Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1182
Revised: December 1, 2006
Accepted: December 30, 2007
Published online: February 28, 2007
AIM: To investigate the hemodynamic changes in a precancerous lesion model of hepatocellular carcinoma (HCC).
METHODS: Hemodynamic changes in 18 Wistar rats were studied with non-invasive magnetic resonance (MR) perfusion. The changes induced by diethylnitrosamine (DEN) developed into liver nodular lesions due to hepatic cirrhosis during the progression of carcinogenesis. The MR perfusion data [positive enhancement integral (PEI)] were compared between the nodular lesions corresponding well with MR images and pathology and their surrounding hepatic parenchyma.
RESULTS: A total of 46 nodules were located by MR imaging and autopsy, including 22 dysplastic nodules (DN), 9 regenerative nodules (RN), 10 early HCCs and 5 overt HCCs. Among the 22 DNs, 6 were low-grade DN (LGDN) and 16 were high-grade DN (HGDN). The average PEI of RN, DN, early and overt HCC was 205.67 ± 31.17, 161.94 ± 20.74, 226.09 ± 34.83, 491.86 ± 44.61 respectively, and their liver parenchyma nearby was 204.84 ± 70.19. Comparison of the blood perfusion index between each RN and its surrounding hepatic parenchyma showed no statistically significant difference (P = 0.06). There were significant differences in DN (P = 0.02). During the late hepatic arterial phase, the perfusion curve in DN declined. DN had an iso-signal intensity at the early hepatic arterial phase and a low signal intensity at the portal venous phase. Of the 10 early HCCs, 4 demonstrated less blood perfusion and 6 displayed minimally increased blood flow compared to the surrounding parenchyma. Five HCCs showed significantly increased blood supply compared to the surrounding parenchyma (P = 0.02).
CONCLUSION: Non-invasive MR perfusion can detect changes in blood supply of precancerous lesions.
- Citation: Guan S, Zhao WD, Zhou KR, Peng WJ, Tang F, Mao J. Assessment of hemodynamics in precancerous lesion of hepatocellular carcinoma: Evaluation with MR perfusion. World J Gastroenterol 2007; 13(8): 1182-1186
- URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1182
The changes of blood flow in dysplastic and regenerative nodules in patients with hepatic cirrhosis have been evaluated in previous studies with computed tomography (CT) arterial portography (CTAP) and CT hepatic arteriography (CTHA) techniques[1-3]. It has been demonstrated that during the late stage of hepatic cirrhosis, sequential hemodynamic changes in blood supply of dysplastic nodules lead to the development of early hepatocellular carcinoma (HCC). The main portal venous blood supply of these nodules gradually changes into the hepatic arterial supply. However, invasive methods require simultaneous catheterization of hepatic artery and superior mesentery artery to inject contrast enhancement substances. Because of their invasiveness, these methods have limited clinical application. With the development of techniques, especially the reduction of scanning time, magnetic resonance imaging (MRI) following intravenous injection of extracellular contrast agents has recently been described and validated to assess perfusion parameters in liver[4-7]. With this method, the contrast agent in blood flow of arterial and portal vein can be measured non-invasively. The purpose of this study was to assess experimentally whether MR perfusion can demonstrate the hemodynamic changes of liver nodular lesions.
The study protocol was reviewed and approved by the Institutional Committee for Animal Care of Fudan University. Experiments were performed on 18 6-wk old male Wistar rats weighing 150 ± 10 g (Experimental Animal Center of Fudan University, Shanghai, China). All animals were housed in independent ventilating cabinets (IVC) at 18-22°C with 55% humidity in a 12 h light/dark cycle with free access to clean diet. Lesions in rats were induced by diethylnitrosamine (DEN, 0.99 mg/mL, Sigma, USA) in order to develop hepatocellular carcinoma during carcinogenesis. The animals received DEN (10 mg/kg per day) in drinking water at 0.01% g/L from a fresh DEN solution prepared every two days for 100 d.
A 1.5 Tesla magnetic resonance (MR) system with a 40 mT/m maximum gradient capability (TwinSpeed, Excite II, double gradient field, General Electric, USA) and 3-inch surface coil was employed and two rats were scanned every week from the 12th wk to the 20th wk after they were exposed to carcinogen. The rats were deeply anesthetized with a mixture of urethane (25%) and diazepam (2 mg) before MR scanning. Plain scanning was performed first in order to show the position of nodules, including sequences of 3-planar-localization scanning, axial and coronal T2 weighted scanning (T2WI), and axial T1 weighted (T1WI) scanning (in-phase and out-phase).
MR perfusion scanning was only performed on the nodules of interest demonstrated by T2WI. Fast spoiled gradient-echo sequence (FSPGR) was selected and the time resolution was 4 sections/2 s. Major protocol parameters included TR/TE: 5.8-8 ms/4-2.7 ms; band width: 31.3-19.2 kHz; section thickness: 2 mm; intersection gap: 0.5 mm; matrix: 128 × 128; excitation time: 1; FOV: 8. The time of acquisition of perfusion-weighted images was at the 8th phase after the starting point of tail vein bolus injection of diluted gadolinium (0.1 mmol/mL, Magnevist; Schering, Guangzhou, China) (0.2 mmol/kg). The total scaning time was 2 min.
Within 24 h after MR imaging, the rats were killed and their liver was removed at autopsy. The whole liver was serially sectioned into 1 mm-thick sections corresponding to the coronal plane MR image. The sections were fixed in a 10% formalin solution for 48 h and paraffin-embedded. Standard hematoxylin-eosin staining was used to assess the nature of nodules and immunohistochemical staining was performed for reticulin protein, CD31, CD34 antibodies. Transmission electron microscopy was also performed to study the ultrastructure of hepatic cancer cells. Pathologic diagnosis was made by two specialists independently and all nodules were defined grossly and microscopically following the criteria and nomenclature of the International Working Party on the Terminology of Nodular Hepatocellular Lesions[8].
Data processing was performed at a workstation with AW 4.2 software package (General Electric, USA). Signal intensity and time curves were derived from manually drawing regions of interest (ROIs) on nodules and surrounding hepatic parenchyma. Positive enhancement integral (PEI) representing the blood perfusion could be obtained by setting the start (8th) and end (12th) phases. Values were expressed as mean ± SD. Statistical analysis was performed with SPSS 11.0. Perfusion data between nodules and parenchyma nearby were compared with t test. P < 0.05 was considered statistically significant.
A total of 46 nodules were located by MR imaging and autopsy, including 22 dysplastic nodules (DN), 9 regenerative nodules (RN), 10 early HCCs and 5 overt HCCs. Among the DN (2-7 mm, average 4.6 mm), 6 were low grade DN (LGDN) and 16 were high grade DN (HGDN). The average positive enhancement integral (PEI) of DN, early and overt HCCs was 205.67 ± 31.17,161.94 ± 20.74,226.09 ± 34.83, 491.86 ± 44.61 respectively, and their adjacent liver parenchyma was 204.84 ± 70.19 (Table 1).
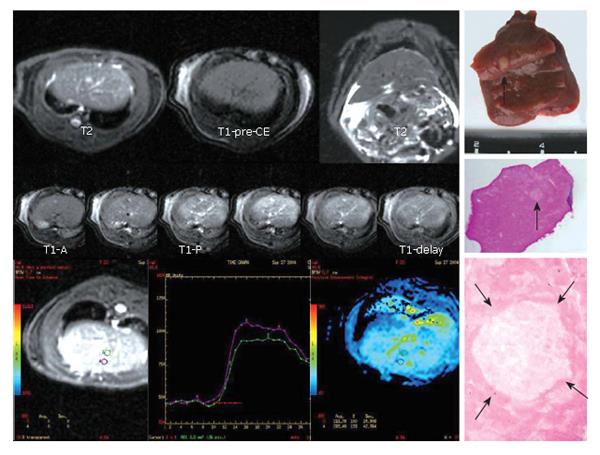
By comparing the blood perfusion index between nodules and their surrounding hepatic parenchyma, we found no difference in RN (t = -5.303, P = 0.06) showing the same signal intensity as its adjacent parenchyma at both hepatic artery phase and portal vein phase of MR perfusion. There were significant differences in dysplastic nodules (t = -3.63, P = 0.02) which manifested decreased blood perfusion. At the later phase of hepatic artery, the perfusion curve in DN declined when compared with surrounding parenchyma. DN showed an iso-signal intensity at hepatic artery phase and a low signal intensity at portal vein phase (Figure 1).
