Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1053
Revised: November 2, 2006
Accepted: November 14, 2006
Published online: February 21, 2007
AIM: To investigate the influence of heme oxygenase-1 (HO-1) gene transfer on the viability and function of cultured rat islets in vitro.
METHODS: Islets were isolated from the pancreata of Sprague-Dawley rats by intraductal collagenase digestion, and purified by discontinuous Ficoll density gradient centrifugation. Purified rat islets were transfected with adenoviral vectors containing human HO-1 gene (Ad-HO-1) or enhanced green fluorescent protein gene (Ad-EGFP), and then cultured for seven days. Transfection was confirmed by fluorescence microscopy and Western blot. Islet viability was evaluated by acridine orange/ propidium iodide fluorescent staining. Glucose-stimulated insulin release was detected using insulin radioimmunoassay kits and was used to assess the function of islets. Stimulation index (SI) was calculated by dividing the insulin release upon high glucose stimulation by the insulin release upon low glucose stimulation.
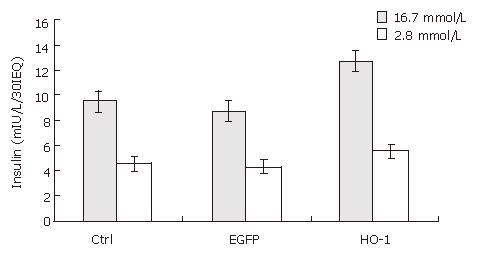
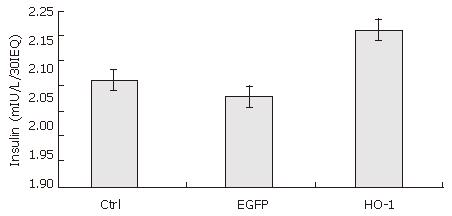
RESULTS: After seven days culture, the viability of cultured rat islets decreased significantly (92% ± 6% vs 52% ± 13%, P < 0.05), and glucose-stimulated insulin release also decreased significantly (6.47 ± 0.55 mIU/L/30IEQ vs 4.57 ± 0.40 mIU/L/30IEQ, 14.93 ± 1.17 mIU/L/30IEQ vs 9.63 ± 0.71 mIU/L/30IEQ, P < 0.05). Transfection of rat islets with adenoviral vectors at an MOI of 20 was efficient, and did not impair islet function. At 7 d post-transfection, the viability of Ad-HO-1 transfected islets was higher than that of control islets (71% ± 15% vs 52% ± 13%, P < 0.05). There was no significant difference in insulin release upon low glucose stimulation (2.8 mmol/L) among Ad-HO-1 transfected group, Ad-EGFP transfected group, and control group (P > 0.05), while when stimulated by high glucose (16.7 mmol/L) solution, insulin release in Ad-HO-1 transfected group was significantly higher than that in Ad-EGFP transfected group and control group, respectively (12.50 ± 2.17 mIU/L/30IEQ vs 8.87 ± 0.65 mIU/L/30IEQ; 12.50 ± 2.17 mIU/L/30IEQ vs 9.63 ± 0.71 mIU/L/30IEQ, P < 0.05). The SI of Ad-HO-1 transfected group was also significantly higher than that of Ad-EGFP transfected group and control group, respectively (2.21 ± 0.02 vs 2.08 ± 0.05; 2.21 ± 0.02 vs 2.11 ± 0.03, P < 0.05).
CONCLUSION: The viability and function of rat islets decrease over time in in vitro culture, and heme oxygenase-1 gene transfer could improve the viability and function of cultured rat islets.
-
Citation: Chen XB, Li YX, Jiao Y, Dong WP, Li G, Chen J, Tan JM. Influence of heme oxygenase-1 gene transfer on the viability and function of rat islets in
in vitro culture. World J Gastroenterol 2007; 13(7): 1053-1059 - URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1053.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1053
Diabetes affects more than 200 million people worldwide[1]. The mainstay treatment for type I diabetic patients is chronic insulin injection. While exogenous insulin therapy has dramatically reduced mortality in diabetes, patients often succumb to the long-term sequelae of diabetic angiopathy, either in the form of nephropathy, neuropathy or retinopathy. Vascularized pancreas transplantation reliably restores normoglycemia and maintains long-term glucose homeostasis, but it has significant surgical morbidity and mortality[2]. In 2000, with the success of the ‘Edmonton protocol’, which had produced insulin independence in 85% of type I diabetic patients one year after allogeneic islets transplantation combined with a non-steroid immunosuppressive regimen, islet transplantation has progressed from research to clinical reality[3].
