Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1108
Revised: December 11, 2006
Accepted: January 29, 2007
Published online: February 21, 2007
AIM: To analyze the clinico-pathological spectrum of primary duodenal neoplasms.
METHODS: A total of 55 primary duodenal neoplasms reported in the last 10 years after excluding ampullary and periampullary tumors were included in the study. Clinical details were noted and routine hematoxylin and eosin stained paraffin sections were studied for histological subtyping of the tumors.
RESULTS: On histopathological examination primary duodenal neoplasms were categorized as: epithelial tumor in 27 cases (49.0%) including 10 cases of adenoma, 15 cases of adenocarcinoma, and 2 cases of Brunner gland adenoma; mesenchymal tumor in 9 cases (16.3%) consisting of 4 cases of gastrointestinal stromal tumor, 4 cases of smooth muscle tumor and I case of neurofibroma; lymphoproliferative tumor in 12 cases (21.8%), and neuroendocrine tumor in 7 cases (12.7%).
CONCLUSION: Although non-ampullary/periampullary duodenal adenocarcinomas are rare, they constitute the largest group. Histopathological examination of primary duodenal tumors is important for correct histological subtyping.
- Citation: Bal A, Joshi K, Vaiphei K, Wig J. Primary duodenal neoplasms: A retrospective clinico-pathological analysis. World J Gastroenterol 2007; 13(7): 1108-1111
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1108
Although the small intestine constitutes 75% of the gastrointestinal tract, tumors arising from it are rare. Small intestine tumors account for about 5% of all alimentary tract tumors and the duodenum has a higher proportion of these tumors than the jejunum and ileum[1]. Duodenal carcinomas account for 20%-25% of all small bowel malignancies, whereas sarcomas, carcinoid and lymphomas are less common[2]. Duodenal tumors pose diagnostic difficulties because of their rarity, non-specific signs and symptoms and the fact that duodenum is usually overlooked during upper gastrointestinal endoscopy. The present study was undertaken to analyze the clinico-pathological spectrum of primary duodenal neoplasms reported in the last 10 years.
A retrospective analysis of duodenal tumors retrieved from the Department of Histopathology, PGIMER, over a period of 10 years (1997-2006) was done. A total of 60 duodenal neoplasms after excluding 163 ampullary/periampullary carcinomas were retrieved from the records. All the clinical details like age, sex, presenting symptoms were noted from the patient record files. Relative laboratory and radiological findings were obtained. The specimens were fixed in buffered formalin and processed for paraffin sections. Routine hematoxylin and eosin-stained paraffin sections were studied for histological subtyping of the tumors. Histochemical stains like periodic acid Schiff (PAS), mucicarmine and immunohistochemical stains like c-kit, smooth muscle antigen (SMA), leukocyte common antigen (LCA), CD3, CD20, cytokeratin (CK) and chromogranin, were performed wherever required for exact categorization of the tumors.
Of the 60 duodenal neoplasms retrieved from records, 2 metastatic tumors and 3 with direct infiltration into duodenum from the subjacent site were excluded from the study. The study comprised of 55 cases of primary duodenal neoplasm. The age of the patients ranged from 7 to 70 years and the male to female ratio was 1.9:1. On histopathological examination primary duodenal neoplasms were categorized as: epithelial tumor, mesenchymal tumor, lymphoproliferative tumor, and neuroendocrine tumor (Table 1).
| Pathological diagnosis | Cases n (%) |
| n = 55 | |
| Epithelial tumors | 27 (49.0) |
| Adenoma | 10 |
| Adenocarcinoma | 15 |
| Brunneroma | 2 |
| Mesenchymal tumors | 9 (16.3) |
| GIST | 4 |
| Benign | 1 |
| Borderline | 1 |
| Malignant | 2 |
| Smooth muscle tumors | 4 |
| Leiomyoma | 2 |
| Leiomyosarcoma | 2 |
| Neurofibroma | 1 |
| Lymphoproliferative tumors | 12 (21.8) |
| Non-Hodgkin’s lymphoma | 12 |
| B-Cell lymphoma | 10 |
| T Cell lymphoma | 2 |
| Neuroendocrine tumors | 7 (12.7) |
| Carcinoid | 5 |
| Gastrinoma | 1 |
| Neuroendocrine carcinoma | 1 |
There were 27 cases (49.0%) of epithelial tumor including 10 cases of adenoma, 15 cases of adenocarcinoma, and 2 cases of Brunner gland adenoma.
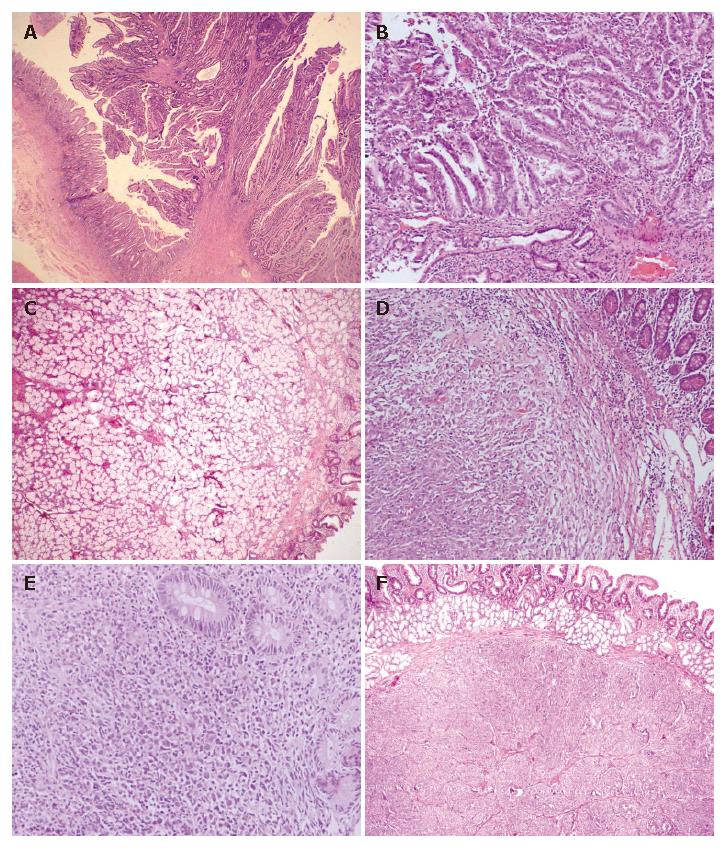
Adenoma: The age range of patients with adenoma was 10-60 years and the male to female ratio was 1:2.3. All adenomas were seen in D1-D2 portion of the duodenum and classified as flat adenoma in 1 case, tubular adenoma in 4 cases, tubulovillous adenoma in 3 cases (Figure 1A), and villous adenoma in 2 cases. Two cases were associated with multiple polyposis coli.
Adenocarcinoma: The age range of patients with adenocarcinoma was 38-70 years and the male to female ratio was 2:1. Of the 15 cases, 10 were found to have adenocarcinoma in D1-D2 portion of the duodenum and 5 were found to have adenocarcinoma in D3-D4 portion of the duodenum. Adenocarcinomas were sub-categorized as well-differentiated adenocarcinoma in 8 cases, moderately-differentiated adenocarcinoma in 2 cases, poorly-differentiated adenocarcinoma in 2 cases, and signet ring cell type adenocarcinoma in 3 cases. One adenocarcinoma was documented to be arising from pre-existing adenoma (Figure 1B).
Brunner gland adenoma: They were both seen in males and in D1 portion of the duodenum. The size of tumor ranged from 3 to 4 cm and microscopic examination showed lobules of Brunner glands separated by fibrovascular septa (Figure 1C).
Nine cases (16.3%) were included in this group including 4 cases of gastrointestinal stromal tumor, 4 cases of smooth muscle tumor and 1 cases of neurofibroma. The age of the patients ranged from 32 to 70 years and the male to female ratio was 1.2:1.
Gastrointestinal stromal tumor (GIST): On patholo-gical examination of 4 cases of GIST, 2 cases were diagnosed as malignant GIST based on the mitotic count of > 10/50HPF and tumor deposits in the omentum. One case was categorized as borderline GIST (5 cm in diameter) with its mitotic count of < 5/50HPF and one case was labeled as benign GIST (Figure 1D). All the cases were positive for c-kit immunostain and negative for SMA and S-100.
Smooth muscle tumor: Leiomyoma was found in 2 cases and leiomyosarcoma in 2 cases, respectively. Leiomyosarcoma showed high mitotic count of > 5/10HPF and areas of necrosis. All the tumors were positive for SMA and negative for c-kit.
Neural tumor: There was a single case of plexiform neurofibroma positive for S-100 immunostain.
Twelve cases (21.8%) of non-Hodgkin’s lymphoma (NHL) were included in this group (Figure 1E). Their age ranged from 7 to 85 years and the male to female ratio was 5:1. All the cases were found to have primary duodenal lymphoma because of the absence of disease in other organs. On immunohistological examination of 12 cases, B-cell phenotype was documented in 10 cases (83.3%) and T-cell phenotype in 2 cases (16.6%). B-cell lymphomas were further categorized as diffuse large cell lymphoma in 8 cases and MALT lymphoma in 2 cases. T-cell duodenal lymphomas were not associated with enteropathy as there was no histologic evidence of villous atrophy.
Seven tumors (12.7%) were located in this histologic category. Of the 7 tumors, 5 were benign and labeled as carcinoid based on their characteristic organoid pattern, granular cytoplasm, salt and pepper nuclear chromatin as well as chromogranin positivity (Figure 1F). One case was suggestive of gastrinoma based upon serum gastrin levels and was associated with parathyroid and adrenal hyperplasia. However, confirmation was not possible on histopathological sections because of non-availability of gastrin immunostain. One case was labeled as malignant as it had metastatic tumor deposits in lymph nodes.
Primary neoplasms of the small intestine constituting 90% of mucosal surface area of the gastrointestinal tract are extremely rare. Duodenum constituting only 4% of the small intestine has a relatively high proportion of all the tumors as compared to the jejunum and ileum. Primary malignant duodenal tumors are uncommon accounting for only 0.3% of all gastrointestinal tumors but about 50% of all small intestinal malignancies[3]. Many hypotheses have been proposed for the low incidence of small intestinal tumors as compared to large intestine including greater fluidity of contents in the small intestine which are less irritating as compared to solid contents, rapid transit time through small intestine thus reducing exposure to potential carcinogens, low bacterial population producing carcinogens and high concentration of lymphoid tissue producing IgA immunoglobulins[4]. It is often difficult to diagnose early duodenal tumors because of their non-specific and insidious presentation.
Adenomas are the most common duodenal tumors including adenomatous polyps and Brunner adenoma. Adenomatous polyps are more common at gastroduodenal junction and have 3%-5% risk of developing adenocarcinoma in life time[5]. Brunner gland adenomas are very rare tumors and less than 150 cases have been reported in the English literature[6]. Size of the tumor is important in differentiating adenoma from Brunner gland hyperplasia. The size less than 1 cm is referred to as Brunner gland hyperplasia. Adenocarcinoma of the duodenum not originating from the region of ampulla is an uncommon neoplasm. Since it was first described in 1746 by Hamburgur, approximately 800 cases have been described in the literature. However in these cases there is a mix of ampullary and peri-ampullary carcinomas[7]. Because of rarity of these tumors, the exact etiological factors have not been defined. Patients with familial adenomatous polyposis and Crohn’s disease or celiac disease have a higher risk of developing duodenal carcinoma[8]. In the present study, there were no such associations. Only one case was arising in the background of adenoma and two cases of tubulovillous adenoma were associated with polyposis coli. Heniford et al[8] reported that D3-D4 is the most common site of primary duodenal adenocarcinoma. However, in the present study 10/15 adenocarcinomas were seen in D1-D2 region. D3-D4 carcinomas have better prognosis as compared to D1-D2 carcinomas because the former behave like hind gut tumors and the latter as foregut tumors[9].
The relative frequency of duodenal stromal tumors is not known. Only one study has presented a series of 190 duodenal stromal tumors[10]. According to their findings, gastrointestinal stromal tumors (GIST), the c-kit positive primary mesenchymal tumor, constitute the largest group of mesenchymal tumors in contrast to smooth muscle tumors i.e. leiomyoma and leiomyosarcoma which are now thought to be rare in the duodenum. In the present series, there were an equal number of GISTs and smooth muscle tumors. However, the number of cases was too small to comment on the relative frequency of these tumors in duodenum. Neural tumors are extremely rare in duodenum and we encountered only one case of neurofibroma. Sub-classification into benign, borderline and malignant stromal tumors based upon size, mitosis, necrosis and metastasis must be made for prognosis[11,12].
Gastrointestinal tract is the most common extranodal site of involvement by non-Hodgkin’s lymphoma (NHL). Primary lymphomas of the small intestine are relatively rare and account for 4%-12% of all NHLs and 19%-38% of small bowel malignancies[13]. In the present study, duodenal lymphomas accounted for 21.8% all duodenal tumors. Recently reports have emerged about the increasing frequency of primary MALT lymphomas in the small intestine and it is proposed that small intestinal MALTs are of gastric origin due to the probable role of H pylori. In this review of duodenal tumors, only two cases were of MALT lymphomas, the remaining were of diffuse large B cell type and T cell lymphomas. It was reported that intestinal T cell lymphomas are more common in small intestine and a term “enteropathy associated T-cell lymphoma” (EATL) has been introduced based upon their existence in coelic disease[14]. However, no such association was seen in our cases. Histopathological examination and exact immunophenotyping of NHLs based on WHO classification are important from the prognosis point of view as T cell lymphomas have poor prognosis as compared to B cell lymphomas. MALT lymphomas in B cell group have a better prognosis.
Duodenal neuroendocrine tumors (NETs) comprise 2%-3% of all gastrointestinal endocrine tumors including gastrinoma, somatostatinoma, nonfunctional NET, and poorly-differentiated NE carcinoma and their frequency is increasing[15]. Although the majority of these tumors are nonfunctional, they can cause Zollinger-Ellison syndrome and other clinical hormonal syndromes. Gastrinomas defined as gastrin secreting tumors are associated with Zollinger-Ellison syndrome (ZES) and hereditary gastrinomas, which are associated with endocrine neoplasia type 1 (MEN1) syndrome. In the present study, there was only one case of gastrinoma associated with parathyroid and adrenal hyperplasia.
In conclusion, although non-ampullary/periampullary duodenal adenocarcinomas are rare, they constitute the largest group. Histopathological examination of primary duodenal tumors is important for the correct histological subtyping of these tumors and collecting prognostic information which influences the management.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Zollinger RM. Primary neoplasms of the small intestine. Am J Surg. 1986;151:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Kaminski N, Shaham D, Eliakim R. Primary tumours of the duodenum. Postgrad Med J. 1993;69:136-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kerremans RP, Lerut J, Penninckx FM. Primary malignant duodenal tumors. Ann Surg. 1979;190:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Negri E, Bosetti C, La Vecchia C, Fioretti F, Conti E, Franceschi S. Risk factors for adenocarcinoma of the small intestine. Int J Cancer. 1999;82:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135:1070-1074; discussion 1074-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Rocco A, Borriello P, Compare D, De Colibus P, Pica L, Iacono A, Nardone G. Large Brunner's gland adenoma: case report and literature review. World J Gastroenterol. 2006;12:1966-1968. [PubMed] |
| 7. | Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996;183:89-96. [PubMed] |
| 8. | Heniford BT, Iannitti DA, Evans P, Gagner M, Henderson JM. Primary nonampullary/periampullary adenocarcinoma of the duodenum. Am Surg. 1998;64:1165-1169. [PubMed] |
| 9. | Tocchi A, Mazzoni G, Puma F, Miccini M, Cassini D, Bettelli E, Tagliacozzo S. Adenocarcinoma of the third and fourth portions of the duodenum: results of surgical treatment. Arch Surg. 2003;138:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Goldblum JR, Appelman HD. Stromal tumors of the duodenum. A histologic and immunohistochemical study of 20 cases. Am J Surg Pathol. 1995;19:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Winfield RD, Hochwald SN, Vogel SB, Hemming AW, Liu C, Cance WG, Grobmyer SR. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg. 2006;72:719-722; discussion 722-723. [PubMed] |
| 13. | Kohno S, Ohshima K, Yoneda S, Kodama T, Shirakusa T, Kikuchi M. Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification. Histopathology. 2003;43:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [PubMed] |
| 15. | Mullen JT, Wang H, Yao JC, Lee JH, Perrier ND, Pisters PW, Lee JE, Evans DB. Carcinoid tumors of the duodenum. Surgery. 2005;138:971-977; discussion 977-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |