Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1175
Revised: October 28, 2006
Accepted: December 28, 2006
Published online: February 28, 2007
AIM: To investigate the growth inhibitory mechanism of NS-398, a selective cyclooxygenase-2 (COX-2) inhibitor, in two hepatocellular carcinoma (HCC) cell lines (HepG2 and Huh7).
METHODS: HepG2 and Huh7 cells were treated with NS-398. Its effects on cell viability, cell proliferation, cell cycles, and gene expression were respectively evaluated by water-soluble tetrazolium salt (WST-1) assay, 4’-6-diamidino-2-phenylindole (DAPI) staining, flow cytometer analysis, and Western blotting, with dimethyl sulfoxide (DMSO) as positive control.
RESULTS: NS-398 showed dose- and time-dependent growth-inhibitory effects on the two cell lines. Proliferating cell nuclear antigen (PCNA) expressions in HepG2 and Huh7 cells, particularly in Huh7 cells were inhibited in a time- and dose-independent manner. NS-398 caused cell cycle arrest in the G1 phase with cell accumulation in the sub-G1 phase in HepG2 and Huh7 cell lines. No evidence of apoptosis was observed in two cell lines.
CONCLUSION: NS-398 reduces cell proliferation by inducing cell cycle arrest in HepG2 and Huh7 cell lines, and COX-2 inhibitors may have potent chemoprevention effects on human hepatocellular carcinoma.
-
Citation: Baek JY, Hur W, Wang JS, Bae SH, Yoon SK. Selective COX-2 inhibitor, NS-398, suppresses cellular proliferation in human hepatocellular carcinoma cell lines
via cell cycle arrest. World J Gastroenterol 2007; 13(8): 1175-1181 - URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1175.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1175
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide[1]. Although HCC is significantly more prevalent in Asia and Africa, a rising incidence has been reported in Western countries. Most patients present at an advanced stage when successful surgical treatment is no longer feasible and current therapeutic options achieve clinical responses in only a small percentage of cases. As a consequence, effective approaches for prevention and treatment need to be established.
Epidemiological and experimental studies have demonstrated the effect of non-steroidal anti-inflammatory drugs (NSAIDs) in the prevention of human cancers, especially those in the gastroenterological tract[2-4]. Epidemiological studies have shown that the rate of mortality of colorectal cancer in individuals taking NSAIDs is 40%-50% lower than that in non-users[4]. NSAIDs inhibit endogenous prostaglandin synthesis. The key step in the enzymatic conversion of arachidonic acid to prostaglandins is catalyzed by cyclooxygenases (COX), and represents the specific target of NSAIDs.
Two forms of cyclooxygenase, COX-1 and COX-2 have been identified. COX-1 is expressed constitutively in most tissues and appears to be responsible for prostaglandin production in various physiological functions such as platelet aggregation and maintenance of gastric mucosa. In contrast, COX-2 is induced by several inflammatory cytokines and growth factors[5] and frequently over-expressed in various tumor cells. Selective COX-2 inhibitors do not affect platelet aggregation or bleeding time and cause far fewer acute gastric erosions than several nonselective inhibitors. Therefore, selective COX-2 inhibitors such as celecoxib and NS-398 are preferable to nonselective COX-2 inhibitors.
The role of COX-2 in hepatocellular carcinogenesis is still unclear. Previous studies have shown increased expression of COX-2 in patients with various liver diseases, suggesting its possible role in chronic liver disease and during HCC progression[6-8]. In addition, some studies have described the possible benefits of treatment with COX-2 inhibitors as indicated by their effects on human HCC cell lines[8-10]. However, contrasting results have also been reported about the expression of COX-2 or the effects of COX-2 inhibitors on HCC cell lines[11]. COX-2 may be a logical therapeutic target in various human cancers including HCC. However, information regarding the mechanisms involved in these effects is scant and sometimes contradictory[12-15].
In this study, we examined the effects and possible mechanism of NS-398, a selective COX-2 inhibitor in two human hepatoma cell lines, HepG2, and Huh7.
Human hepatoma cell lines HepG2 (showing wild-type p53) and Huh7 (showing mutant-type p53) were obtained from the American Type Culture Collection (Rockville, MD, USA). Each cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), essential amino acids, 100 units/mL penicillin (Invitrogen), and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). All cells were maintained in a humidified atmosphere containing 950 mL/L air and 50 mL/L CO2 at 37°C. NS-398 was purchased from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in dimethyl sulfoxide (DMSO).