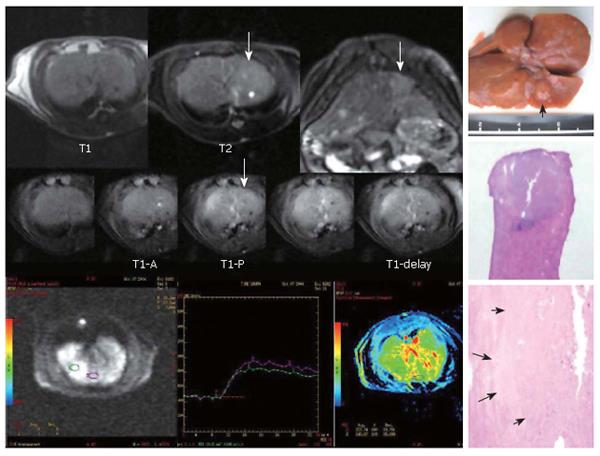
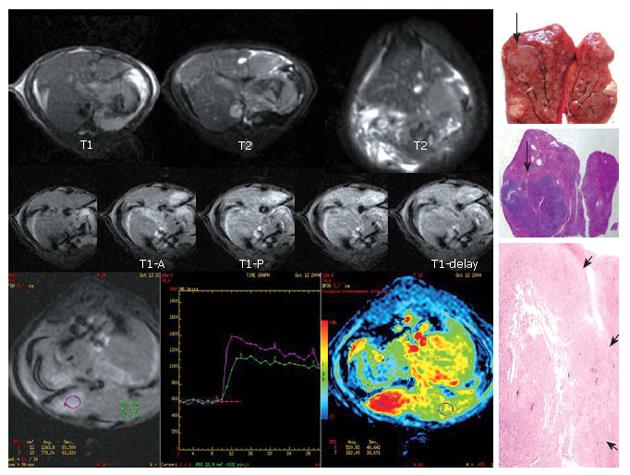
Of the 10 early HCCs, 4 manifested low perfusion and the average PEI value was smaller than that of adjacent parenchyma, showing an iso-signal intensity at the phase of artery and a low signal intensity at portal vein phase. The PEI values were higher in 6 early HCCs than in parenchyma nearby, which had a relatively higher signal intensity at hepatic artery phase and a low signal intensity at portal vein phase (Figure 2). Five overt HCCs had a remarkably higher signal intensity at hepatic artery phase and their perfusion curves ran up when compared with the surrounding parenchyma at early artery phase. The PEI value of HCC was much higher than that of adjacent parenchyma and the difference was significant (t = 3.74, P = 0.02) (Figure 3).
There is cumulative evidence that RN at the late stage of hepatic cirrhosis can develop into HCC through an intermediate step of DN which has been considered as a precancerous lesion by the International Working Party since 1995[8,9], even though the hepatocarcinogenesis has not been clarified thoroughly. Early detection of HCC is critical to patient treatment and survival because of improved surgical techniques for resection and transplantation and new alternative therapeutic options, such as transcatheter chemoembolization or radiofrequency ablation. For surveillance of patients at risk, accurate methods capable of revealing not only HCC but also the premalignant precursor, namely DN, are required.
The imaging characteristics of DN have been previously reported[10-12]. However, the diagnosis of precancerous lesions is still difficult because of the overlapping between DN and RN, DN and early HCC on ultrasound, CT and MRI. Meanwhile, some researchers demonstrated that RN has almost the same blood flow as liver, which is mainly supplied by portal vein, and hepatic artery accounting for only a small part of total blood flow[13]. When RN progresses into DN, the blood flow in most parts of DN decreases (mainly portal vein blood supply) compared with surrounding parenchyma, and HCC always has increased blood flow (mainly hepatic artery blood supply) than its adjacent parenchyma. Until now this change has been regarded as the key-point to differentiate the nodules in liver. CTAP and CTHA are considered the best ways to make their differential diagnosis. But the application is limited in clinical practice due to their invasiveness. Non-invasive methods at present mainly focus on multi-detector spiral CT, MRI, PET and harmonic ultrasound[14-17]. To our knowledge, hemodynamic changes in hepatic nodules studied with MR perfusion have not been previously reported.
In the present study, MR perfusion demonstrated hemodynamic changes during the transition of RN to DN, early and overt HCC. The results obtained by ultra-fast MR perfusion scanning are consistent with those by invasive methods of CTAP or CTHA[18]. Moreover, hepatic artery segment of the perfusion curve in nodules declined slightly compared with surrounding parenchyma at the stage of HGDN and early HCC, suggesting that the preexisting hepatic artery is destructed in nodules. Otherwise, the positive enhancement integral representing the total blood flow would show polarization during this period. Part of them presented as increased blood flow (6/10 in early HCC; 5/16 in HGDN), but decreased blood flow was also observed at the same time in others (4/10 in early HCC; 11/16 in HGDN). The results of our pathology showed that the blood vessels in DN (including HGDN and LGDN) decreased compared with its surrounding hepatic parenchyma, and an increased number of tumor vessels were seen in HCC and even in early HCC. However, sinusoidal capillarization inside the nodules became gradually prominent during the process from RN, LGDN to HGDN and finally to HCC. This discrepancy cannot be clearly explained at present. The explanation of this phenomenon and the answer to its relationship with the alteration of nodular characteristics, and whether it corresponds with the different changes in tumor cells or in microvessles, depend on further pathologic studies.
The main advantage of MR perfusion is that this procedure provides ample information not only on the hemodynamic changes of nodular lesions in liver, but also on the parenchyma of the tumor. Therefore, this technique may be more valuable for the differential diagnosis of nodules in liver. The sequence we used in this study was fast spin gradient echo (FSPGR) which is as fast as echo-planar imaging (EPI). The ratio of time resolution was 0.5 s/section, but its signal-noise ratio (SNR) and spatial resolution were much higher than those of EPI, suggesting that this sequence is suitable for liver MR perfusion.
Nowadays, although CT perfusion (CTP) and PET are also used to study the hemodynamics, MR perfusion shows more advantages when small nodular lesions in liver and precancerous lesion of HCC are analyzed. It has been reported that MRI is more sensitive than CT and PET in revealing the small nodules in liver, especially in the hepatic parenchyma with the background of serious cirrhosis[19,20]. Moreover, MR perfusion has no side effects of radiation and has a better prospect than CT in study of hemodynamics. However, both MR imaging and CT have their technical defects at present. The scan of perfusion could only focus on ROI of one or several sections and could not cover the whole liver, while nodular lesions liver are usually multiple and located in different lobes., thus limiting its application in clinical practice. Newly developed harmonic ultrasound showing the vascular structure inside nodules more clearly, may have a broader prospect. Reports on the application of this technique in liver are few and further investigation is needed[21].
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Moriyama N, Takayama T, Yamasaki S, Sakamoto M, Hirohashi S. Early hepatocellular carcinoma: appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology. 1995;194:101-105. [PubMed] |
| 2. | Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Yokoyama R, Nakamura H. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. AJR Am J Roentgenol. 1997;168:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Mikami S, Kubo S, Hirohashi K, Shuto T, Kinoshita H, Nakamura K, Yamada R. Computed tomography during arteriography and arterial portography in small hepatocellular carcinoma and dysplastic nodule: a prospective study. Jpn J Cancer Res. 2000;91:859-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Materne R, Smith AM, Peeters F, Dehoux JP, Keyeux A, Horsmans Y, Van Beers BE. Assessment of hepatic perfusion parameters with dynamic MRI. Magn Reson Med. 2002;47:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Cha S. Perfusion MR imaging: basic principles and clinical applications. Magn Reson Imaging Clin N Am. 2003;11:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Zhao JG, Feng GS, Kong XQ, Li X, Li MH, Cheng YS. Changes of tumor microcirculation after transcatheter arterial chemoembolization: first pass perfusion MR imaging and Chinese ink casting in a rabbit model. World J Gastroenterol. 2004;10:1415-1420. [PubMed] |
| 7. | Zhao JG, Feng GS, Kong XQ, Li X, Li MH, Cheng YS. Assessment of hepatocellular carcinoma vascularity before and after transcatheter arterial chemoembolization by using first pass perfusion weighted MR imaging. World J Gastroenterol. 2004;10:1152-1156. [PubMed] |
| 8. | Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 686] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 9. | Su Q, Bannasch P. Relevance of hepatic preneoplasia for human hepatocarcinogenesis. Toxicol Pathol. 2003;31:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Efremidis SC, Hytiroglou P. The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol. 2002;12:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Kim MJ, Lim JH, Lee SJ, Kim SH, Lee WJ, Lim HK, Park JM, Park CK. Correlation between the echogenicity of dysplastic nodules and their histopathologically determined fat content. J Ultrasound Med. 2003;22:327-334. [PubMed] |
| 12. | Lim JH, Choi BI. Dysplastic nodules in liver cirrhosis: imaging. Abdom Imaging. 2002;27:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [PubMed] |
| 14. | Lim JH, Kim MJ, Park CK, Kang SS, Lee WJ, Lim HK. Dysplastic nodules in liver cirrhosis: detection with triple phase helical dynamic CT. Br J Radiol. 2004;77:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Kapanen MK, Halavaara JT, Häkkinen AM. Assessment of vascular physiology of tumorous livers: comparison of two different methods. Acad Radiol. 2003;10:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ward J, Robinson PJ. How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol. 2002;12:2258-2272. [PubMed] |
| 17. | Theise ND, Park YN, Kojiro M. Dysplastic nodules and hepatocarcinogenesis. Clin Liver Dis. 2002;6:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 20. | Krinsky GA, Lee VS, Theise ND. Focal lesions in the cirrhotic liver: high resolution ex vivo MRI with pathologic correlation. J Comput Assist Tomogr. 2000;24:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Honda T, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Toyoda H, Ishiguro H, Ogawa S, Takeshima K. Comparison of contrast-enhanced harmonic ultrasonography and power Doppler ultrasonography for depicting vascularity of hepatocellular carcinoma identified by angiography-assisted CT. Hepatol Res. 2003;27:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |