Published online Mar 7, 2007. doi: 10.3748/wjg.v13.i9.1445
Revised: December 27, 2006
Accepted: March 7, 2007
Published online: March 7, 2007
AIM: To investigate the effects of KN-93, a CaMKII selective inhibitor on cell proliferation and the expression of p53 or p21 protein in human hepatic stellate cells.
METHODS: Human hepatic stellate cells (LX-2) were incubated with various concentrations (0-50 μmol/L) of KN-93 or its inactive derivative, KN-92. Cell proliferation was measured by CCK-8 assay, and the expression of two cell cycle regulators, p53 and p21, was determined by SDS-PAGE and Western blotting.
RESULTS: KN-93 (5-50 μmol/L) decreased the proliferation of human hepatic stellate cells in a dose-dependent manner from 81.76% (81.76% ± 2.58% vs 96.63% ± 2.69%, P < 0.05) to 27.15% (27.15% ± 2.86% vs 96.59% ± 2.44%, P < 0.01) after 24 h treatment. Incubation of 10 μmol/L KN-93 induced the cell growth reduction in a time-dependent manner from 78.27% at 8 h to 11.48% at 48 h. However, KN-92, an inactive derivative of KN-93, did not inhibit cell proliferation effectively. Moreover, analysis of cell cycle regulator expression revealed that KN-93 rather than KN-92 reduced the expression of p53 and p21.
CONCLUSION: KN-93 has potent inhibitory effect on proliferation of LX-2 cells by modulating the expression of two special cell cycle regulators, p53 and p21.
-
Citation: An P, Zhu JY, Yang Y, Lv P, Tian YH, Chen MK, Luo HS. KN-93, a specific inhibitor of CaMKII inhibits human hepatic stellate cell proliferation
in vitro . World J Gastroenterol 2007; 13(9): 1445-1448 - URL: https://www.wjgnet.com/1007-9327/full/v13/i9/1445.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i9.1445
Many forms of acute and chronic liver injury, such as viral infection (especially hepatitis B and C), cholestasis, metabolic diseases, persistent alcohol abuse, and autoimmune liver diseases, result in hepatic fibrogenesis[1,2]. In the wound-healing process, retinoid-storing quiescent hepatic stellate cells (HSCs) are activated turning to myofibroblasts which are the major cell type responsible for producing excessive extracellular matrix, and play a pivotal role in the development of hepatic fibrosis[3-5]. For the outstanding importance in hepatic fibrosis, more and more researches focus on investigating the proliferation and apoptosis of HSC.
Among many signaling pathways of proliferation, intracellular calciumol/L has been extensively demonstrated to be very important[6]. In cytoplasm, calciumol/L binds to calmodulin, and then activates the Ca2+/calmodulin (CaM) dependent kinases (CaMKs) which are a family of structurally related serine/threonine protein kinases including CaMKI-IV[7]. Recent studies have suggested that CaMKII, a multi functional protein kinase, is ubiquitously involved in many physiological processes including control of cell cycle, apoptosis, gene expression, and neurotransmission[8,9]. Despite the important role of CaMKII in cell cycle, no studies on CaMKII and its inhibitors in HSC proliferation have been reported.
KN-93, as a membrane permeant compound of CaMKII-selective inhibitor, can intensively prevent CaMK-II activation by antagonizing CaM binding[10], and has been used in functional studies on living cells. In contrast to KN-93, KN-92 is a congener of KN-93 without CaM kinase inhibitory activity and has been used as an experimental control[11]. It was reported that KN-93 not only suppresses CaMKII activity but is a strong inhibitor of cell proliferation[12].
This study was to explore the effects of KN-93 on proliferation of human HSC and its mechanism.
KN-93 and KN-92 were provided by Calbiodhem (La Jolla, CA). Dimethyl sulfoxide (DMSO) trypsin, PMSF, aproptin and SDS were obtained from Sigma Chemical Company (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), glutamine, and penicillin-streptomycin were purchased from Gibco (Invitrogen, Carlsbad, CA). Fetal bovine serum (FBS) was from Hyclone. Primary antibody against β-actin, p53 and p21 were obtained from Santa Cruze Biotechnology (Santa Cruz, CA). WST-8 cell proliferation and cytotoxicity assay kit was from Beyotime Institute of Biotechnology (Jiangshu, China).
The human hepatic stellate cell line LX-2[13] was obtained from Institute of Liver Diseases, Shanghai University of Traditional Chinese Medicine. Cells were propagated in DMEM supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 g/L) under standard culture conditions (37°C, 950 mL/L humol/lidified air and 50 mL/L CO2). A stock solution of 10 mmol/L KN-93 and 10 mmol/L KN-92 in DMSO was prepared, and final concentrations (1, 5, 10, 25, 50 μmol/L) in DMEM were prepared from stock solution immediately before use. DMEM with an equal volume of DMSO served as a negative control.
The proliferation of LX-2 cells was estimated by CCK-8 assay according to the manufacturer’s guidelines. LX-2 cells treated with DMSO were used as a control. This assay is based on the cleavage of the tetrazolium salt WST-8 by mitochondrial dehydrogenase in viable cells. Briefly, LX-2 cells (2 × 103 cells/well) were incubated with 100 μL of culture medium in 96-multiwell plates. After incubated with KN-93 or KN-92 at indicated concentrations for 24 h or with 10 μmol/L of KN-93 or KN-92 for indicated periods of time, the media were removed and 100 μL of DMEM containing CCK-8 (10 μL) was added to each well. After a further 2 h incubation at 37°C, the absorbance at 450 nm of each well was measured with a Thermomax microplate reader. Each experiment was repeated three times, and the data represent the mean of all measurements.
A total of 5 × 105 LX-2 cells were plated in 6-well dishes. After attachment, KN-93 or KN-92 at indicated concentrations or vehicle control was added and incubated for 24 h. For Western blot analysis, the procedure was performed as described previously[14]. Briefly, an equal amount of protein lysates (40 μg) was electrophoresed on a 10% polyacrylamide gel and then electophoretically transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat dry milk for 2 h at room temperature and incubated with the indicated primary antibody overnight at 4°C. After washing, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ). Subsequently, the blots were developed by using the ECL kit (Santa Cruz Biotechnology, Santa Cruz, CA) and exposed to X-ray film. Protein bands were quantified by densitometric scanning using a Bio-Rad GS-800-calibrated densitometer. Each experiment repeated three times.
Data were expressed as mean ± SD. Statistical comparisons between groups were analyzed by the Student’s t test. P < 0.05 was considered statistically significant.
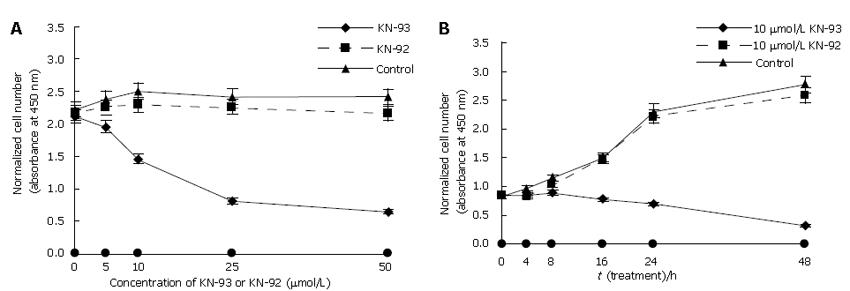
To assess the biological activity of KN-93 in LX-2 cell proliferation, KN-93 and its inactive derivative, KN-92 were chosen in CCK-8 assay. KN-93 significantly inhibited cell proliferation. After incubation of KN-93 at various concentrations for 24 h, the number of LX-2 cells reduced dramatically (Figure 1A) from 81.76% (5 μmol/L) to 27.15% (50 μmol/L) (Table 1). Exposure to 10 μmol/L KN-93 also significantly declined cell proliferation in a time-dependent manner (Figure 1B). The proliferation of LX-2 cells was reduced after KN-93 was incubated for 8 h (P < 0.01) and more obviously after further treatments (Table 2). Despite KN-93 had such a powerful influence on human hepatic stellate cells, KN-92 was ineffective in blocking cell growth (Figure 1, Tables 1 and 2). These results implied that KN-93 inhibited proliferation of LX-2 cells in a dose- and time-dependent manner.
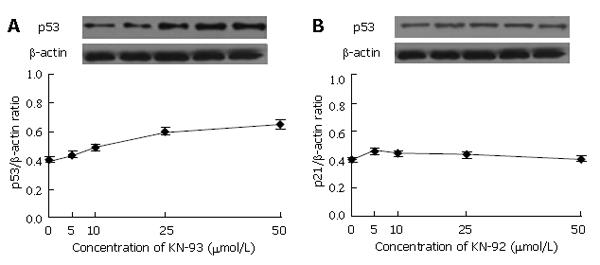
Next, we concentrated our studies on whether KN-93 influences the expression of cell cycle regulator, p53. The results from Western blotting and data analysis are exhibited in Figure 2. It was much clear that KN-93 at each concentration improved the expression of p53, especially at the concentration of 50 μmol/L (Figure 2A). As expected, KN-92 showed little impact on p53 expression (Figure 2B), demonstrating that it was KN-93 rather than KN-92 increased p53 expression in a dose-dependent manner.
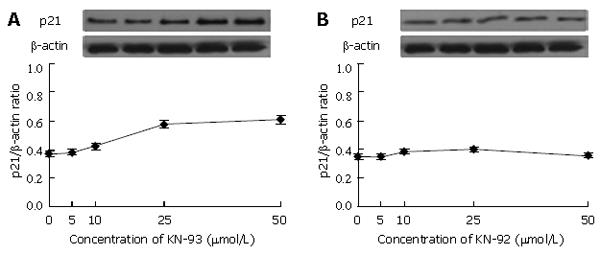
Another important regulator involving cell cycle, p21, was examined. The level of p21 increased with the increase of KN-93 concentration (Figure 3A). Few changes in p21 expression were observed when KN-92 was added (Figure 3B).
During liver fibrosis, HSCs perform a phenotype change from vitamin A-rich cells to proliferative, contractile and fibrogenic myofibroblasts. The activated HSCs produce excessI and III collagens which deposit and lead to fibrotic tissue in liver[15,16].
KN-93, a specific inhibitor of CaMKII is verified to have a powerful capability of suppressing the activation of CaMKII[17]. It was reported that KN-93 is involved in cell cycle arrest and proliferation[12], indicating that CaMKII is necessary for cell cycle progression through G1 and is a key regulator in the transduction signals of cell growth. In Hela cells, KN-93 can also inhibit cell proliferation and elevate some cell cycle regulators such as CKD1[18]. These results indicate that CaMKII is one of the most important factors for cell survival.
Our study suggested that KN-93 could significantly inhibit human hepatic stellate cell LX-2 proliferation in a dose-dependent manner. The inhibition increased with increase of incubation time. The effect of KN-92 on cell growth demonstrated the specificity of KN-93 in LX-2. These obvious results indicate that KN-93 might not only inhibit human HSC proliferation but also exert toxic effects on cells despite its negative effect on KN-92. In this study, 10 mmol/L KN-93 inhibited LX-2 cell proliferation in a time-depended manner, which is in agreement with the reported data showing the effect of KN-93 on apoptosis in NIH 3T3 cells[12], suggesting that HSCs and CaMKII can enhance cell proliferation.
Since KN-93 inhibits cell growth, we studied the regulators of cell cycle. P53, a suppressor protein, is a nuclear transcription factor controlling cell cycle progression[19,20]. Abriss et al[21] showed that constitutive expression of p53 is sufficient to induce cell arrest in HSCs. P53 can reduce proliferation of activated HSCs. In the present study, KN-93 significantly down-regulated the expression of p53 compared with KN-92 KN-92. Although KN-93 inhibited LX-2 proliferation, the exact mechanism by which KN-93 affects p53 is not clear and needs further study.
P21 is an inhibitor of cyclin-dependent kinases that control the cells to enter the S-phase[22]. P21 is regulated by the protein product of the gene p53, and is the downstream point. In this study, p21 expression was obviously decreased, suggesting that p21 is involved in KN-93-induced inhibition of HSC proliferation.
In conclusion, KN-93 has inhibitory effects on the proliferation of hepatic stellate cells. However, further studies are needed to explore the mechanism of KN-93 underlying the proliferation and apoptosis of hepatic stellate cells.
S- Editor Liu Y L- Editor Wang XL E- Editor Zhou T
| 1. | Friedman SL, Schiff E, Sorrell M, Maddrey W. Diseases of the Liver. 8th ed. Philadelphia: Lippincott-Raven 1998; 371–386. |
| 2. | Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 5. | Maher JJ, Bissell DM, Friedman SL, Roll FJ. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988;82:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24:719-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 359] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 643] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Heist EK, Schulman H. The role of Ca2+/calmodulin-dependent protein kinases within the nucleus. Cell Calcium. 1998;23:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Mamiya N, Goldenring JR, Tsunoda Y, Modlin IM, Yasui K, Usuda N, Ishikawa T, Natsume A, Hidaka H. Inhibition of acid secretion in gastric parietal cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-93. Biochem Biophys Res Commun. 1993;195:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Rodriguez-Mora OG, LaHair MM, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent kinase I and calcium/calmodulin-dependent kinase kinase participate in the control of cell cycle progression in MCF-7 human breast cancer cells. Cancer Res. 2005;65:5408-5416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Tombes RM, Grant S, Westin EH, Krystal G. G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase). Cell Growth Differ. 1995;6:1063-1070. [PubMed] |
| 13. | Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 845] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 14. | Han SW, Lei ZM, Rao CV. Up-regulation of cyclooxygenase-2 gene expression by chorionic gonadotropin during the differentiation of human endometrial stromal cells into decidua. Endocrinology. 1996;137:1791-1797. [PubMed] |
| 15. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [PubMed] |
| 16. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Muthalif MM, Karzoun NA, Benter IF, Gaber L, Ljuca F, Uddin MR, Khandekar Z, Estes A, Malik KU. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II--induced vascular hyperplasia and hypertension. Hypertension. 2002;39:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Rasmussen G, Rasmussen C. Calmodulin-dependent protein kinase II is required for G1/S progression in HeLa cells. Biochem Cell Biol. 1995;73:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155-168. [PubMed] |
| 20. | Cox LS, Lane DP. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995;17:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Abriss B, Hollweg G, Gressner AM, Weiskirchen R. Adenoviral-mediated transfer of p53 or retinoblastoma protein blocks cell proliferation and induces apoptosis in culture-activated hepatic stellate cells. J Hepatol. 2003;38:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5482] [Cited by in RCA: 5464] [Article Influence: 195.1] [Reference Citation Analysis (0)] |