To evaluate the inhibitory growth effect of NS-398, we confirmed its cytotoxicity and proliferating activity by water-soluble tetrazolium salt (WST-1) assay (Roche Diagnostics, Mannheim, Germany). The WST-1 assay is a colorimetric method in which the dye intensity is proportional to the number of viable cells. Cells were seeded into 96-well microtiter plates at a concentration of 5 × 103 cells/well. After 12-h incubation, cells were treated with serum-free media containing various concentrations of NS-398 (0, 50, 100, and 200 μmol/L) for 24, 48, and 72 h. Because we used DMSO-dissolved NS-398, cells cultured in the serum-free media containing an equivalent volume of DMSO without NS-398 respectively were used as controls to ensure DMSO not to promote unwanted cellular effects. The final concentration of DMSO was < 0.3%. After incubation, the cells were washed with PBS and the cell proliferation reagent WST-1 was added with cell culture medium, and incubated for 4 h. Sample absorbence was analyzed using a bichromatic ELISA reader at 450 nm. All experiments were performed in triplicate.
Characterization of apoptosis and/or necrosis was carried out after propidium iodide (PI) and Annexin V-FITC staining with apoptosis detection kitI(Pharmingen, San Diego, CA, USA) followed by flow cytometric analysis after 24, 48 and 72 h of 100 μmol/L NS-398 treatment in HepG2 and Huh7 according to the manufacturer’s instructions. For histogram analysis after PI staining, the cells were fixed with 70% ethanol in PBS for 15 min at -20°C. The fixed cells were incubated with RNase A (100 μg/mL) for 30 min at 37°C and then stained with PI (50 mg/mL in PBS). Fluorescence of individual nuclei and intact cells was determined using a FACScalibur flow cytometer (Beacton Dickinson, San Jose, CA).
For detection of apoptosis, cells were washed twice with ice cold PBS and stained simultaneously with FITC-conjugated Annexin V and PI for 20 min on ice in the dark with a binding buffer. Within the next hour, cells were analyzed for apoptosis. The total number of apoptotic cells was determined by calculation of Annexin V+ and PI-cells (reflecting early apoptosis) together with annexin V+ and PI+ cells (reflecting late apoptosis/secondary necrosis).
4’-6-diamidino-2-phenylindole (DAPI), a DNA-binding fluorescent dye, was used to determine whether the mechanism of growth inhibition upon NS-398 treatment is apoptosis. After treatment with 100 μmol/L NS-398 for 0, 24, 48, and 72 h, the cells were washed three times with PBS, fixed in a 3.7% formaldehyde solution for 10 min, fixed once in 1 mL of methanol, and then stained with 4 μg/mL DAPI (Oncor, Gaithersburg, MD) for 10 min. Results were determined by visual observation of nuclear morphology via fluorescence microscopy.
HepG2 and Huh7 cells were seeded on 100-mm dishes at a density of 1.5 × 106 cells/dish. After seeding and overnight incubation, cells were serum-starved for 24 h and incubated in serum-free media with 100 μmol/L NS-398 for 0, 24, 48 and 72 h. Attached and floating cells were harvested together. Cells cultured in the same condition without NS-398 were used as controls. Proteins extracted from either treated or control cells were measured by Bradford assay, and subjected to SDS-PAGE. The proteins were then transferred electrophoretically to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) at 100 V for 1 h at 4°C. To stain the proteins and to validate that equal amounts of protein were loaded in each lane and transferred efficiently, the membrane was immersed in 0.5% Ponceau S (Sigma Chemical Co., St. Louis, MD) in 1% acetic acid. The membrane was washed three times with TBS containing 0.1% Tween 20 (TBS-T). After blocking with 5% skim milk in TBST buffer for 1 h, the membrane was incubated with primary antibodies (anti-mouse COX-2 antibody, 1:500; anti-rabbit caspase-9, caspase-3 and caspase-8, 1:1000; anti-mouse poly [ADP-ribose] -polymerase (PARP) antibody, 1:1000; anti-mouse proliferating cell nuclear antigen (PCNA) antibody, 1:1000 and anti-mouse β-actin antibody, 1:2500) overnight at 4°C. The membrane was incubated with secondary antibodies for 1 h and detected with ECL reagent (Amersham-Pharmacia, UK).
One-way analysis of variance (ANOVA) was performed to test the differences in cellular proliferation and apoptosis of the two cell lines treated with different NS-398 concentrations for varying times. P < 0.05 was considered statistically significant. Data were analyzed using the Statistical Package for Social Sciences (SPSS) (version 11.0).
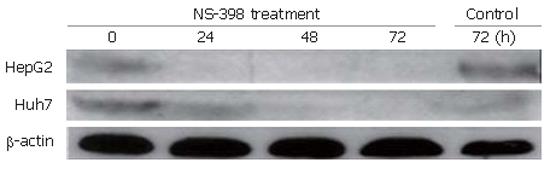
We first characterized the hepatoma cell lines by their expression of COX-2. Samples containing 50 μg of extracted protein were loaded onto the gel. Immunoblot analysis revealed that both cell lines expressed COX-2 protein. The expression of COX-2 was inhibited by NS-398. Immunoblot analysis also demonstrated different COX-2 expressions after treatment with 100 μmol/L NS-398 for different periods of time (Figure 1).
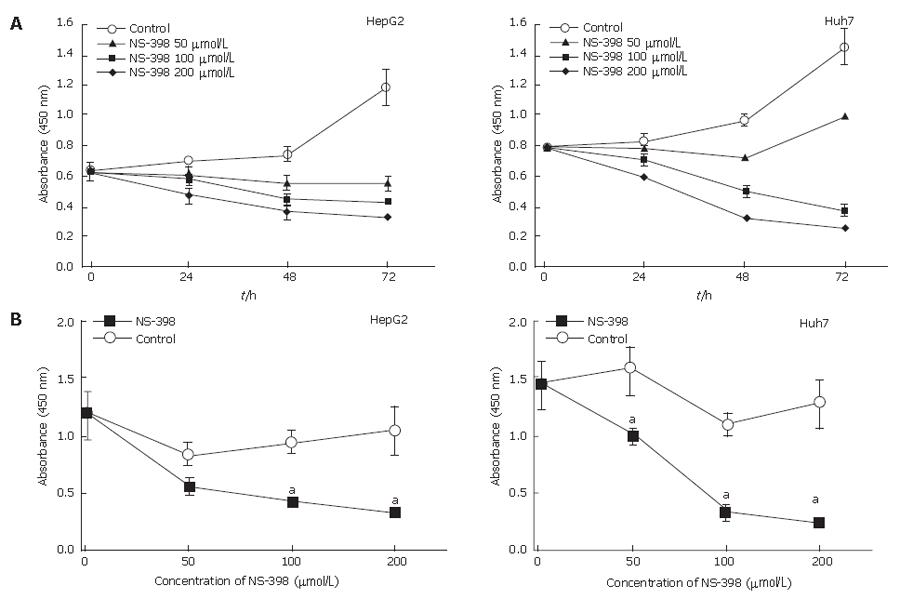
To determine the effect of the selective COX-2 inhibitor NS-398 on HepG2 and Huh7 cells, cell viability was determined by WST-1 assay. Growth-inhibitory experiments were performed by treating cells with various doses of NS-398 for different periods of time. As shown in Figure 2, NS-398 reduced cell viability in a time- and dose-dependent manner on both cell lines. However, treatment with only DMSO as a control did not alter HepG2 and Huh7 cell proliferation (P > 0.05).
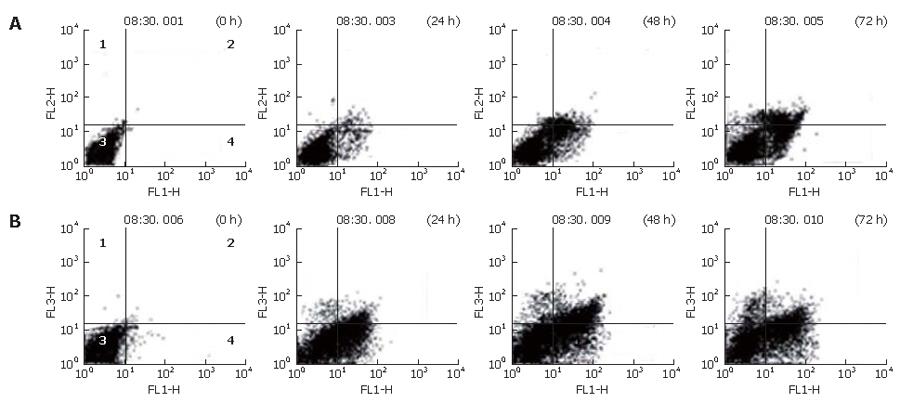
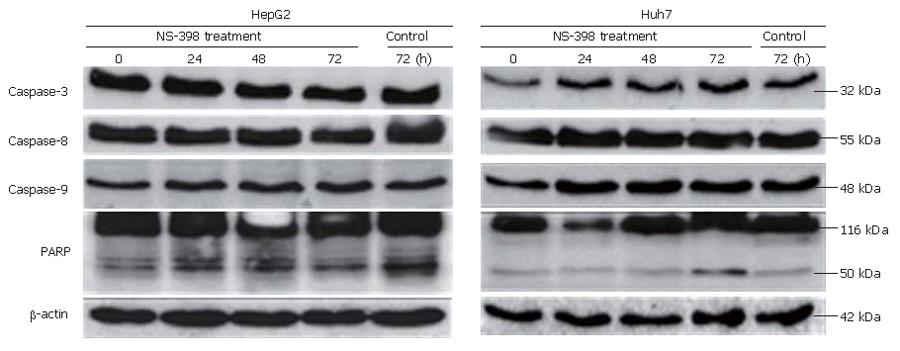
To confirm that NS-398 induces apoptosis of COX-2-expressing HepG2 and Huh7 cells, a double-staining method using FITC-conjugated Annexin V and PI, DAPI staining and Western blotting were done. In the Annexin and PI staining, the percentages of apoptotic and necrotic cells at 0, 24, 48 and 72 h after 100 μmol/L of NS-398 treatment were not significantly different in HepG2 and Huh7 cells (Figure 3). Moreover, no condensed and fragmented nuclei were found in DAPI staining (data not shown). Immunoblot analysis showed that there was no activation of caspase-3, caspase-8, caspase-9 and PARPs in HepG2 and Huh7 cells treated with 100 μmol/L of NS-398 (Figure 4). Taken together, NS-398 did not induce apoptosis in HepG2 and Huh7 cells.
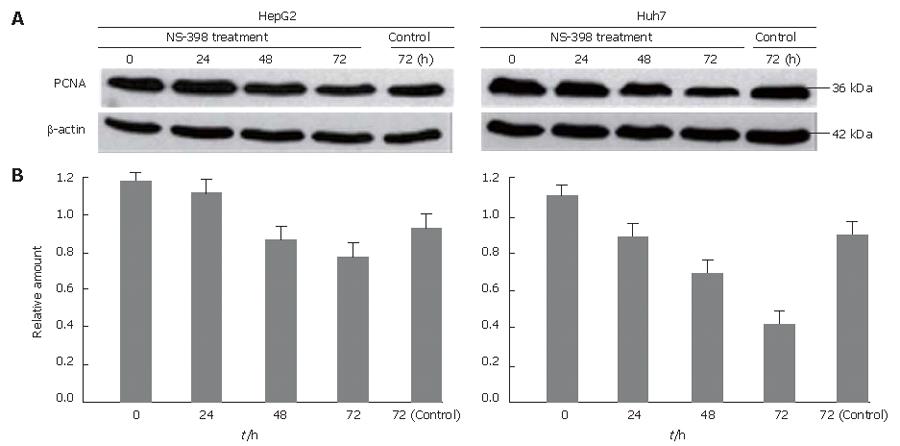
To test the effects of NS-398 on cellular proliferation, the two HCC cell lines were treated with 100 μmol/L of NS-398. We detected PCNA protein by IB analysis. As shown in Figure 5, both cell lines showed a down-regulation of PCNA expression after treatment with 100 μmol/L of NS-398 in a time dependent manner. In particular, NS-398 treatments significantly suppressed the cellular proliferation in Huh7 cells.
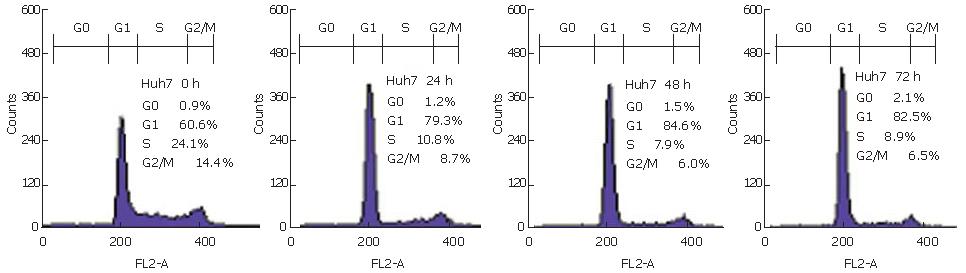
To determine whether suppressions of proliferation in Huh7 cells induced by NS-398 are associated with cell-cycle arrest, we measured DNA content by flow cytometry. We observed that the population of cells in sub-G1 phase increased after 100 μmol/L NS-398 treatment in both cell lines for different periods of time. A significant correlation was observed between the growth inhibition and accumulation of cells in sub-G1 phase for Huh7 at 48 h (Figure 6).
COX-2 is involved in several pathological processes such as inflammation and cancer, and inhibition of apoptosis is regarded as one mechanism by which COX-2 contributes to tumorigenesis. Recent studies demonstrated that selective COX-2 inhibitors (including NS-398) which can inhibit cell proliferation or induce apoptosis are found not only in HCC cell lines but also in colorectal[2,3], esophageal[14], bladder[15], prostatic[16], lung[17], cervix[18] carcinoma cells.
Over-expression of COX-2 has been demonstrated in patients with HCC and high COX-2 expression in non-tumor liver tissue is significantly associated with inflammatory activity[6,8,19]. These findings indicate that COX-2 expression plays an important role in hepatic inflammation and malignant transformation of hepatocytes[20]. However, the key mechanism by which COX-2 affects HCC cell growth remains unclear.
In this study, we detected the expression of COX-2 protein in HepG2 and Huh7 cells by Western blotting. Decreased changes in COX-2 protein expression were observed during the course of growth inhibition by NS-398 treatment (Figure 1). Our findings suggest that the growth-inhibitory effect on hepatocellular carcinoma cell lines is dependent on COX-2 expression[10]. However, several other studies have shown that there is no correlation between COX-2 expression and growth-inhibitory effects induced by NSAIDs[9,11,21].
NS-398-induced inhibition of cell growth is both dose- and time-dependent (Figure 2). The association of COX-2 inhibition with suppression of tumor cell proliferation remains unclear in HCC. Our findings demonstrate that NS-398 may inhibit cell growth in a dose- and time-dependent manner, due to the result of evident cell cycle arrest at G1 (Figures 2 and 6)[11]. Decreased PCNA expression supports the finding of a reduced number of cells in S phase by cell cycle analysis, which is an important event in cell cycle arrest of HepG2 (wild-type p53) and Huh-7 (p53-220Cys) induced by NS-398. In particular, NS-398 treatment significantly suppressed Huh7 proliferation. Recent reports indicate a complex relationship between the tumor suppressor p53 and COX-2[22,23]. While wild-type p53 over-expression leads to increased COX-2 levels[24], opposite effects where p53 inhibits COX-2 transcription have been reported[25]. Thus, different chemosensitivity to the selective COX-2 inhibitor NS-398 might be associated with different p53 status, including Huh-7 (mutated p53) and HepG2 (wild-type p53).
Several other studies, however, have shown that NS-398 inhibits cell proliferation and induces apoptosis via caspase-dependent or caspase-independent pathway in hepatoma cell lines[7,26,27]. But in our experiments, we obtained all negative data in Western blots and DAPI staining. This discrepancy may be due, at least in part, to sub-clone variation and different culture conditions[9,28].
Alteration of apoptosis is considered an important mechanism of carcinogenesis[21,26]. Caspases can be divided, based on their activity, into initiator caspases (caspase-8 and -9) and effector caspases (caspases-3, -6 and -7). In our study, no definite evidence of apoptosis was found after NS-398 treatment of both cell lines, suggesting that the major mechanism of growth inhibition by NS-398 may be anti-proliferation by cell cycle arrest rather than apoptosis.
In conclusion, selective COX-2 inhibitor, NS-398, inhibits the growth of HepG2 and Huh7 cells by inducing cell cycle arrest and is a potential candidate as an effective chemopreventive tool against human HCC.
S- Editor Wang J L-Editor Wang XL E- Editor Chin GJ
| 1. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 454] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679-1683. [PubMed] |
| 4. | Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97-102. [PubMed] |
| 5. | Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3-21. [PubMed] |
| 6. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Rahman MA, Dhar DK, Masunaga R, Yamanoi A, Kohno H, Nagasue N. Sulindac and exisulind exhibit a significant antiproliferative effect and induce apoptosis in human hepatocellular carcinoma cell lines. Cancer Res. 2000;60:2085-2089. [PubMed] |
| 8. | Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1410-1418. [PubMed] |
| 9. | Cheng AS, Chan HL, Leung WK, Wong N, Johnson PJ, Sung JJ. Specific COX-2 inhibitor, NS-398, suppresses cellular proliferation and induces apoptosis in human hepatocellular carcinoma cells. Int J Oncol. 2003;23:113-119. [PubMed] |
| 10. | Hu KQ, Yu CH, Mineyama Y, McCracken JD, Hillebrand DJ, Hasan M. Inhibited proliferation of cyclooxygenase-2 expressing human hepatoma cells by NS-398, a selective COX-2 inhibitor. Int J Oncol. 2003;22:757-763. [PubMed] |
| 11. | Cheng J, Imanishi H, Amuro Y, Hada T. NS-398, a selective cyclooxygenase 2 inhibitor, inhibited cell growth and induced cell cycle arrest in human hepatocellular carcinoma cell lines. Int J Cancer. 2002;99:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Cheng J, Imanishi H, Liu W, Nakamura H, Morisaki T, Higashino K, Hada T. Involvement of cell cycle regulatory proteins and MAP kinase signaling pathway in growth inhibition and cell cycle arrest by a selective cyclooxygenase 2 inhibitor, etodolac, in human hepatocellular carcinoma cell lines. Cancer Sci. 2004;95:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kern MA, Schöneweiss MM, Sahi D, Bahlo M, Haugg AM, Kasper HU, Dienes HP, Käferstein H, Breuhahn K, Schirmacher P. Cyclooxygenase-2 inhibitors suppress the growth of human hepatocellular carcinoma implants in nude mice. Carcinogenesis. 2004;25:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Yu HP, Shi LY, Lu WH, Su YH, Li YY, Xu SQ. Expression of cyclooxygenase-2 (COX-2) in human esophageal cancer and in vitro inhibition by a specific COX-2 inhibitor, NS-398. J Gastroenterol Hepatol. 2004;19:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Choi EM, Kwak SJ, Kim YM, Ha KS, Kim JI, Lee SW, Han JA. COX-2 inhibits anoikis by activation of the PI-3K/Akt pathway in human bladder cancer cells. Exp Mol Med. 2005;37:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245-4249. [PubMed] |
| 17. | Hida T, Leyton J, Makheja AN, Ben-Av P, Hla T, Martinez A, Mulshine J, Malkani S, Chung P, Moody TW. Non-small cell lung cancer cycloxygenase activity and proliferation are inhibited by non-steroidal antiinflammatory drugs. Anticancer Res. 1998;18:775-782. [PubMed] |
| 18. | Kim SH, Song SH, Kim SG, Chun KS, Lim SY, Na HK, Kim JW, Surh YJ, Bang YJ, Song YS. Celecoxib induces apoptosis in cervical cancer cells independent of cyclooxygenase using NF-kappaB as a possible target. J Cancer Res Clin Oncol. 2004;130:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005-4012. [PubMed] |
| 20. | Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1047] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 21. | Huang DS, Shen KZ, Wei JF, Liang TB, Zheng SS, Xie HY. Specific COX-2 inhibitor NS398 induces apoptosis in human liver cancer cell line HepG2 through BCL-2. World J Gastroenterol. 2005;11:204-207. [PubMed] |
| 22. | Lee DW, Park SW, Park SY, Heo DS, Kim KH, Sung MW. Effects of p53 or p27 overexpression on cyclooxygenase-2 gene expression in head and neck squamous cell carcinoma cell lines. Head Neck. 2004;26:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Choi EM, Heo JI, Oh JY, Kim YM, Ha KS, Kim JI, Han JA. COX-2 regulates p53 activity and inhibits DNA damage-induced apoptosis. Biochem Biophys Res Commun. 2005;328:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Corcoran CA, He Q, Huang Y, Sheikh MS. Cyclooxygenase-2 interacts with p53 and interferes with p53-dependent transcription and apoptosis. Oncogene. 2005;24:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Liu XH, Kirschenbaum A, Yu K, Yao S, Levine AC. Cyclooxygenase-2 suppresses hypoxia-induced apoptosis via a combination of direct and indirect inhibition of p53 activity in a human prostate cancer cell line. J Biol Chem. 2005;280:3817-3823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Foderà D, D'Alessandro N, Cusimano A, Poma P, Notarbartolo M, Lampiasi N, Montalto G, Cervello M. Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Ann N Y Acad Sci. 2004;1028:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |