Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1243
Revised: December 1, 2006
Accepted: December 18, 2006
Published online: February 28, 2007
AIM: To investigate the relationship between myelo-peroxidase polymorphisms as a host-related factor and atrophy caused by H pylori.
METHODS: Our study enrolled 77 patients. Biopsy materials obtained during gastrointestinal endoscopies were evaluated for the presence of H pylori. Polymerase chain reaction-restriction fragment length polymorphism assay was used to characterize myeloperoxidase genotypes.
RESULTS: Forty four patients (57.1%) were Hp (+) and 33 (42.9%) were Hp (-). Sixty six (85.7%) had GG genotype, 10 (12.9%) had GA genotype and 1 (1.29%) had AA genotype. The change in atrophy in relation to neutrophil infiltration was significant in Hp (+) patients (P = 0.0001). The change in atrophy in relation to neutrophil infiltration in patients with GG genotype was significant (P = 0.002). However, the change in atrophy in relation to neutrophil infiltration was not significiant in patients with Hp (+) GG genotype (r = 0.066, P = 0.63).
CONCLUSION: Myeloperoxidase genotype is critical for development of atrophy in relation to the severity of inflammation. However, it is interesting to note that, H pylori does not show any additive effect on development of atrophy.
-
Citation: Yilmaz &, Dursun H, Gürsan N, Pirim İ, Yılmaz A, Okcu N. Effects of the myeloperoxidase 463 gene polymorphisms on development of atrophy in
H pylori infected or noninfected gastroduodenal disease. World J Gastroenterol 2007; 13(8): 1243-1246 - URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1243
H pylori is an agent that produces chronic infection in more than half of the world population[1]. The most important characteristic of H pylori infection is that it causes chronic active inflammation in the gastric mucosa, which involves neutrophils and monocytes[2]. While 100% of the persons carrying this microbe develops gastritis, the lifetime risk of peptic ulcer is 15%-20% and that of gastric cancer is 1%-3%[1].
The role of host-related factors in the pathogenesis of diseases caused by H pylori has been greatly ignored up to date[3,4]. It was demonstrated that the ability of the host to regulate the production of cytokines is influenced by the presence of polymorphisms in the promoter region of the relevant genes[5]. The polymorphisms in the genes affecting the production of cytokines might be one of the factors that lead to the interpersonal differences in the severity of gastric inflammation[3,5].
Myeloperoxidase (MPO) is a lysosomal enzyme found in the azurophilic granules of polymorphonuclear leukocytes (PNL)[6-9]. There are two promoter regions affecting MPO; -463G/A and -129G/A[10]. Allele A of this polymorphism reduces mRNA expression and thus, tissue damage in local inflammation is decreased[8,9,11]. Allele G has 25 times more transcriptional efficacies compared to allele A. The G/G genotype confers higher risk for persistent H pylori infection[12,13].
H pylori activates the oxidative metabolism in neutrophils[14]. Reactive oxygen products such as free oxygen radicals released from neutrophils, (O2-), H2O2 and hydroxyl ion (OH-) reacts with MPO and hypochloric acid (HOCI) is formed. Monochloroamines (NH2CI) are formed when HOCI reacts with the ammonium produced by the urease enzyme of H pylori. Monochloroamines are oxidizing agents that are able to induce DNA fragmentation[4,8,14-16]. Thus, it has been advocated that myeloperoxidase is involved in gastric damage induced by H pylori[4,11,17]. We assessed the association between neutrophil infiltration and atrophy caused by H pylori and MPO gene polymorphisms.
We enrolled 77 patients (46 males, 31 females) who had undergone endoscopic examinations due to epigastric pain, dyspepsia, nausea and vomiting or weight loss. Patients who received antibiotic therapy, proton-pump inhibitors or non-steroid anti-inflammatory drugs within 3 mo prior to endoscopies were excluded.
Based on the endoscopic findings, 24 patients had gastritis, 26 had ulcers (duodenal or gastric ulcers) and 27 had gastric cancers. Diagnosis of patients who showed malignant findings in their endoscopies was confirmed by pathologic examination. In the light of endoscopic findings, two biopsies were obtained each from the antrum, angulus and corpus mucosa of the tissue adjacent to ulcer region and regions distant to malignant lesions. Biopsy materials were transferred into 3 separate small bottles containing formaldehyde and sent to the laboratory for evaluation of H pylori and pathologic examinations. Also, peripheral blood samples (3 mL) were collected from enrolled patients simultaneously and transferred into EDTA hemogram tubes. The tubes were transferred to the genetic laboratory under appropriate conditions avoiding coagulation.
The materials brought to the pathology laboratory were embedded in paraffin after follow-up procedures. Subsequently, cross-sections of 3-4 microns thick were obtained from the paraffin blocks. These cross-sections were stained by hematoxylin-eosin, Giemsa and Warthin-Starey stains, respectively. Preparations were divided into two groups as negative or positive based on the presence of H Pylori during direct visualization under a light microscope. Biopsy materials were staged according to neutrophilic activity, chronic inflammatory cell infiltration, glandular atrophy, intestinal metaplasia and H pylori density using criteria from the modified Sydney classification system. Each feature was evaluated as none (0), mild (1), moderate (2) or apparent (3)[18]. After all patients were scored according to 4 parameters of Sydney classification, sum of the parameters was calculated for three biopsy regions.
Patient blood samples transferred into EDTA tubes were used for DNA isolation. DNA isolation was performed using the column method (Gentra DNA isolation kit). Myeloperoxidase polymorphism analysis was performed by PCR and restriction fragment length polymorphism (RFLP) methods. Primers to be used were designed to detect Codone 463 of the myeloperoxidase gene.
Twenty microlitres DNA from each patient was transferred into 0.2 mL Eppendorf tubes. After addition of two different primers (forward pimer 5’-CCGTATAGGCAGAGAATGGTGAG-3’ and reverse primer 5’-GCAATGGTTCAAGCGATTCTTC-3’), 1.5 μL; dNTP mix, 1 μL; Taq DNA polymerase, 1 μL; PCR buffer, 10 μL and distilled water, 15 μL, these tubes were placed into a PCR machine. PCR conditions were primer annealing at 56°C for 1 min, polymerization at 72°C for 1 min, and denaturation at 94°C for 1 min. Thirty cycles were carried out[19]. The PCR product (amplicon) was incubated with Acl-I enzyme at 37°C for 1.5 h and separated on a 2% agarose gel. DNA fragments on the gel were visualized after staining with 0.5 μg/mL ethidium bromide (EtBr).
Statistical analyses were performed using SPSS 11.0 for Windows statistical software, Kendall’s Tau test and Linear regression analysis. P values < 0.05 were considered significant.
The mean age of the patients was 54.9 ± 14.1 years (19-82). Pathologic examination revealed that 44 of them were Hp (+) and 33 were Hp (-). Based on MPO gene polymorphism, 66 patients (85.7%) had GG genotype, 10 (12.9%) had GA and 1 (1.29%) had AA genotype. The patient with genotype A was an Hp (-) ulcer patient (Table 1).
| Hp (+) | Hp (-) | Total | |
| n (%) | 44 (57.1) | 33 (42.9) | 77 (100.0) |
| Gastritis | 13 | 11 | 24 |
| Ulcer (gastric or duodenum ) | 18 | 8 | 26 |
| Gastric cancer | 13 | 14 | 27 |
| Myeloperoxidase GG | 37 | 29 | 66 |
| Myeloperoxidase GA | 7 | 3 | 10 |
| Myeloperoxidase AA | - | 1 | 1 |
| Neutrophil infiltration | 2.80 ± 1.941 | 0.88 ± 1.24 | 1.97 ± 1.91 |
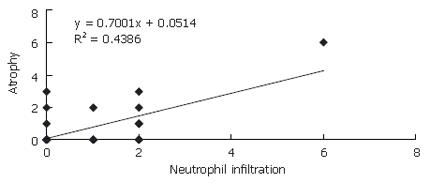
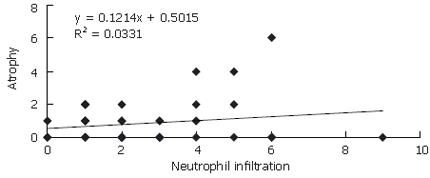
There was a difference between H pylori (+) and H pylori (-) patients with respect to PNL infiltration (P = 0.0001). The change in atrophy in relation to neutrophil infiltration was significant in Hp (+) patients (y = 0.700x + 0.051) (P = 0.0001) (Figure 1). No such correlation was found for Hp (-) patients (y = 0.1214x + 0.50) (y: atrophy, x: neutrophil infiltration) (Figure 2) .
We found a significant correlation between atrophy and neutrophil infiltration in patients with GG genotype (n = 66) (y = 0.252x + 0.239) (P = 0.002). There was an insignificant correlation between atrophy and neutrophil infiltration in patients with GA genotype (n = 10) (y = 0.15x + 1.387) (P = 0.56).
Among the Hp (+) patients, the correlation between atrophy and neutrophil infiltration was insignificant for patients with both GG (n = 37) and GA genotypes (n = 7) (r = 0.066, P = 0.63; r = -0.474, P = 0.18, respectively). Among the patients with Hp (-), we found an insignificant correlation between atrophy and neutrophil infiltration in patients with both GG (n = 29) and GA genotypes (n = 3) (r = 0.316, P = 0.06; r = 0.816, P = 0.22, respectively). Since our sample size was small, a statistical analysis for MPO AA (n = 1) genotype could not be performed.
About 60% of the world population is infected with H pylori[1]. Hyperproliferation induced by H pylori gastritis has been assumed as a starting point for events that trigger gastric cancer. Moreover, this hyperproliferation has been suggested to initiate changes in DNA[4,20,21]. The World Health Organization (WHO) has declared H pylori as the leading carcinogenic factor. In a cohort study conducted by WHO in 3 different regions, anti-Hp antibody levels were measured in blood samples obtained within the last 24 years from patients diagnosed with gastric cancer. It was stated that H pylori seropositivity increased the relative risk for developing gastric cancer[1]. Yamagato et al[22] reported that 3% of 1721 patients with H pylori developed gastric cancer during 9 years of follow up. Also, Uemura et al[23] reported that 2.9% of 1246 Hp (+) patients developed gastric cancer during 7.6 years of follow up. While there was a significant difference in Hp (+) patients with respect to atrophy and neutrophil infiltration, no such correlation was found for Hp (-) patients. Thus, inflammation induced by H pylori could be one of the predisposing factors in carcinogenesis.
Recently studies have been published on cytokine production in several conditions caused by H pylori infection as a host response and genetic polymorphisms[4]. Zambon et al[3] found that bacterial and virulence factors were associated with mucosal inflammation and severity of the illness. They found that H pylori virulence genes and a host genotype of IL-1 RN were directly correlated with peptic ulcer and intestinal metaplasia and they suggested that the interaction between cytokine genotypes and bacterial virulence factors are fundamental to development of H pylori-related lesions. Nardone et al[24] reported that, first atrophy localized in the antrum regresses or disappears subsequent to H pylori eradication. The changes seen in these lesions as a result of eradication show that, neutrophil infiltration plays a major role in these events above all.
Polymorphisms, defined as known changes in the genomic sequence, occur frequently throughout the human genome. It is known that in some cases, they modify the expression or function of gene products. Any polymorphism could affect susceptibilities to and outcome of an illness through the interaction of environment and genetics[25].
The question of how the variations in the genes associated with inflammation affect the inflammatory response induced by H pylori and accompanying gastric pathologies is an interesting one[26]. It has been stated that MPO itself might modulate susceptibilities to clinical outcomes of several illnesses where neutrophils are involved[10]. In a number of studies, the association between MPO polymorphisms and several conditions including chronic granulomatous illness, Alzheimer’s disease, malignancies such as the lung or pharyngeal cancer, some types of leukemia, atherosclerosis, periodontal diseases, multiple sclerosis and cystic fibrosis were reported[4,8,11-13]. Hamajima et al[12] investigated a myeloperoxidase genotype with low expression, which was negatively correlated with H pylori infection in 241 patients having complaints of dyspepsia, without a history of cancer. They found 79.7% of GG genotype, 19.5% of GA genotype and 0.8% of AA genotype. In another study assessing the association between MPO polymorphisms, atrophy and neutrophil infiltration, Roe et al[4] found 81.9% of GG genotype, 18.1% of GA genotype and 0.0% of AA genotype. It was indicated that while there was a positive correlation between the degree of atrophy and neutrophil infiltration in individuals with GG polymorphism, no such association was found in individuals with GA polymorphism. Also, in our study we found that while the correlation between atrophy and neutrophil infiltration was significant in patients with GG genotype, it was insignificant in patients with GA genotype.
Our results show that generally myeloperoxidase GG genotype is a critical host-related factor for development of atrophy that occurs in the setting of inflammation, and is considered as a cancer precursor. However, it is interesting to note that on the contrary to what is expected, GG genotype and H pylori positivity does not potentiate each other for development of atrophy. More studies are needed to elucidate this point in larger patient groups.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Sugiyama T. Development of gastric cancer associated with Helicobacter pylori infection. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S12-S20. [PubMed] |
| 2. | Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, Okamoto S, Maeda H. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect Immun. 2000;68:4378-4383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Zambon CF, Basso D, Navaglia F, Germano G, Gallo N, Milazzo M, Greco E, Fogar P, Mazza S, Di Mario F. Helicobacter pylori virulence genes and host IL-1RN and IL-1beta genes interplay in favouring the development of peptic ulcer and intestinal metaplasia. Cytokine. 2002;18:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Roe I, Nam S, Kim J, Shin J, Bang W, Yang M. Association of the myeloperoxidase -463G--> A polymorphism with development of atrophy in Helicobacter pylori-infected gastritis. Am J Gastroenterol. 2002;97:1629-1634. [PubMed] |
| 5. | Hwang IR, Hsu PI, Peterson LE, Gutierrez O, Kim JG, Graham DY, Yamaoka Y. Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2003;8:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Bombardier C. An evidence-based evaluation of the gastrointestinal safety of coxibs. Am J Cardiol. 2002;89:3D-9D. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157-33160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1314] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 8. | Cascorbi I, Henning S, Brockmöller J, Gephart J, Meisel C, Müller JM, Loddenkemper R, Roots I. Substantially reduced risk of cancer of the aerodigestive tract in subjects with variant--463A of the myeloperoxidase gene. Cancer Res. 2000;60:644-649. [PubMed] |
| 9. | Pakakasama S, Chen TT, Frawley W, Muller CY, Douglass EC, Lee R, Pollock BH, Tomlinson GE. CCND1 polymorphism and age of onset of hepatoblastoma. Oncogene. 2004;23:4789-4792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Rutgers A, Heeringa P, Giesen JE, Theunissen RT, Jacobs H, Tervaert JW. Neutrophil myeloperoxidase activity and the influence of two single-nucleotide promoter polymorphisms. Br J Haematol. 2003;123:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Matsuo K, Hamajima N, Suzuki R, Nakamura S, Seto M, Morishima Y, Tajima K. No substantial difference in genotype frequencies of interleukin and myeloperoxidase polymorphisms between malignant lymphoma patients and non-cancer controls. Haematologica. 2001;86:602-608. [PubMed] |
| 12. | Hamajima N, Matsuo K, Suzuki T, Nakamura T, Matsuura A, Tajima K, Tominaga S. Low expression myeloperoxidase genotype negatively associated with Helicobacter pylori infection. Jpn J Cancer Res. 2001;92:488-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Katsuda N, Hamajima N, Tamakoshi A, Wakai K, Matsuo K, Saito T, Tajima K, Tominaga S. Helicobacter pylori seropositivity and the myeloperoxidase G-463A polymorphism in combination with interleukin-1B C-31T in Japanese health checkup examinees. Jpn J Clin Oncol. 2003;33:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Mizuki I, Shimoyama T, Fukuda S, Liu Q, Nakaji S, Munakata A. Association of gastric epithelial apoptosis with the ability of Helicobacter pylori to induce a neutrophil oxidative burst. J Med Microbiol. 2000;49:521-524. [PubMed] |
| 15. | Bhattacharjee M, Bhattacharjee S, Gupta A, Banerjee RK. Critical role of an endogenous gastric peroxidase in controlling oxidative damage in H. pylori-mediated and nonmediated gastric ulcer. Free Radic Biol Med. 2002;32:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Karmeli F, Okon E, Rachmilewitz D. Sulphydryl blocker induced gastric damage is ameliorated by scavenging of free radicals. Gut. 1996;38:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Tran CD, Huynh H, van den Berg M, van der Pas M, Campbell MA, Philcox JC, Coyle P, Rofe AM, Butler RN. Helicobacter-induced gastritis in mice not expressing metallothionein-I and II. Helicobacter. 2003;8:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3527] [Article Influence: 121.6] [Reference Citation Analysis (3)] |
| 19. | Le Marchand L, Seifried A, Lum A, Wilkens LR. Association of the myeloperoxidase -463G--> a polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:181-184. [PubMed] |
| 20. | Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J Clin Pathol. 2002;55:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Cahill RJ, Kilgallen C, Beattie S, Hamilton H, O'Morain C. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996;38:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Yamagata H, Kiyohara Y, Aoyagi K, Kato I, Iwamoto H, Nakayama K, Shimizu H, Tanizaki Y, Arima H, Shinohara N. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160:1962-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3160] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 24. | Nardone G, Staibano S, Rocco A, Mezza E, D'armiento FP, Insabato L, Coppola A, Salvatore G, Lucariello A, Figura N. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Foster CB, Lehrnbecher T, Mol F, Steinberg SM, Venzon DJ, Walsh TJ, Noack D, Rae J, Winkelstein JA, Curnutte JT. Host defense molecule polymorphisms influence the risk for immune-mediated complications in chronic granulomatous disease. J Clin Invest. 1998;102:2146-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, Gerhard M, Prinz C. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |