Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1067
Revised: October 28, 2006
Accepted: November 14, 2006
Published online: February 21, 2007
AIM: To evaluate the safety and feasibility of bone marrow cell (BMC) transplantation in patients with chronic liver disease on the waiting list for liver transplantation.
METHODS: Ten patients (eight males) with chronic liver disease were enrolled to receive infusion of autologous bone marrow-derived cells. Seven patients were classified as Child-Pugh B and three as Child-Pugh C. Baseline assessment included complete clinical and laboratory evaluation and abdominal MRI. Approximately 50 mL of bone marrow aspirate was prepared by centrifugation in a ficoll-hypaque gradient. At least of 100 millions of mononuclear-enriched BMCs were infused into the hepatic artery using the routine technique for arterial chemoembolization for liver tumors. Patients were followed up for adverse events up to 4 mo.
RESULTS: The median age of the patients was 52 years (range 24-70 years). All patients were discharged 48 h after BMC infusion. Two patients complained of mild pain at the bone marrow needle puncture site. No other complications or specific side effects related to the procedure were observed. Bilirubin levels were lower at 1 (2.19 ± 0.9) and 4 mo (2.10 ± 1.0) after cell transplantation that baseline levels (2.78 ± 1.2). Albumin levels 4 mo after BMC infusion (3.73 ± 0.5) were higher than baseline levels (3.47 ± 0.5). International normalized ratio (INR) decreased from 1.48 (SD = 0.23) to 1.43 (SD = 0.23) one month after cell transplantation.
CONCLUSION: BMC infusion into hepatic artery of patients with advanced chronic liver disease is safe and feasible. In addition, a decrease in mean serum bilirubin and INR levels and an increase in albumin levels are observed. Our data warrant further studies in order to evaluate the effect of BMC transplantation in patients with advanced chronic liver disease.
- Citation: Lyra AC, Soares MBP, Silva LFMD, Fortes MF, Silva AGP, Mota ACA, Oliveira SA, Braga EL, Carvalho WA, Genser B, Santos RRD, Lyra LGC. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol 2007; 13(7): 1067-1073
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1067
Chronic liver disease may progress to end-stage liver disease (ESLD), liver failure and death. Patients with ESLD may experience serious complications such as encephalopathy, ascites and esophagogastric variceal hemorrhage. Liver transplantation is the only available therapy for patients with chronic liver failure. Because of the shortage of donated organs, up to 10%-15% of these patients die without receiving an organ in developed countries[1]. In some undeveloped countries the number of deaths on the waiting list might be greater. In some regions of Brazil it takes an average of more than two years until liver transplantation. Therefore, alternative methods such as cell therapy are necessary to increase patient survival on the liver transplant waiting list. Several sources of stem cells have been proposed for cell therapy. Embryonic stem cells are the most potent in terms of their differentiation potential but may be oncogenic when transplanted in vivo and their use has been a matter of controversy because of ethical issues[2]. A number of studies during the last decade have identified cells both within and outside the liver that have properties of hepatic stem cells and might differentiate into hepatocytes or bile duct epithelial cells[3-5]. Hematopoietic tissue is most accessible and of special interest , and it has been demonstrated that bone marrow contains multipotent adult progenitor cells that can generate a variety of cell types found in other tissues[6-8].
Studies in animal models of liver diseases have demonstrated that bone marrow cell (BMC) transplantation may accelerate the liver regeneration process, reduce hepatic fibrosis and improve liver function and survival rate[9-11]. However, its mechanism is still controversial. Several studies have suggested that hematopoietic cells may generate hepatocyte-like cells, while others hypothesized that they should act mainly by fusion with hepatocytes or by paracrine effect[12-15]. Fusion between hepatocytes and hematopoietic cells produces heterokaryotic hybrid cells that initially contain the genetic elements and organelles of both cell types and may then express the hepatocyte phenotype. Several studies suggest that cytokines and growth factors produced by infused hematopoietic cells may support liver function and repair in adult animals without forming new hepatocytes from the infused cells[15]. Based on the findings from other studies that suggest improvement of liver fibrosis in experimental models of liver disease and because bone marrow cell transplantation itself is already an established treatment for hematological and oncological diseases, we conducted a clinical trial to evaluate the safety and feasibility of autologous BMC therapy in patients with chronic liver disease on the waiting list for liver transplantation.
The study group comprised 10 patients with chronic liver disease (8 males, 2 females, age: 24 to 70 years) on the waiting list for liver transplantation. They were enrolled to receive infusion of autologous BMC from September 2005 to January 2006. Informed written consent was obtained from all subjects. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Hospital São Rafael, Salvador, Brazil and by the Brazilian National Ethics Committee (CONEP). Eligible patients had all the following inclusion criteria: age range between 18-75 years, advanced chronic liver disease of different etiologies classified as Child Pugh B or C, absence of liver tumors and appropriate use of contraception method for women of child-bearing potential. Patients were excluded from the study if they had one or more of the following exclusion criteria: risk for bone marrow aspiration, sepsis, human immunodeficiency virus infection, active hepatic encephalopathy, liver tumor, history of malignant neoplasm except for non-melanoma skin cancer, decompensated heart failure, platelet count < 30 000/mm3, international normalized ratio (INR) > 2.2, renal failure (creatinine > 2.5 mg/dL), participation in other clinical trials, pregnancy or lactation.
Baseline assessment included complete clinical and laboratorial evaluation as well as abdominal magnetic resonance imaging to exclude liver tumor. Laboratory tests consisted of complete blood count, serum bilirubin levels, prothrombin time, serum blood glucose, urea, creatinine, alpha-fetoprotein, total proteins and albumin levels, serum aminotransferase concentrations, alkaline phosphatase and gamma-glutamyl transferase levels, thyroid stimulating hormone (TSH) concentrations. Patients were followed up for adverse events with clinical and laboratory evaluations on d 1, 2, 3, 7, 15, 30, 45, 60, 90, 120 after BMC transplantation.
Approximately 50 mL of bone marrow was aspirated from the iliac crest. An enriched fraction of bone marrow mononuclear cells was prepared by centrifugation of total bone marrow in a ficoll-hypaque gradient. At least 100 millions of mononuclear-enriched BMC suspended in 20 mL of saline was infused into the hepatic artery of included patients by a catheter, using the routine technique for arterial chemoembolization of liver tumors[16].
Exploratory data analysis was conducted to calculate means, standard deviations and 95% confidence intervals of measurements (laboratory tests, Child Pugh score at baseline at 1 and 4 mo). Relative mean changes at 1 and 4 mo with respect to the baseline level were calculated. To examine the development of laboratory parameters after BMC infusion individual response profiles were plotted as a function of weeks after intervention. In addition, estimates of the mean response profiles and 95% confidence intervals were obtained by calculating moving averages of patients’ measurements over a period of 4 weeks and by applying linear interpolation. All statistical analyses were conducted by the statistical software package STATA (StataCorp. 2005. Stata Statistical Software: Release 8. College Station, TX: StataCorp LP).
The median age of the study population was 52 years (range 24-70 years). Four patients had alcoholic liver disease, 4 had chronic hepatitis C, one had chronic cholestatic liver disease and one cryptogenic cirrhosis (Table 1). Seven patients were classified as Child-Pugh B and 3 patients as Child-Pugh C. None had a liver tumor.
| PatientID | Age(yr) | Sex | Etiology ofcirrhosis | BMCs transplanted(n) |
| JWOR | 49 | Male | Alcohol + HCV | 2.8 × 108 |
| FMB | 60 | Male | Alcohol | 5.2 × 108 |
| EAS | 63 | Female | HCV | 3.2 × 108 |
| RNR | 24 | Male | Cholestatic | 13.1 × 108 |
| IAN | 49 | Male | Alcohol + HCV | 2.6 × 108 |
| NPA | 55 | Female | Cryptogenic | 3.5 × 108 |
| RBC | 49 | Male | Alcohol | 4.8 × 108 |
| RMV | 66 | Male | HCV | 3.4 × 108 |
| GAFL | 70 | Male | Alcohol | 1.6 × 108 |
| ABSF | 40 | Male | HCV | 2.4 × 108 |
The number of BMCs infused in each patient is shown in Table 1. All patients were discharged 48 h after BMC infusion. Two patients complained of mild pain at the bone marrow needle puncture site. No other complications or specific side effects related to the infusion procedure were reported. A 70-year old patient with a history of previous episodes of hepatic encephalopathy developed reversible grade I encephalopathy 33 d after BMC infusion. The episode was controlled without requirement of hospitalization and was not considered an adverse event since 3 identical episodes were documented for this patient in the last year before his inclusion in the study.
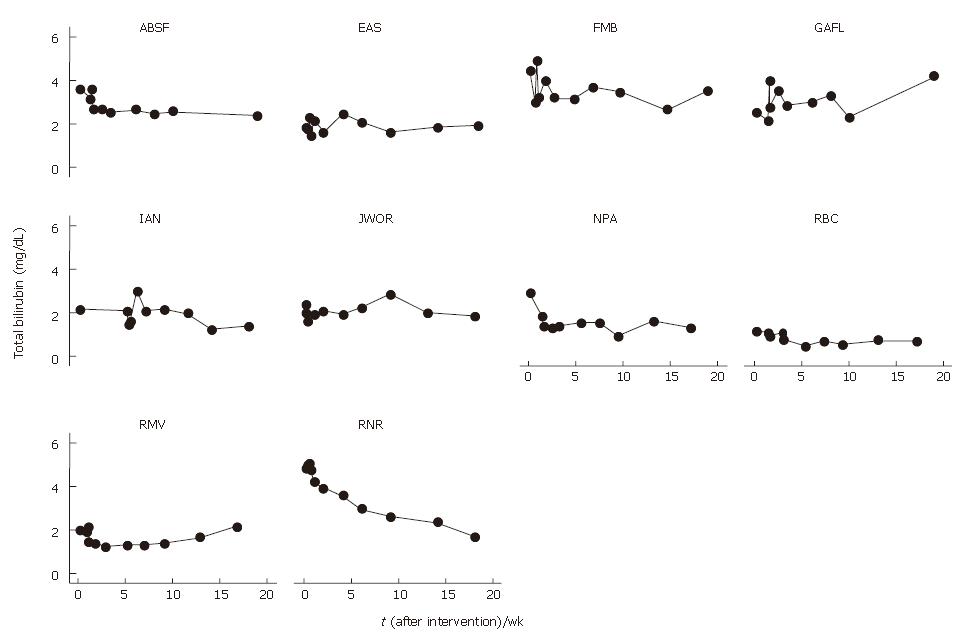
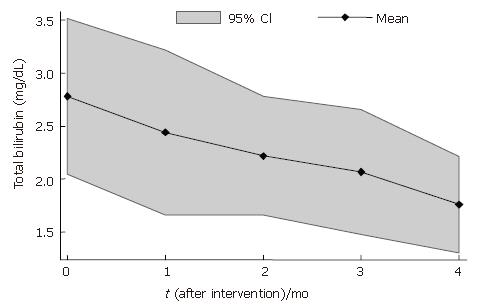
Total bilirubin levels were 21% lower at 1 mo (2.19 ± 0.91) and 24% lower at 4 mo (2.10 ± 1.04) after BMC transplantation that baseline levels (2.78 ± 1.16) (Table 2). When individual response profiles were evaluated, a decrease in total bilirubin levels was observed in 7 of 10 patients after BMC transplantation (Figure 1). The mean profile of bilirubin levels after BMC infusion is shown in Figure 2.
| Bilirubin(mg/dL) | Minimum | Maximum | Mean | Median | Standarddeviation | Relative meanchange frombaseline (%) |
| Baseline | 1.2 | 4.83 | 2.78 | 2.45 | 1.16 | |
| 1 mo | 0.5 | 3.56 | 2.19 | 2.28 | 0.91 | -21 |
| 4 mo | 0.72 | 4.16 | 2.1 | 1.87 | 1.04 | -24 |
| Albumin (unit) | ||||||
| Baseline | 2.5 | 4.4 | 3.47 | 3.5 | 0.51 | |
| 1 mo | 2.9 | 4.5 | 3.44 | 3.25 | 0.52 | -1 |
| 4 mo | 3.1 | 4.8 | 3.73 | 3.6 | 0.51 | 7 |
| INR (unit) | ||||||
| Baseline | 1.08 | 1.89 | 1.46 | 1.48 | 0.23 | |
| 1 mo | 1.1 | 1.94 | 1.44 | 1.43 | 0.23 | -1 |
| 4 mo | 1.16 | 1.75 | 1.42 | 1.43 | 0.18 | -3 |
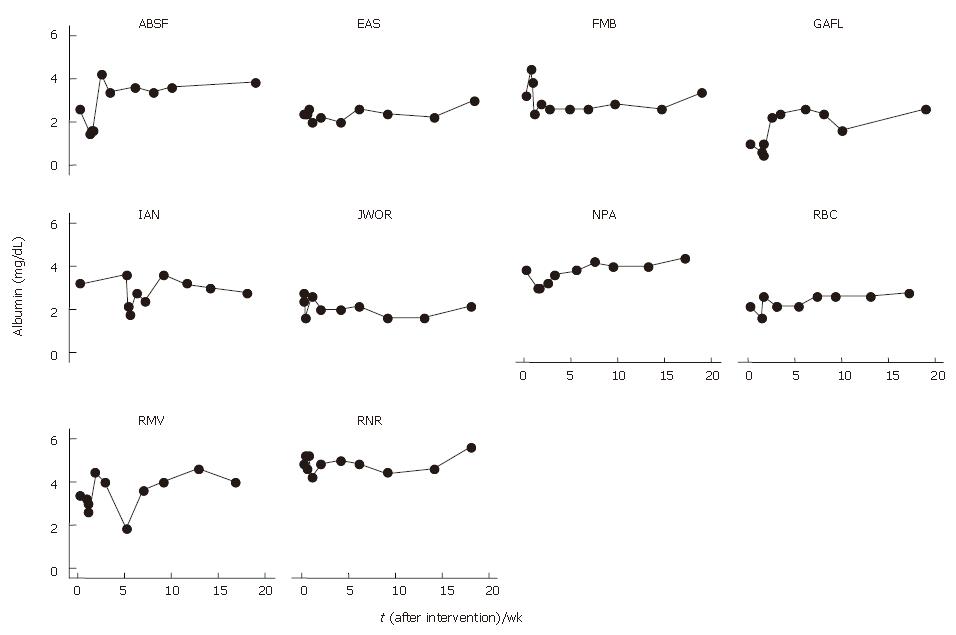
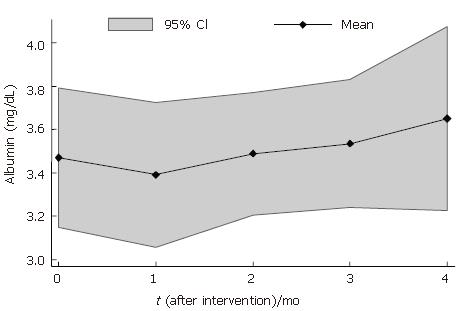
Albumin levels were 1% lower at 1 mo (3.44 ± 0.52) and 7% higher at 4 mo after BMC infusion (3.73 ± 0.51) that baseline levels (3.47 ± 0.51) (Table 2). The analysis of individual levels showed an increase in albumin levels in 6 patients and a reduction in 1 patient 4 mo after BMC transplantation (Figure 3). The mean profile of serum albumin levels is shown in Figure 4.
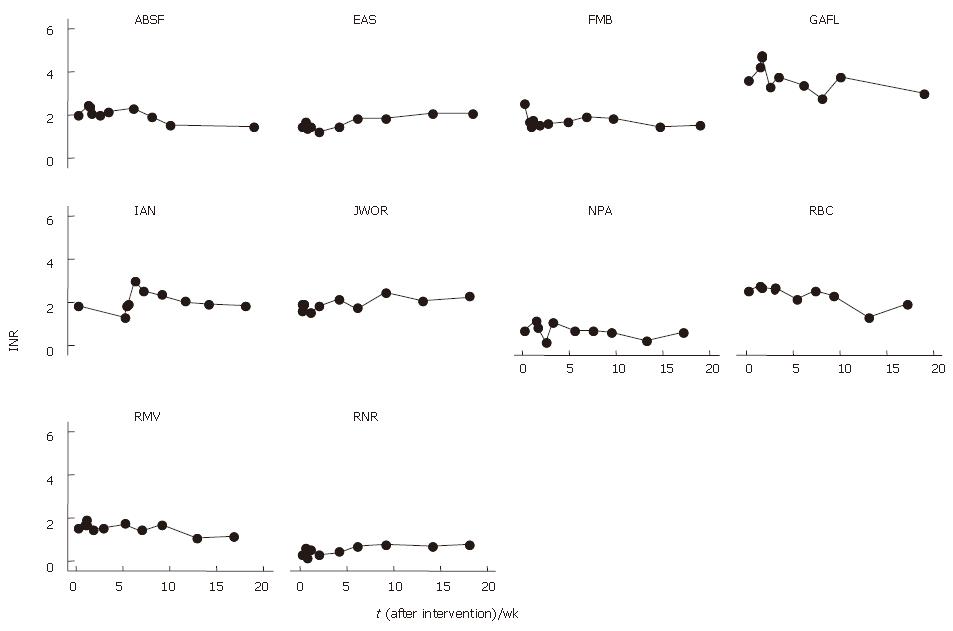
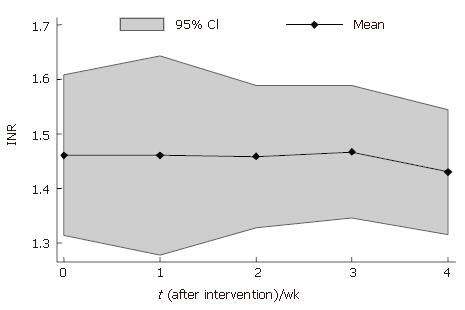
INR levels were 1% lower at 1 mo (1.44 ± 0.23) and 4% lower at 4 mo (1.42 ± 0.18) after BMC transplantation that baseline levels (1.46 ± 0.23) (Table 2). INR levels reduced in 7 patients and increased in 1 patient after BMC transplantation (Figure 5). The mean profiles of INR levels are shown in Figure 6.
The data about other relevant laboratory parameters (WBC, hemoglobin, ALT, AST, GGT, creatinine) before and after the intervention are shown in Table 3.
| Minimum | Maximum | Mean | Median | Standarddeviation | Relative meanchange frombaseline (%) | |
| Hemoglobin (g/dL) | ||||||
| Baseline | 11.30 | 16.90 | 13.16 | 12.2 | 1.90 | |
| 1 mo | 9.50 | 16.00 | 12.26 | 11.95 | 1.81 | -8 |
| 4 m | 10.30 | 15.10 | 12.80 | 12.7 | 1.47 | -3 |
| WBC (x 103/mm3) | ||||||
| Baseline | 2.60 | 5.40 | 3.94 | 4 | 0.97 | |
| 1 mo | 2.30 | 5.10 | 3.35 | 2.85 | 1.04 | -15 |
| 4 mo | 2.30 | 6.00 | 3.60 | 3.25 | 1.14 | -9 |
| GGT (U/L) | ||||||
| Baseline | 50.00 | 543.00 | 172.20 | 139 | 147.50 | |
| 1 mo | 48.00 | 474.20 | 157.20 | 134.5 | 129.00 | -9 |
| 4 mo | 47.00 | 489.67 | 163.50 | 132.5 | 133.25 | -5 |
| AST (U/L) | ||||||
| Baseline | 29.00 | 192.00 | 100.60 | 92.5 | 56.24 | |
| 1 mo | 26.00 | 173.00 | 86.90 | 79 | 47.58 | -14 |
| 4 mo | 29.00 | 180.00 | 85.80 | 64 | 46.87 | -15 |
| ALT(U/L) | ||||||
| Baseline | 11.00 | 120.00 | 56.00 | 43 | 35.54 | |
| 1 mo | 22.00 | 115.00 | 54.80 | 42.5 | 32.71 | -2 |
| 4 mo | 17.00 | 131.00 | 53.50 | 41 | 34.97 | -4 |
| Creatinine (mg/dL) | ||||||
| Baseline | 0.60 | 1.50 | 0.86 | 0.8 | 0.26 | |
| 1 mo | 0.70 | 1.30 | 0.90 | 0.85 | 0.20 | 5 |
| 4 mo | 0.60 | 1.20 | 0.87 | 0.85 | 0.19 | 1 |
There is an urgent need to search for alternatives to whole organ transplantation. Based on the ability of stem cells to differentiate into specific cell types according to their environment, cell transplantation has become an attractive therapeutic method for the treatment of patients with liver disease aiming, at least, at a temporary support of hepatic function until a liver becomes available for organ transplantation.
This phase I study aimed to evaluate the feasibility and safety of autologous bone marrow cell transplantation into the hepatic artery of patients with advanced chronic liver disease. In the present study, we infused BMCs into the hepatic artery using the same routine technique for arterial chemoembolization of liver tumors which is feasible and not associated with serious local side effects[16], and used the hepatic artery for cell infusion since the blood inflow to the liver could be mostly secured. Our results confirmed the feasibility and safety of BMC infusion into hepatic artery in such patients. BMC transplantation was well tolerated to all patients and only two patients complained of mild pain at the bone marrow needle puncture site. We did not detect any other side effects. The episode of reversible hepatic encephalopathy 33 d after BMC infusion in a 70-year old man with alcoholic cirrhosis was not interpreted as an adverse event related to the procedure because identical episodes were observed in the previous year.
In addition to the evaluation of safety and feasibility, our results indicated that there were alterations of liver function parameters after transplantation of autologous BMCs in patients with advanced chronic liver disease. Four months after BMC transplantation the mean serum bilirubin and INR levels decreased while the mean albumin levels increased. Of note, 7 patients had their bilirubin levels reduced while 6 patients had their albumin levels increased. The greatest reduction in the bilirubin levels was observed in a 24-year old patient (R.N.R.) with chronic cholestatic liver disease who had the highest amount of transplanted BMCs (13.1 × 108) compared to the others (Table 1). The number of transplanted BMCs was lower in patients with cirrhosis caused by HCV and in the 70-year old patient.
Recently, two phase I studies using granulocyte-colony stimulating factor (G-CSF) to mobilize BMCs in patients with chronic liver disease were conducted[17,18]. Gaia et al[17] evaluated the feasibility and safety of BMC mobilization in 8 patients with end stage liver cirrhosis following G-CSF administration. Mobilization (monitored by the number of CD34 + ve cells) was observed in all patients after G-CSF administration, which was well tolerated and free of adverse events. Child-Pugh score decreased by 2 or more points in four patients, increased in one patient, while it was unchanged (or decreased by less than 2 points) in three patients. Overall, the MELD score decreased from a median pre-treatment value of 17.5 (range 11-20) to 14.5 (range 9-20) at end of follow-up. In another study Gordon et al[18] evaluated the safety and tolerability of autologous CD34+ cell injection into five patients with liver insufficiency. Included patients were given subcutaneously 520 μg G-CSF and CD34+ cells were collected and then returned to the patients via the hepatic artery or the portal vein. No major complications or specific side effects related to the procedure were observed. Three of the five patients showed a reduction in serum bilirubin and four of five showed an increase in serum albumin. The results of these two studies appear to be in agreement with ours. The choice of utilizing purified stem cells instead of mononuclear cell fraction depends on a future comparison of the efficacy in the two cell populations. In addition, purification procedures to obtain a specific cell population increase the costs of the therapy.
Studies in animal models of liver diseases have demonstrated that bone marrow cell transplantation decreases hepatic fibrosis and improves survival rate. Sakaida et al[11] investigated the effect of BMC transplantation on mice with established liver fibrosis induced by carbon tetrachloride (CCl4) administration. Four weeks after BMC transplantation, the mice had significantly reduced liver fibrosis, as assessed by hydroxyproline content in the livers, compared to mice treated with CCl4 alone. Similar results have been observed in other studies[9,19,20]. In addition, improvement in several parameters of liver function, such as albumin and bilirubin levels and prothrombin time has been reported[8,12,21]. These experimental studies suggest that BMC infusion may be responsible for the potential beneficial effects on liver function as observed in our clinical study.
Our study was not able to evaluate the efficacy of BMC infusion in patients with liver disease due to its design. Controlled studies would be required to address this issue. Further studies are also necessary to determine the number of BMCs required for achievement of therapeutic effect, which may vary with the patient’s age and the etiology of liver disease.
In summary, BMC infusion into the hepatic artery of patients with advanced chronic liver disease is safe and feasible. In addition, in most patients there is a decrease in mean serum bilirubin and INR levels, and an elevation of serum albumin. Our results warrant further studies in order to evaluate the effects of BMC transplantation in patients with advanced chronic liver disease.
We thank Dr Patricia Seixas A Brandrao, Heleni F de Carvalho, Andrea da S Sant’Ana, Dr. Paulo Engracio M de Souza, Dr Sonia M Duarte Cathala and Dr. Gabriela Fortes for their invaluable assistance in the care of the patients and Carol O Ramos, Luciano Coelho, Amanda A. Mascarenhas and Carlos Daniel VB de Carvalho for their support in the organization of the clinical data.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Allen KJ, Buck NE, Williamson R. Stem cells for the treatment of liver disease. Transpl Immunol. 2005;15:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005;37:285-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Haque S, Haruna Y, Saito K, Nalesnik MA, Atillasoy E, Thung SN, Gerber MA. Identification of bipotential progenitor cells in human liver regeneration. Lab Invest. 1996;75:699-705. [PubMed] |
| 4. | Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 485] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology. 1997;25:329-334. [PubMed] |
| 6. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Braun KM, Sandgren EP. Cellular origin of regenerating parenchyma in a mouse model of severe hepatic injury. Am J Pathol. 2000;157:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1631] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 9. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Med Mol Morphol. 2005;38:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 898] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 13. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1114] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 14. | Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV, Fabregat I, Bueren JA, Theise ND, Segovia JC. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology. 2006;43:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. [PubMed] |
| 20. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |