Published online Mar 7, 2007. doi: 10.3748/wjg.v13.i9.1435
Revised: December 25, 2006
Accepted: January 18, 2007
Published online: March 7, 2007
AIM: To investigate the use of Daclizumab (Dmab) as an immunosuppressive agent in an experimental model of hepatocyte xenotransplantation (XenoTx) in rats with fulminant hepatic failure (FHF).
METHODS: Two white male New Zealand rabbits were used as donors and 68 Wistar rats as recipients. FHF was induced by intravenous application of dimethylnitrosamine (DMNA). The isolated hepatocytes of the rabbits were xenotransplanted into the spleen of the rats 24 h after FHF induction. Group A (n = 13): no treatment; Group B (n = 14): FHF and XenoTx; Group C (n = 14): FHF and XenoTx and cyclosporin (CsA); Group D (n = 14): FHF and XenoTx and Dmab; Group E (n = 13): FHF and XenoTx and CsA and Dmab. The rats were followed for 15 d.
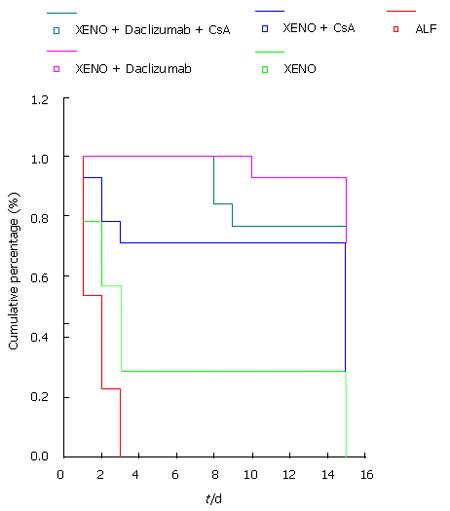
RESULTS: Statistical analysis showed better survival among groups D (92.86%) and E (76.92%) compared to group A (all rats died after 72 h), group B (28.57%) or group C (71.43%), although the differences were not statistically significant. Biochemical evaluation of the liver enzymes and histology confirmed satisfactory function and engraftment, respectively.
CONCLUSION: This experimental model has shown the safe, effective and beneficial use of Dmab in a xenotransplantation model of rabbit hepatocytes in rats.
- Citation: Papagoras D, Papalois A, Tsaroucha A, Lytras D, Kyriazanos J, Giannakou N, Laftsidis P, Simopoulos C. Beneficial effect of an antibody against interleukin-2 receptor (daclizumab) in an experimental model of hepatocyte xenotransplantation. World J Gastroenterol 2007; 13(9): 1435-1437
- URL: https://www.wjgnet.com/1007-9327/full/v13/i9/1435.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i9.1435
The lack of a sufficient number of organ transplants constitutes the main problem in the field of organ transplantation. As far as the transplantation of hepatic cells is concerned, the same difficulty exists in collecting transplants. Other attractive alternatives as transgenetic xenotransplantion, xenotransplantation of hepatic cells and the artificial liver, are related to economic, technical or ethical issues that make their extensive clinical application very difficult[1].
Recent studies stress the important aspect of xeno-transplantation as a possibility of a bridging therapy in patients with organ failure[2]. In the current experimental study we evaluate the safety and efficiency of Daclizumab, a monoclonal anti-CD25 antibody, and an IL-2 receptor antagonist, which selectively blocks IL-2 receptors on activated T helper cells in rats with acute liver failure and xenotransplantation of rabbit hepatocytes.
Sixty-eight adult male Wistar rats weighing 200-400 g were obtained from the Pasteur Institute. Rats were acclimatized to our laboratory conditions for 1 wk prior to use in experiments. Rats ate commercial rat chow and had water ad libitum. Rabbits were obtained from the Pasteur Institute as well. All experimental procedures described below were approved by the Animal Care Committee of the Veterinary Directorate of the Local Prefecture, according to the European Union Act and the Greek Law 160, A-64, May 1991. Also all animals received proper care in compliance with the “Principles of Laboratory Animal Care” and the Guide for the Care and Use of Laboratory Animals, prepared by the Academy of Science and published by the National Institute of Health.
Fulminant hepatic failure (FHF) was induced in 68 wistar rats with intravenous application of N-dimethynitrosamine (N-DMNA) (Sigma, N-3632) in a dose of 18 mg/kg body weight. This dose has been shown to result in reproducible hepatic necrosis and high mortality rates. Twenty-four hours after the induction of toxic acute hepatic failure 55 rats received randomly approximately 108 viable rabbit hepatocytes injected intrasplenically. Two male white New Zealand rabbits weighing 2600 g were used for the isolation of hepatocytes according to the method described by Papalois et al[3]. Viability was 92% ± 6% by the trypan blue exclusion test (Serva 23 850) while contamination of non-parenchymal cell was less than 2%.
During the experimental period the animals had free access to water and regular diet. After fulminant hepatic failure (FHF) and intrasplenic xenotransplantation (XenoTx) of the rabbit hepatocytes, the rats were rand-omly assigned to 5 study groups: (1) Group A (n = 13): Toxic FHF with no treatment; (2) Group B (n = 14): FHF and XenoTx; (3) Group C (n = 14): FHF and XenoTx and cyclosporin (Cyclosporin-CsA); (4) Group D (n = 14): FHF and XenoTx and daclizumab (Daclizumab- Dmab); (5) Group E (n = 13): FHF and XenoTx and Dmab and CsA.
Dmab was administered in a dose of 0.05 mg/kg body weight immediately after the XenoTx and at the seventh day of the experiment (instructions of the manufacturer). CsA was administered immediately after the XenoTx in a dose of 20 mg/kg body weight per os/day. Fifteen days after the acute liver failure induction, the rats were sacrificed and blood was taken for biochemical evaluation (liver enzymes, bilirubin, cholestatic enzymes). The liver, spleen and kidney were resected for histopathology examination. Specimens were fixed in 10% neutral formalin and stained with hematoxylin-eosin. A histopathology grading score for the quantification of the liver damage was adopted from Ishak et al[4].
Quantitative data is presented as median (min-max) and qualitative data as frequencies (n) and percentages (%). All the results were computed with the SPSS version 12 package. To evaluate the differences in survival, the Kaplan-Meier survival analysis was performed and a log rank test was used for the evaluation of differences. Mann-Whitney and Kruskall-Wallis tests were used for the comparisons of histological and biochemical variables between groups. Statistical significance was set at a value of P < 0.05.
None of the rats with acute toxic hepatic failure and no further treatment survived beyond 48 h after the injection of N-DMNA. Rats treated with XenoTx and Dmab showed the best survival rate (Table 1 and Figure 1). In accordance to this survival rate, the Dmab group also had the best histopathology grading score (Table 2). The statistical analysis of the histopathological score also showed better results in the Dmab group. Values of hepatic enzymes, direct total bilirubin in the rats of the study groups are shown in Table 3. Statistical analysis confirmed the improvement of liver function, especially in the Dmab group.
| SGOT1 | SGPT2 | Direct TBi3 | |
| Median (Min-Max) | Median (Min-Max) | Median (Min-Max) | |
| FHF | 565.00 (3-1077) | 257.00 (111-759) | 0.03 ( 0.02-0.08)e |
| XenoTx | 143.00 (83-154 )a | 60.00 ( 58-67)c | |
| XenoTx + CsA | 58.00 (43-88)a | 112.00 (85-183)c | 0.03 (0.02-0.05)e |
| XenoTx + Dmab | 246.00 (101-365)a | 92.00 (50-339)c | 0.02 (0.00-0.10) |
| XenoTx + Dmab + CsA | 142.50 (98-247)a | 58.50 (41-77)c | 0.01 (0.00-0.03) |
Despite the fact that XenoTx is far from a general use, most experts in the field of transplantation point out that a certain amount of research should be directed to this specific area of transplantation[5,6]. The main problem in organ transplantation, i.e. organ shortage, is also adherent to the collection, isolation and allotransplantation of human hepatocytes. XenoTx of hepatocytes could therefore represent an interesting form of “bridging therapy” in patients with fulminant hepatic failure[2,7].
We chose a xenotransplantation model in which rabbit hepatocytes were transplanted intrasplenically in rats with toxic acute liver failure. N-DMNA induced hepatic failure has the advantage of a non-invasive experimental model that mimics in certain degree the pathophysiology of acute liver failure in humans[8,9]. Daclizumab was used successfully in the clinical pancreatic islet transplantation program by Shapiro et al[10], and still is one of the most promising immunosuppressive agents[11,12]. Daclizumab has also been successfully used in an experimental model of allotransplantation of hepatocytes in rats with acute hepatic failure[13]. In the present experimental protocol, only 4 (28%) of the rats with acute liver failure survived by xenotransplantation without immunosuppressive treatment for 15 d. Regarding the survival rate of the study groups, the acute hepatic failure with no treatment group had the highest score in the liver injury grading system, whereas the Daclizumb group had the lowest score. As shown by the results, daclizumab was excellently tolerated, and so was the combination of cyclosporin and daclizumab. This monoclonal antibody has already been evaluated by clinical trials focusing on organ transplantation with excellent results[14]. The safety and effectiveness of cyclosporin was studied by Wen et al[15] in a xenograft model, whereas the safety and effectiveness of daclizumab was studied only in rats with acute liver failure and intrasplenic transplantation of rat hepatic cells[13]. To our knowledge, our data are the first about the safety and effectiveness of daclizumab in xenotransplantated rats with acute toxic liver failure.
S- Editor Liu Y L- Editor Zhu LH E- Editor Zhou T
| 1. | Leckel K, Blaheta RA, Markus BH. State of hepatocyte transplantation: a risk or a chance? Zentralbl Chir. 2003;128:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Papalois A, Arkadopoulos N, Kostopanagiotou G, Theodorakis K, Peveretos P, Golematis B, Papadimitriou J. Experimental xenotransplantation of fresh isolated and cryopreserved pig hepatocytes: a biochemical and morphological study. Transplant Proc. 1997;29:2096-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3784] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 5. | Auchincloss H, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998;16:433-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 330] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Inverardi L, Pardi R. Early events in cell-mediated recognition of vascularized xenografts: cooperative interactions between selected lymphocyte subsets and natural antibodies. Immunol Rev. 1994;141:71-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Bloom ET. Xenotransplantation--federal regulatory considerations. Curr Top Microbiol Immunol. 2003;278:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Arkadopoulos N, Papalois A, Pataryas TH, Golematis B, Papadimitriou J. Experimental transplantation of hepatocytes in cases of toxic acute liver failure. An allograft model. Transpl Int. 1994;7 Suppl 1:S171-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Contini S, Pezzarossa A, Sansoni P, Mazzoni MP, Botta GC. Hepatocellular transplantation in rats with toxic induced liver failure: results of iso-, allo- and xenografts. Ital J Surg Sci. 1983;13:25-30. [PubMed] |
| 10. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3837] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 11. | Sandrini S. Use of IL-2 receptor antagonists to reduce delayed graft function following renal transplantation: a review. Clin Transplant. 2005;19:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Jorga A, Johnston A. Novel therapies in transplantation. Expert Opin Investig Drugs. 2005;14:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tsiolis I, Papalois A, Loukopoulos I, Gravvanis A, Lykoudis E, Theodossopoulou E, Chairakakis A, Dimitroulopoulos D, Sfiniadakis I, Vassiliou I. Experimental isolation and transplantation of hepatocytes with the use of antibody against interleukin-2 receptor (daclizumab) as immunosuppressive agent. Transplant Proc. 2005;37:1929-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Vidhun JR, Sarwal MM. Corticosteroid avoidance in pediatric renal transplantation: can it be achieved? Paediatr Drugs. 2004;6:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Wen L, Grudé P, Conti F, Honiger J, Capeau J, Nordlinger B, Weill B, Calmus Y. Suppression of humoral immunization against encapsulated xenogeneic hepatocytes and prolongation of their function by 2-week cyclosporine treatment in the rat. Surgery. 2000;127:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |