Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1098
Revised: November 15, 2006
Accepted: January 12, 2007
Published online: February 21, 2007
AIM: To investigate the incidence of KIT immunoho-stochemical staining in (GI) stromal tumors (GISTs), and to analyze the clinical manifestations of the tumors and prognostic indicators.
METHODS: We retrospectively analyzed 50 cases of previously diagnosed GISTs. Tissue samples were assessed with KIT (CD117 antigen), CD34, SMA, desmin, S-100, NSE, PCNA, Ki-67, and BCL-2 for immunohistochemical study and pathological characteristics were analyzed for prognostic factors.
RESULTS: Fifteen tumors (30%) were negative in KIT staining. A significant association was observed between gender (male patients: 14/15) and KIT-negative staining (P = 0.003).The patients's mean age was 56.6 years. Tumors developed in stomach (n = 8), small intestine (n = 5), large intestine (n = 1) and oesophagus (n = 1). The mean tumor size was 5.72 cm. The mitotic count ranged from 0-29/50 HPF (mean: 3.4) and 73% of tumors showed no necrosis. The majority of the tumors (67%) had dual or epithelioid differentiation. Tumors were classified as very low or low risk (n = 7), intermediate risk (n = 5), and high risk (n = 3) groups. Twelve (80%) patients were alive without evidence of residual tumor for an average period of 40.25 mo (12-82 mo); three patients developed metastatic disease to the liver and eventually died within 2-12 mo (median survival: 8.6 mo).
CONCLUSION: A small subgroup of GISTs fulfils the clinical and morphological criteria of these tumors, and lacks KIT expression. These tumors predominantly developed in the stomach, being dual or epithelioid in morphology, which are classified as low risk tumors and presented a better survival status than KIT-positive tumors. The ability to diagnose GISTs still depends on immunohistochemical staining but the research should extend in gene mutations.
- Citation: Kontogianni-Katsarou K, Lariou C, Tsompanaki E, Vourlakou C, Kairi-Vassilatou E, Mastoris C, Pantazi G, Kondi-Pafiti A. KIT-negative gastrointestinal stromal tumors with a long term follow-up: A new subgroup does exist. World J Gastroenterol 2007; 13(7): 1098-1102
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1098
Most gastrointestinal mesenchymal neoplasms are gastrointestinal stromal tumors (GISTs). Their definitions follow the WHO histological classification where the term GIST is now used for a specific group of tumors comprising the majority of all gastrointestinal stromal tumors[1]. Typically, GISTs are immunohistochemically positive for KIT tyrosine kinase receptor which is perhaps their single best defining feature[2]. Most GISTs are positive for KIT (CD117 antigen), which may show membrane, diffuse cytoplasmic or a perinuclear accentuation pattern.
Histological assessment of malignancy is essentially based on mitotic counts, the size of the lesion and presence or absence of metastasis[3-5]. A proportion of GISTs, especially the malignant tissues show mutations in the regulatory juxtamembrane domain (exon 11) of the KIT gene[6]. Until now, the treatment with selective tyrosine kinase inhibitors, such as imatinib mesylate, for patients with GISTs has hinged on the KIT positive immunostaining tumors. Although the KIT positivity by immunohistochemistry becomes invaluable in the diagnosis of GISTs, some authors believe that a small subgroup of these tumors fulfils the clinical and morphological criteria of GISTs, and lacks KIT expression. The biological features of these tumors have rarely been addressed.
Our aim was to investigate the incidence of KIT immunohostochemical staining in 50 cases of previously diagnosed GI stromal tumors, to carried out a comprehensive examination of GISTs that are negative in CD117 expression, and to analyze the clinical manifestations and prognostic indicators of the tumors.
Using the database of Surgery and Pathology Departments of “Evaggelismos” General Hospital and Areteion University Hospital, we collected records with a pathologic diagnosis of stromal tumor of GI tract. Fifty patients with the diagnosis of GIST between 1994-2004 were retrieved from the archives. Patient age, gender, clinical manifestations, tumor size, pathological characteristics, the presence of distant metastasis and the outcome were recorded.
Tumor specimens were fixed in 10% buffered formalin after gross examination and embedded in paraffin. Histologic sections stained with hematoxylin and eosin were evaluated for all cases. Tumors were classified as very low risk, low risk, intermediate or high risk groups based on histological parameters according to NIH Consencus Guidelines for Grading[4].
The tumor samples from all 50 cases were examined for various markers using commercially available immunohistochemical antibodies against KIT (CD117 antigen), (A4502, polyclonal, Dako, USA; 1:50 dilution), CD34 (clone QBEnd/10) (Novocastra Labs; 1:50), S-100 (clone S1/61/69) (Novocastra Labs; 1:40), smooth-muscle actin (SMA) (clone asm-1) (Dako; 1:200), desmin (clone DE-R-11) (Novocastra Labs; 1:100), neuron-specific enolase (NSE) (clone 5E2) (Novocastra Labs; 1:100), neurofilament protein (NFL) (clone NR4) (Novocastra Labs; 1:50), bcl-2 (clone 124) (Dako; 1:40), proliferating cell nuclear antigen (PNA) (clone PC10) (Novocastra Labs; 1:200), Ki-67 (clone MM1) (Novocastra Labs; 1:200) by a standard three-step immunoperoxidase procedure (APAAP, DAKO, Glostroup, Danmark). Appropriate positive controls were run concurrently for all antibodies tested. According to the percentage of tumor cells showing an immunopositive reaction among the total tumor cells, tumors were reported as negative (≤ 10%) or positive (> 10%).
Data was analyzed using statistical software SPSS version 12.0. Chi-square test or Fisher’s exact test was done for categorical variables to assess differences among baseline patient features. Overall survival was computed by the Kaplan-Meier method. Comparison of survival between subgroups was performed by the log-rank test. The relative importance of prognostic factors for the survival was analyzed with Cox’s proportional hazard model. Statistical significance would be inferred at a two-tailed P value < 0.05.
Thirty-one (62%) patients were male and 19 (38%) female. Their age at diagnosis ranged from 26 to 89 years (mean: 62 ± 14.5). The most common symptoms were abdominal pain (72%). The most common anatomic sites of tumor origin were the small intestine (n = 23) and the stomach (n = 19). Three tumors were located in oesophagus and 5 tumors in large intestine.
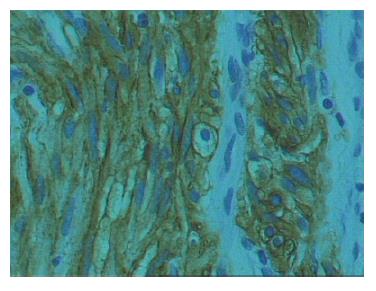
The size of the tumor ranged from 0.2 cm to 30 cm (mean: 4.58 ± 5.2). The mitotic count was 0-29 per 50 HPF (× 400) (mean: 4.25 ± 2). Necrosis was present in 13 (26%) tumors. Twenty-four (48%) tumors showed evidence of dual differentiation toward smooth muscle and neural elements. Reactivity for either SMA or desmin (epithelioid features) was observed in 8 (16%) cases. There was neural differentiation (spindled features) in 7 (14%) cases. No evidence of differentiation toward either cell type, was formed even after exhaustive immunohistochemistry in 11 (22%) cases. Of the 50 tissues tested, 35 (70%) were positive for KIT staining (Figure 1), while 15 (30%) tumors lacked KIT expression. The high incidence of KIT-positive staining (57%) was in tissues diagnosed as “high risk” tumors. Twenty-four (48%) tumors were CD34 positive. The proliferative activity (PCNA labeling index) was high (> 10% labeled nuclei) in 62% of our specimens. Only 6 (12%) cases were characterized by high (> 20% labeled nyclei) Ki-67 immunoreactivity percentages. Bcl-2 protein was positively expressed in the cytoplasm of tumor cells in 26 (52%) specimens.
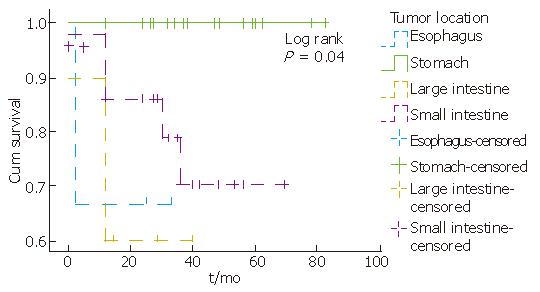
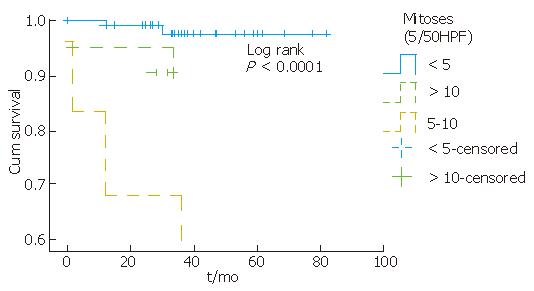
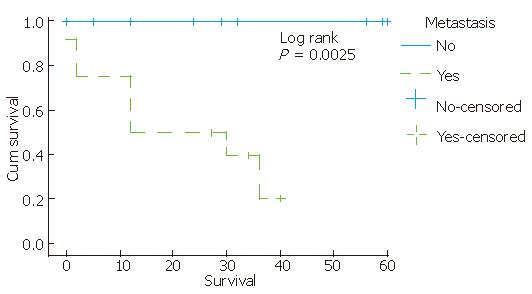
Complete information on patients’ clinical course could be obtained in 50 (100%) cases. According to the available follow-up, patients with KIT-positive staining tumors were alive without evidence of residual tumor for an average period of 32.3 mo (12-82 mo). Tumor location, mitotic counts, risk group and metastasis seem to be related to survival, since partial likelihood ratio test of Cox regression for each of these patient’s feature was less than 0.05 (Figure 2, Figure 3, Figure 4). There was an indication of association between tumor size and mitoses (P = 0.055, Fisher’s Exact test).
Of the 50 tissues tested, a small subgroup of tumors (n = 15) fulfiled the clinical and morphological criteria of GISTs and lacked KIT antigen immunoexpression. The clinicopathological features of KIT-negative cases are shown in Table 1. A highly significant association was observed between gender and CD117 staining (P = 0.003). All, except one, KIT-negative tumors were observed in male patients, while the majority of female patients (18/19) expressed CD117 immunostaining (Table 2).
| CaseNo. | Sex | Age (yr) | Symptoms | Site | Size | Mitoses(/50 HPF) | Presenceof necrosis | Morphology | Risk category | Clinicalstatus | Survivaldata/mo |
| (cm) | |||||||||||
| 1 | M | 60 | Yes | Small Intestine | 4.30 | 1 | No | Epithelioid | Low risk | Primary | Alive/12 |
| 2 | M | 46 | No | Stomach | 0.50 | 0 | No | Mixed | Very low risk | Primary | Alive/24 |
| 3 | M | 50 | No | Small Intestine | 0.50 | 0 | No | Anaplastic | Very low risk | Primary | Alive/24 |
| 4 | M | 64 | Yes | Large Intestine | 4.50 | 10 | Yes | Epithelioid | High risk | Liver metastasis | Dead/12 |
| 5 | M | 78 | Yes | Small Intestine | 7.00 | 1 | Yes | Mixed | Intermediate risk | Liver metastasis | Dead/12 |
| 6 | F | 53 | Yes | Stomach | 5.00 | 0 | No | Spindled | Low risk | Primary | Alive/47 |
| 7 | M | 43 | Yes | Esophagus | 7.00 | 0 | No | Mixed | Intermediate risk | Primary | Alive/25 |
| 8 | M | 51 | Yes | Small Intestine | 6.00 | 2 | Yes | Epithelioid | Intermediate risk | Primary | Alive/12 |
| 9 | M | 69 | No | Stomach | 2.00 | 0 | No | Mixed | Very low risk | Primary | Alive/37 |
| 10 | M | 54 | Yes | Stomach | 3.50 | 0 | No | Spindled | Low risk | Primary | Alive/62 |
| 11 | M | 70 | Yes | Stomach | 6.00 | 0 | No | Anaplastic | Intermediate risk | Primary | Alive/82 |
| 12 | M | 42 | Yes | Stomach | 6.00 | 2 | No | Anaplastic | Intermediate risk | Primary | Alive/56 |
| 13 | M | 26 | No | Stomach | 0.50 | 0 | No | Epithelioid | Very low risk | Primary | Alive/78 |
| 14 | M | 82 | Yes | Stomach | 30.00 | 6 | Yes | Mixed | High risk | Primary | Alive/82 |
| 15 | M | 61 | Yes | Small Intestine | 3.00 | 29 | No | Mixed | High risk | Liver metastasis | Dead/2 |
| CD117 expression | ||||
| Negative | Positive | Total | ||
| Gender | Male | 14 | 17 | 31 |
| 45.20% | 54.80% | 100% | ||
| Female | 1 | 18 | 19 | |
| 5.30% | 94.70% | 100% | ||
| Total | 15 | 35 | 50 | |
Patients' age at diagnosis ranged from 26 to 82 years (mean: 56.6). The majority of them (11/15) presented at the hospital with symptoms, as abdominal pain. KIT-negative GISTs developed in stomach (n = 8), small intestine (n = 5), large intestine (n = 1) and oesophagus (n = 1) (Table 3).
| Tumor location | CD117 expression | |||
| Negative | Positive | Total | ||
| Esophagus | 1 | 2 | 3 | |
| 33.30% | 66.70% | 100% | ||
| Stomach | 8 | 11 | 19 | |
| 42.10% | 57.90% | 100% | ||
| Small intestine | 5 | 18 | 23 | |
| 21.70% | 78.30% | 100% | ||
| Large intestine | 1 | 4 | 5 | |
| 20% | 80% | 100% | ||
| Total | 15 | 35 | 50 | |
| 30% | 70% | 100% | ||
Tumors size ranged from 0.5 cm to 30 cm (mean: 5.72). The majority of tumors were smaller than 5 cm (9/15), and only one was > 10 cm. The mitotic count ranged from 0-29 per 50 HPF (× 400) (mean: 3.4). Twelve (80%) tumors contained less than 5/50 HPF mitoses, 2 (13%) tumors contained mitoses between 5 and 10/50 HPF and 1 (7%) tumor contained mitoses > 10/50 HPF. Absence of necrosis was present in 73% (11/15) of tumors.
Of the 15 KIT-negative samples, 6 (40%) cases had dual differentiation showing histologically mixed spindled and epithelioid type features, four (27%) cases showed histologically predominantly epithelioid type features, two (13%) cases spindled type features, and 3(20%) cases were classified as anaplastic (Table 4).
| Immunohistochemistry | ||||||
| CaseNo. | CD117 | α-SMA | Desmin | S-100 | NSE | CD34 |
| 1 | Negative | Positive | Positive | - | - | Positive |
| 2 | Negative | - | Positive | Positive | - | - |
| 3 | Negative | - | - | - | - | Positive |
| 4 | Negative | Positive | - | - | - | Positive |
| 5 | Negative | Positive | - | - | Positive | - |
| 6 | Negative | - | - | Positive | Positive | - |
| 7 | Negative | Positive | Positive | Positive | - | - |
| 8 | Negative | Positive | - | - | - | Positive |
| 9 | Negative | Positive | Positive | - | Positive | - |
| 10 | Negative | - | - | Positive | Positive | Positive |
| 11 | Negative | - | - | - | - | Positive |
| 12 | Negative | - | - | - | - | Positive |
| 13 | Negative | Positive | Positive | - | - | - |
| 14 | Negative | Positive | - | - | Positive | Positive |
| 15 | Negative | Positive | - | Positive | - | - |
KIT-negative tumors were diagnosed as “very low” and “low risk” (benign) (n = 7), “intermediate risk” (uncertain malignant potential) (n = 5), and “high risk” tumors (malignant potential) (n = 3).
The clinical status was primary presentation in 12 patients. According to the available follow-up, twelve patients (80%) were alive without evidence of residual tumor for an average period of 40.25 mo (12-82 mo); three patients developed metastatic disease to the liver and eventually died within 2-12 mo (median survival: 8.6 mo).
The classification of GISTs has been a continually evolving process reflecting our increasing understanding of the biological nature of these tumors. One of the most important concepts of the recent years is that GISTs show the differentiation of the interstitial cells of Cajal (ICC)[2]. Mesenchymal tumors of GI can be identified based on the features of ICCs and can therefore designated as GISTs[4].
Histological and immunohistochemical advances, and molecular genetics provide a new era for GISTs. KIT, a type III tyrosine kinase growth factor receptor, is the common denominator in most GISTs[7-9]. CD117, the epitope for KIT, is introduced as a new phenotypic marker for distinguishing between GISTs and non-GIST spindle cell tumors of the GI. A small subgroup of GISTs that fulfill the clinical and morphological criteria of these tumors is essentially KIT-negative by immunohistohemistry. The biological features of these tumors have rarely been addressed. In the absence of CD117 immunopositivity, the diagnosis of GISTs is challenging.
Based on this and using the latest clinical and histological criteria, we screened 50 cases of gastrointestinal stromal tumors with a long term follow-up. Generally, as it was expected, tumor location, mitotic counts, risk group and metastasis were significantly associated with survival. Of the 50 tissues tested, 35 (70%) were positive for CD117 staining and 15 (30%) were negative. A significant association was observed between gender and KIT (CD117) immunostaining. KIT-negative tumors were observed in male patients, while the majority of female patients expressed CD117 immunostaining.
The majority of KIT-negative tumors developed in stomach while KIT-positive tumors developed in small intestine. This finding is in accordance with recent studies[10]. The majority of KIT-negative tumors were smaller than 5 cm (9/15), and 80% contained mitoses less than 5/50 HPF.
Of the 15 CD117-negative samples, 6 cases had evidence of dual differentiation, 4 cases showed histologically epithelioid type features, two had spindle type features, and 3 cases were negative for all markers, but positive for CD34 staining. Our findings support previously published data[10,11], suggesting that there is a subgroup of KIT–negative GISTs that exhibit the same clinical and morphological features as the KIT-positive tumors.
The majority of KIT-negative tumors were diagnosed as “very low” or “low risk” tumors, while the highest incidence of KIT-positive staining was found in “high risk” tumors. The majority of patients with KIT-negative tumors (80%) were alive without evidence of residual tumor for an average period of 40.25 mo and presented a better survival status than the patients with KIT-positive tumors.
Benign and malignant GISTs carry mutations in KIT gene. It is still not clear whether mutations are independent prognostic factors[12,13]. We believe that a search for gene mutation, as the c-kit gene, in KIT-negative staining tumors might clarify the diagnosis status (unpublished observations), as other authors believe that KIT mutations[11] or intragenic platelet-derived growth factor-alpha (PDGFR-a) activating mutations are present in some of these tumors[10]. The pharmaceutical development and therapeutic implications of protein tyrosine kinase inhibitors has refocused the attention on GIST. Until recently, no patient with complete response to therapy was reported[14]. Is there any value in separating these tumors with epithelioid or dual differentiation because they are often KIT-antigen negative[15]? Is this going to be the result of a better differentiation status, detection of certain molecular alterations or it may be related to more traditional criteria as size and mitosis rate? There is still challenge to identify those patients who would benefit from receiving the new therapy.
In conclusion, our study confirms that traditional histologic criteria alone are not enough to confirm GISTs diagnosis, but are still the only criteria to estimate biological behavior in these tumors. A small subgroup of GISTs fulfils the clinical and morphological criteria of these tumors, and lacks KIT expression. These tumors predominantly develop in stomach, showing dual or epithelioid morphology; they are classified as “low risk” tumors, and present with a better survival status than KIT-positive staining tumors. The ability to diagnose GISTs still depends on the immunohistochemical staining but the research should expand in gene mutations.
S- Editor Liu Y L- Editor Ma JY E-Editor Lu W
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 2. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 3. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 4. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 5. | Berman J, O'Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol. 2001;32:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Kitamura Y, Hirota S, Nishida T. Molecular pathology of c-kit proto-oncogene and development of gastrointestinal stromal tumors. Ann Chir Gynaecol. 1998;87:282-286. [PubMed] |
| 7. | Koay MH, Goh YW, Iacopetta B, Grieu F, Segal A, Sterrett GF, Platten M, Spagnolo DV. Gastrointestinal stromal tumours (GISTs): a clinicopathological and molecular study of 66 cases. Pathology. 2005;37:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 9. | Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Debiec-Rychter M, Wasag B, Stul M, De Wever I, Van Oosterom A, Hagemeijer A, Sciot R. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol. 2004;202:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Tzen CY, Mau BL. Analysis of CD117-negative gastrointestinal stromal tumors. World J Gastroenterol. 2005;11:1052-1055. [PubMed] |
| 12. | Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Miettinen M. Gastrointestinal stromal tumors: parameters that determine biological potential and guide therapy from a surgical pathologist point of view. Society for Ultrastructural Pathology. USCAP Meeting;. 2003;16-20. |
| 14. | Demetri GD. Identification and treatment of chemoresistant inoperable or metastatic GIST: experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571). Eur J Cancer. 2002;38 Suppl 5:S52-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Herrera GA. Histological perspective: a journey through evolution of classification schemes. Society for Ultrastructural Pathology. USCAP Meeting;. 2003;1-6. |