Degree of differentiation | |||||
| Well | 14 | 8.38 ± 4.60 | 0.119 | 21.28 ± 9.38 | 0.962 |
| Moderately & poorly | 118 | 10.56 ± 4.95 | 21.44 ± 11.58 | ||
| Lymph node metastasis | |||||
| Yes | 74 | 12.01 ± 4.90 | < 0.001 | 24.00 ± 11.98 | 0.003 |
| No | 58 | 8.18 ± 4.12 | 18.14 ± 9.58 | ||
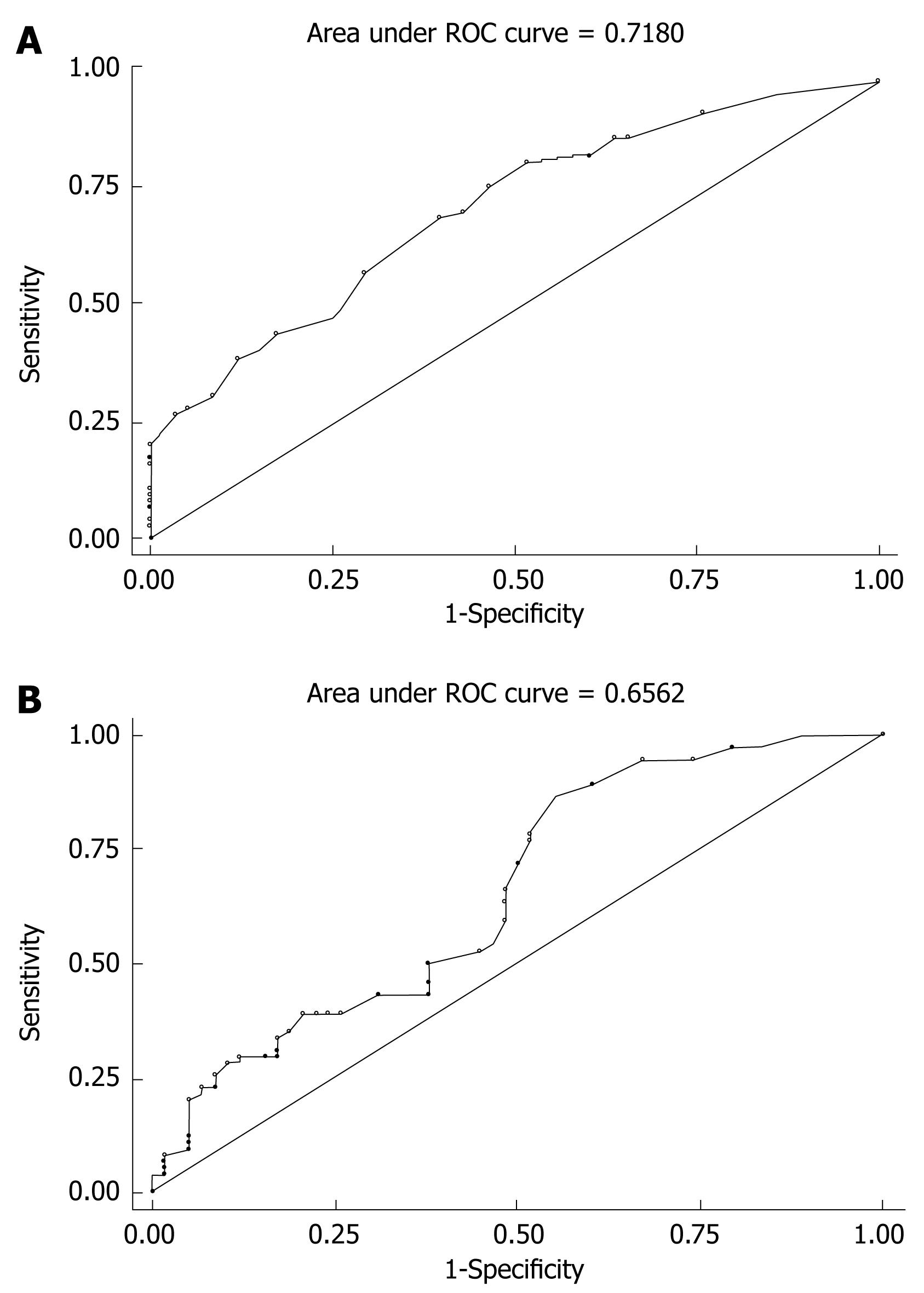
A logistic model was built with LVD as an independent and metastasis as dependent variable (Prob > chi2 = 0.0000, Pseudo R2-0.1176). According to the analysis for of the logistic model, we found an OR value of 1.45, meaning that for each lymphatic vessel there is an 1.45-fold increase in the risk of metastasis in SCRC. The specificity and sensitivity of LVD in predicting metastasis or non-metastasis in SCRC were 71.62% and 56.90%, respectively, and the corresponding LVD was 5 (Figure 2A).
There were abundant microvessels labeled by vWF in SCRC, with the similar distribution pattern to lymphatic vessels. The distribution feature and quantity of microvessels were different within one tumor sample. That is, microvessels at surrounding part of tumor were abundant (mean = 21.24 ± 11.98) and had large and open lumina (Figure 1D). But, microvessels at central portions were seldom and often had a reticular architecture with numerous tiny and ill-defined lumina (Figure 1E). The MVD was obviously higher in the colorectal carcinoma samples with metastasis (23.74 ± 12.02) than in those without (18.00 ± 9.44) (P = 0.003). There was no significant correlation between MVD with age, gender, tumor size, location, degree of differentiation, or invasive depth (P > 0.05) (Table 1).
The specificity of D2-40 for lymphatic vessels and vWF for microvessels was confirmed by double labeling immunohistochemistry. There was no cross-reaction between the two antibodies. Lymphatic vessels and microvessels distributed separately in tumor borders without obvious relationship (Figure 1F).
A l-Logistic model with MVD as an independent and metastasis as the dependent variable could be established (Prob > chi2 = 0.0022, Pseudo R2 = 0.0519). According to this model, we found an OR of 1.11, meaning that for each microvessel there is an 1.11-fold increase in the risk of metastasis in SCRC. According to ROC curve analysis, the specificity and sensitivity of MVD in predicting metastasis or non-metastasis in SCRC were 71.62% and 56.90%, respectively (Figure 2B).
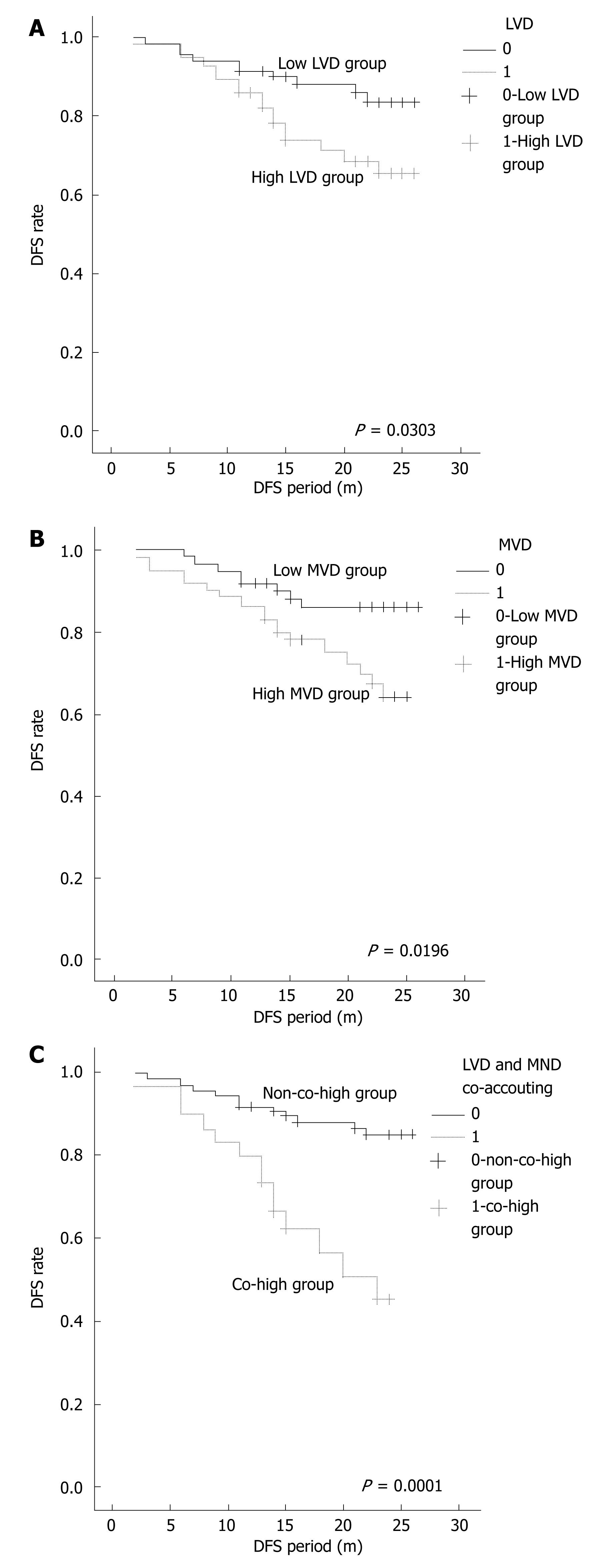
LVD = 5 was defined as demarcation value according to the ROC curve of LVD. LVD > 5 was high LVD group and LVD < 5 was low LVD group. The median of MVD (median = 9.33) was defined as demarcation value. MVD > 9.33 was high MVD group and MVD < 9.33 was low MVD group. LVD > 5 and MVD > 9.33 was co-high group. In univariate analysis with Kaplan-Meier for disease-free survival (DFS), high LVD (P = 0.0303), high MVD (P = 0.0196), co-high LVD and MVD (P < 0.0001) were associated with patient’s poor DFS (Puni < 0.05) (Figure 3).
All factors were brought into a Cox regression model, including some clinico-pathologic parameters such as patients’ age, gender, tumor size, location, degree of differentiation, invasive depth and metastasis, LVD, MVD, and co-accounting of LVD and MVD. Multivariate analysis indicated that besides metastasis (P = 0.004), gender (P = 0.012), and location (P = 0.028), co-accounting of LVD and MVD was an independent prognostic factor of colorectal carcinoma (P = 0.014).
This study is one of the first attempts to quantify colorectal carcinoma lymphangiogenesis and angiogenesis in the same sample by microvessel density using the novel lymphatic marker D2-40 and vWF. We compared the distribution pattern and density of lymphatic vessels and blood vessels, and related the results to clinicopathologic parameters and the outcome of colorectal carcinoma. Moreover, specificity of the two antibodies was confirmed by double labeling immunohistochemistry.
The lymphatic system is the primary pathway of metastasis for most human cancers. Lymphangiogenesis refers to the development and proliferation of new lymphatics from host vessels. Recently, antibodies specific for lymphatic endothelium have become available, providing important new insights into the process of tumor-associated lymphangiogenesis and its possible clinical relevance. Many studies have reported that tumors are able not only to induce lymphangiogenesis, but also to enhance lymphatic metastasis[89].
There are some antibodies for lymphatic endothelium now, including VEGFR-3/flt4 (vascular endothelial growth factor receptor 3/fms-like tyrosine kinase 4)[10], LYVE-1 (lymphatic endothelial hyaluronan receptor)[11], Prox-1[12], CD31[13], and podoplanin[14]. VEGFR-3, the receptor for vascular endothelial growth factors (VEGFs) C and D, is expressed on lymphatic endothelium and may play a role in lymphangiogenesis[1516]. But, some studies indicated that VEGFR-3 was also involved in blood vessel angiogenesis in the adult and it was not a specific antibody for lymphatic endothelium[17]. CD31 also stained both in blood vessel and in lymphatic vessel. But, the monoclonal antibody D2-40 is a highly selective marker of lymphatic endothelium in sections of both frozen and formalin-fixed paraffin-embedded normal and neoplastic tissues. In a direct comparison of D2-40 and CD31 on paraffin sections of a series of tumors derived from lymphatic endothelium (lymphangiomas) and blood vessel endothelium (hemangiomas), D2-40 stained all lymphagiomas (10/10) but no hemangiomas (0/10). Conversely, CD31 stained a fraction of lymphangiomas (5/10) but all hemangiomas (10/10)[13].
Pusztaszeri et al[18] found D2-40 to be a sensitive and relatively specific marker for lymphatic endothelium in all parenchymatous organs. It stained lymphatic endothelium only and it has been demonstrated to recognize tumor-associated lymphatic vessels in many tumors[1920]. Lymphatic vessels stained by D2-40 in our study often had a defined lumen with thin walls lacking erythrocytes, which were all the features of lymphatic vessels. There was no cross-reaction between D2-40 for lymphatic vessels and vWF for microvessels in double labeling immunohistochemistry. D2-40 is specific for lymphatic vessels[2122].
We also found lymphatic vessels to show a different distribution between tumor central portions and tumor borders in SCRC. Lymphatic vessels at central portions were seldom, even absent in some cases, but abundant at tumor borders. This phenomenon may be due to the inside pressure of the tumor. The LVD was obviously higher in the cases of colorectal carcinoma with metastasis than that in cases without, indicating that tumor cells might migrate from a primary site to lymph nodes through lymphatic vessels at the tumor borders. The increase of LVD was related to lymph nodes metastasis in SCRC. According to the logistic model and ROC curve analysis, we found an OR of 1.45, meaning that for each lymphatic vessel there is a 1.45-fold increase in the risk of metastasis in SCRC. The specificity and sensitivity of LVD in predicting metastasis or non-metastasis in SCRC were 71.62% and 56.90%, respectively, and the corresponding LVD was 5. The univariate analysis showed high LVD to be associated with patient’s poor DFS. Thus, LVD was an important factor to predict metastasis and prognosis for the patients with SCRC. Lymphatic vessels were composed of a single, non-fenestrated endothelial layer with wide and irregular lumina. Endothelial cells, with scant cytoplasm were often overlapping. But there were infrequent interendothelial tight junctions and no basement membrane and encircling pericytes on them. The morphology of lymphatic vessels differed from that of blood microvessels consequently made it was easy for fluid, particles, and tumor cells to pass into the lymphatic vessels. The surface area between tumor cells and lymphatic endothelial cells increased with the enhancement of LVD, which promoted the migration of tumor cells to lymph nodes[2]. So lymphangiogenesis is associated with an increased incidence of lymph nodes metastasis, and it is possible that this step is essential to the metastatic process. Recently, some studies on gastric cancer[23] and breast cancer[2425] demonstrated LVD to be correlated with lymph nodes metastasis.
Angiogenesis, the formation of new blood vessels from the endothelium of the existing vasculature, is fundamental in tumor growth, progression and metastasis, especially for colorectal carcinoma[26]. The complex network of tumor blood microvessels guarantees adequate supply of tumor cells with nutrients and oxygen and provides efficient drainage of metabolites. In addition to primary tumor growth, metastatic tumor growth depends upon neovascularization in at least two steps: First, malignant cells must exit from a primary tumor into the blood circulation after the tumor becomes neovascularized. Second, after arrival at distant organs, metastatic cells must again induce angiogenesis for a tumor to expand to a detectable size[2728]. Zhao et al[29] suggests MVD as one of the important prognostic factors for gastric cancer patients by immunohistochemical staining of endothelial protein factor VIII-related antigen. Romani et al[30] evaluated retrospectively the effect of CD105-assessed (a marker of neovascularization in solid malignancies) angiogenesis on the risk of developing metastatic disease in colorectal cancer. One hundred and twenty-five paraffin-embedded samples were analyzed by immunohistochemical methods using CD105 monoclonal antibody. The CD105-vessel count was found to be strongly correlated with the occurrence of metastatic disease. The median CD105-positive vessels in patients with and without metastatic disease were 24.7 and 13.2 vessels/mm2, respectively (P < 0.001). For each one microvessel increase in the vessels count per 400 × field, there was a 1.42-fold increase in the risk of metastatic disease (P < 0.001). We found the distribution feature and quantity of microvessels were different within one tumor sample labeled by vWF, which is similar to the distribution pattern of lymphatic vessels. That is, microvessels at surrounding part of tumor were abundant (mean = 21.24 ± 11.98) and had large and open lumina. In contrast, microvessels at central portions were less frequent and often had a reticular architecture with tiny and ill-defined lumina. The MVD was obviously higher in samples from colorectal carcinoma patients with metastasis than in those without. Our results indicated metastasis of SCRC to be associated with MVD. According to the logistic model, we found an OR of 1.11, meaning for each microvessel there is an estimated 1.11-fold higher risk of metastasis in SCRC. According to ROC curve analysis, the specificity and sensitivity of MVD in predicting metastasis or non-metastasis in SCRC were 71.62% and 56.90%, respectively. The univariate analysis showed high MVD to be associated with patient’s poor DFS. The lymphatic system was the most common pathway of metastasis for SCRC, accounting 60%. And the second primary pathway was blood vessels. Compared with LVD, the specificity and sensitivity of MVD in predicting metastasis or non-metastasis was lower. But MVD still played a role in roughly predicting prognosis of patients with SCRC.
Lymphangiogenesis and angiogenesis are essential for metastasis of tumor cells. We evaluated the specificity (71.62% vs 66.22%) and sensitivity (56.90% vs 51.72%) of LVD and MVD in SCRC. The specificity and sensitivity of LVD was slightly higher than that of MVD. Thus, neither LVD nor MVD alone were sufficiently specific and sensitive to predict metastasis or prognosis. We thus combined LVD with MVD to co-account. Univariate analysis indicated that co-accounting of high LVD and high MVD were closely associated with patient’s poor DFS and multivariate analysis indicated besides metastasis, gender and location, co-accounting of LVD and MVD to be independently predictive. Thus, evaluating lymphangiogenesis and angiogenesis are thought to be clinically important, particularly for the estimation of the metastatic risk and prognosis.
In summary, D2-40 is a new specific antibody for lymphatic endothelial cells. Lymphogenesis and angiogenesis are commonly seen in SCRC, especially at tumor borders. The detection of LVD and MVD at tumor borders may be useful in predicting metastasis and prognosis in patients with SCRC, and especially, the co-accounting of LVD and MVD might be used as a prognostic factor of SCRC.
More and more researchers focus on the importance of lymphogenesis in tumorigenesis and metastasis because of the development and update of new markers for lymphatic vessels. It is a common phenomenon that metastatic local lymph node in sporadic colorectal carcinoma (SCRC), but the relationship between the lymphogenesis and metastasis is not clear.
D2-40 is a new specific marker for lymphatic endothelium in normal and neoplastic tissue. Recent studies demonstrated D2-40 to be probably useful for diagnosis of malignant mesothelioma and research on lymphatic spread of cancer cells.
We investigated the role of lymphogenesis and angiogenesis using D2-40 and their distribution patterns in Sporadic colorectal carcinoma (SCRC). Moreover, we assessed how to predict metastasis by the increase of Microvessel density (MVD) and Lymphatic vessel density (LVD) and determined specificity and sensitivity.
Our study found LVD and MVD to be related to prognosis of SCRC. We calculated the risk ratio in predicting metastasis in SCRC by statistical procedures. In the future, we will be able to give an estimate on the prognosis and the survival of patients with colorectal carcinoma by accounting LVD and MVD.
SCRC: Sporadic colorectal carcinoma; LVD: Lymphatic vessel density; MVD: Microvessel density.
The results of this paper are reliably to the conclusions. The innovative and significant points conform to the background, objectives, materials and methods, results and conclusions. There is no conflict of interest, nor ethics problems. This is significant research.
| 1. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. |
| 3. | Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573-583. |
| 4. | Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569-578. |
| 5. | Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46:396-402. |
| 6. | Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255-1257. |
| 7. | Sheehan KM, Steele C, Sheahan K, O'Grady A, Leader MB, Murray FE, Kay EW. Association between cyclooxygenase-2-expressing macrophages, ulceration and microvessel density in colorectal cancer. Histopathology. 2005;46:287-295. |
| 8. | Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883-1886. |
| 9. | Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786-1790. |
| 10. | Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566-3570. |
| 11. | Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789-801. |
| 12. | Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769-778. |
| 13. | Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. |
| 14. | Ordonez NG. Podoplanin: a novel diagnostic immuno-histochemical marker. Adv Anat Pathol. 2006;13:83-88. |
| 15. | Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12:6865-6868. |
| 16. | Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672-682. |
| 17. | Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490-497. |
| 18. | Pusztaszeri MP, Seelentag W, Bosman FT. Immuno-histochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385-395. |
| 19. | Adachi Y, Nakamura H, Kitamura Y, Taniguchi Y, Araki K, Shomori K, Horie Y, Kurozawa Y, Ito H, Hayashi K. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol Int. 2007;57:171-177. |
| 20. | Fogt F, Zimmerman RL, Ross HM, Daly T, Gausas RE. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol Rep. 2004;11:47-50. |
| 21. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. |
| 22. | Walgenbach-Bruenagel G, Tolba RH, Varnai AD, Bollmann M, Hirner A, Walgenbach KJ. Detection of lymphatic invasion in early stage primary colorectal cancer with the monoclonal antibody D2-40. Eur Surg Res. 2006;38:438-444. |
| 23. | Shimizu K, Kubo H, Yamaguchi K, Kawashima K, Ueda Y, Matsuo K, Awane M, Shimahara Y, Takabayashi A, Yamaoka Y. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328-333. |
| 24. | Schoppmann SF, Birner P, Studer P, Breiteneder-Geleff S. Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer. Anticancer Res. 2001;21:2351-2355. |
| 25. | Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183-191. |
| 26. | Rmali KA, Puntis MC, Jiang WG. Tumour-associated angiogenesis in human colorectal cancer. Colorectal Dis. 2007;9:3-14. |
| 27. | Cao Y, O'Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest. 1998;101:1055-1063. |
| 28. | Eichhorn ME, Kleespies A, Angele MK, Jauch KW, Bruns CJ. Angiogenesis in cancer: molecular mechanisms, clinical impact. Langenbecks Arch Surg. 2007;392:371-379. |
| 29. | Zhao HC, Qin R, Chen XX, Sheng X, Wu JF, Wang DB, Chen GH. Microvessel density is a prognostic marker of human gastric cancer. World J Gastroenterol. 2006;12:7598-7603. |
| 30. | Romani AA, Borghetti AF, Del Rio P, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446-455. |