The technique to maintain isolated islet preparations in tissue culture has been adopted by most islet transplant centers. Islet culture for a brief period (24 to 72 h) has emerged as the current standard procedure prior to clinical transplantation[4,5]. It offers advantages over immediate infusion post-isolation, enabling assessment of islet quality and safety, and reducing islet immunogenicity as well as recipient travel to the transplantation site and immunosuppression before transplantation[6]. But in vitro culture of islets has been shown to result in loss of viable tissue over time, and a decrease in glucose responsiveness has been observed in those islets which survive[7,8].
Several phenomena, including activation of free radicals, apoptosis and necrosis, may be responsible for these effects. Therefore, approaches towards enhancing islets resistance to these insults would facilitate both clinical and investigative trials. As a cellular graft, islets are especially suited for gene therapy. An attractive strategy for protecting islets in in vitro culture is to use gene therapy to transduce islets with cytoprotective genes that can make islets more resistant to injury.
Heme oxygenase-1 (HO-1) is the rate-limiting enzyme in the heme degradative pathway that catalyzes the oxidation of heme into biliverdin, carbon monoxide (CO), and free iron[9,10], and it has been described as a ubiquitous inducible stress protein capable of cytoprotection via radical scavenging and apoptosis prevention. Overexpression of HO-1 by chemical induction or gene therapy has been used to reduce the deleterious effects of oxidative stress and apoptosis in various cell types and animal models[11-14].
Compared with chemical induction, gene transfer can provide effective, targeted, and relatively persistent expression of HO-1. The aim of the present study was to investigate the influence of HO-1 gene transfer on the viability and function of cultured rat islets, and to explore the potential value of HO-1 gene transfer in islet transplantation.
Twenty male Sprague-Dawley rats weighing 250 to 300 g were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences.
Rat islets were isolated from the pancreata of the outbred male Sprague-Dawley rats by a collagenase digestion technique and discontinuous Ficoll density gradient centrifugation[15]. The main bile duct was located and clamped at both ends. Ten milliliters of collagenase P (Roche Applied Science, Indianapolis, Ind, USA) solution (1 g/L, pH 7.8) was injected into the duct and then the distended pancreas was surgically resected, and incubated at 38°C for 15 min. The digested gland was vigorously shaken for 10 s and the digestion was stopped by Hank’s solution (4°C) with 100 mL/L fetal calf serum (Gibco, BRL, USA). The tissue was filtered through a 600 μm screen, and then washed by Hank’s solution twice. Islets were purified by centrifugation at 3000 r/min for 20 min on discontinuous Ficoll (Pharmacia Fine Chemicals, Uppsala, Sweden) gradients. After several washes with Hank’s solution, islets were suspended in RPMI-1640 medium (Gibco, BRL, USA) containing 100 mL/L fetal calf serum (Gibco, BRL, USA), 20 mmol/L HEPES (Sigma-Aldrich Chemicals, Louis Mo, USA), 100 kU/L of penicillin and 100 g/L of streptomycin at 37°C in a humidified atmosphere of 50 mL/L CO2. Islets purity was assessed by dithizone (Sigma-Aldrich Chemicals, Louis Mo, USA) staining, and islets were counted and scored in size. An algorithm was used for the calculation of 150 μm-diameter islet equivalent number (IEQ).
Adenoviral vectors were prepared by use of the AdEasy system (Stratagene, Baltimore, USA). Human HO-1 cDNA was cloned into pAdTrack-CMV. Once constructed, the shuttle vector was linearized with Pme I and co-transformed into E. coli BJ5183 together with pAdEasy-1, the supercoiled viral DNA plasmid. Transformants were selected on kanamycin and the recombinants were subsequently identified by restriction digestion. Once a recombinant was identified, it was produced in bulk using the recombination-deficient XL10-Gold® strain. Purified recombinant adenoviral plasmid DNA was then linearized by Pac I to expose its inverted terminal repeats (ITR) and transfected into HEK293 cells where deleted viral genes necessary for virus assembly were complemented in vivo. The pAdEasy-1 vector will not replicate in cells other than complementing cells (293 cells). This vector has been developed to infect but not replicate in non-permissive target cells. Ad-EGFP was generated using the same system and supplied by the Institute of Genetics of Fudan University. Viral titers were determined by plaque assay and expressed as plaque forming units per mL (pfu/mL). Viral titers of Ad-HO-1 and Ad-EGFP were 1.96 × 109 and 1.99 × 109 pfu/mL, respectively.
Aliquots of 30 IEQ were resuspended in 0.5 mL serum-free culture medium and placed in a 24-well culture plate and incubated with Ad-HO-1 and Ad-EGFP vectors at a multiplicity of infection (MOI) of 20 at 37°C for 4 h with agitation every 1 h. MOI was calculated using the assumption that islets contain on average 1000 cells. After infection, islets were washed twice with culture medium and incubated for at least 48 h before further analysis to allow for transgene expression. Control islets were mock infected. Mock infected islets underwent a similar procedure, but were not exposed to viruses during the incubation period and were not incubated with any vectors.
Islet cells (48 h post-transfection) were washed with cold phosphate buffered saline (PBS) and lysed in 2% SDS, Tris-HCl 60 mmol/L (pH 6.8) buffer, incubated at 95°C, sonicated in a water bath at 37°C and centrifuged at 12 000 r/min for 15 min. Assessment of the total protein content was carried out with the BCA detection kit (Pierce Biotechnology, Rockford, IL, USA). Aliquots corresponding to 100 μg of protein were subjected to electrophoresis on a 15% SDS-PAGE pre-cast gel and transferred electrophoretically to a nitrocellulose membrane. The membranes were incubated with 50 g/L non-fat dry milk in TBS (20 mmol/L Tris, 500 mmol/L NaCl, pH 7.5) overnight at 4°C to block non-specific binding. The blots are then incubated with the murine antihuman HO-1 monoclonal antibodies (StressGen, Victoria, BC, Canada) at a dilution of 1:200 for 2 h at room temperature; this was followed by a 1 h incubation with the AP-conjugated rabbit antimouse polyclonal antibody (Promega, USA) at a dilution of 1:1000. The protein bands were visualized by the NBT + BCIP staining system (Haoyang Biological Manufacture Co, Ltd, Tianjin, China).
Islet viability was evaluated by acridine orange (AO)/propidium iodide (PI) (Sigma-Aldrich Chemicals, Louis Mo, USA) fluorescent staining. The fluorescent dye, containing 10 μL AO (670 μmol/L) and 1mL PI (750 μmol/L), was used at a 1:10 dilution. After the islets were washed twice with Hank’s solution, the fluorescent dye was added to each well. Ten minutes later, islets were analyzed under a fluorescence microscope. Living cells were identified by green staining (AO), whereas dead cells showed a brown-red staining (PI).
In vitro function of freshly isolated islets and cultured islets was assessed by glucose-stimulated insulin release. Islets of different groups were washed with PBS twice, and incubated first in low (2.8 mmol/L) and then in high (16.7 mmol/L) concentrations of glucose in culture medium. The static incubation assay was performed in a 24-well flat-bottomed culture plate with 30 IEQ/well and 3 duplicate wells for each islet group. Supernatant from each well was collected after each 1 h incubation, and the concentration was measured using an insulin radioimmunoassay kit (Jiuding Biotech Co, Ltd, Tianjin, China). Stimulation index (SI) was calculated by dividing the insulin release upon high glucose by the insulin release upon low glucose stimulation.
Data are expressed as mean ± SD. Statistical and graphical analysis was performed with software of SPSS10.0. Analyses were performed using the two-tailed Student’s t-test where appropriate.
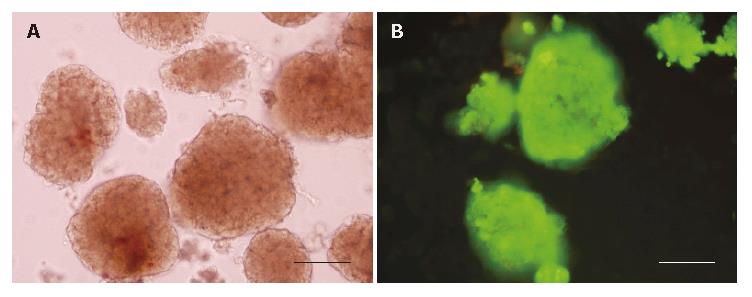
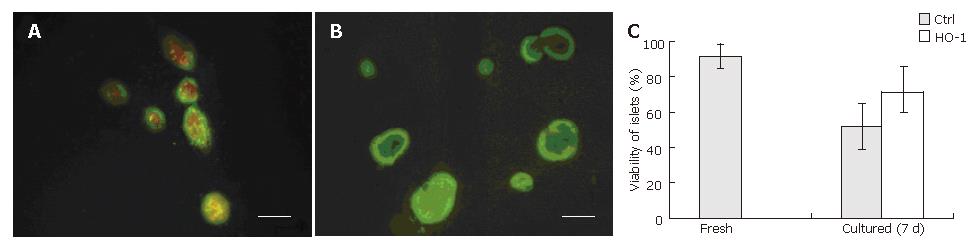
The purity of freshly isolated islets was above 90% calculated from the ratio of dithizone stained cells to dithizone nonstained cells as a percentage of the total cell number (Figure 1A). The viability of freshly isolated islets was above 90% calculated from the ratio of AO staining cells (living cells) to PI staining cells (dead cells) as a percentage of the total cell number (Figure 1B).
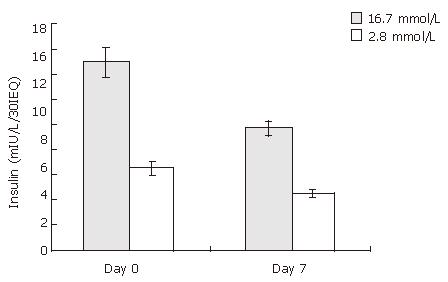
The glucose-stimulated insulin release was used to assess the function of cultured rat islets. As shown in Figure 2, after 7 d culture, the insulin release upon low and high glucose stimulation decreased from 6.47 ± 0.55 to 4.57 ±0.40 mIU/L/30IEQ, and from 14.93 ± 1.17 to 9.63 ± 0.71 mIU/L/30IEQ, respectively. In other words, islet function decreased conspicuously over time in in vitro culture.
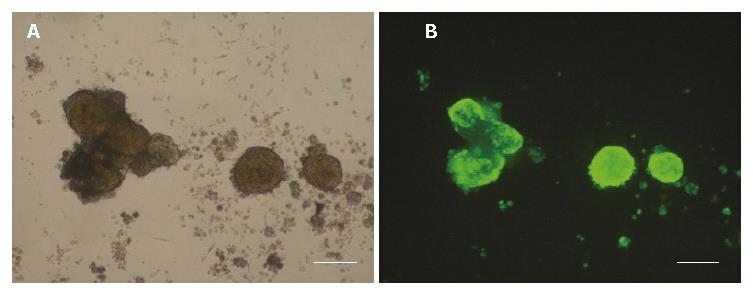
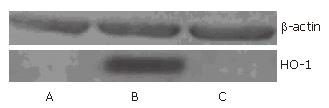
Typical fluorescence micro-photographs of rat islets transfected with Ad-EGFP at an MOI of 20 were taken at 48 h post-transfection. As shown in Figure 3A and B, the fluorescence in Ad-EGFP transfected islets was intense. Western blot was used to detect the expression of human HO-1 protein in three groups of islets. Figure 4 shows that human HO-1 protein was detected in Ad-HO-1 transfected islets but not in uninfected or Ad-EGFP transfected islets. Therefore, adenovirus mediated exogenous gene transfer into rat islets was successful.
The viability of islets was assessed by AO/PI fluorescent staining.
Representative pictures are shown in Figure 5. The viability of control islets was greatly reduced as shown by the large number of dead cells within the islets (Figure 5A). Nevertheless, the number of dead cells was reduced when islets were transfected with HO-1 gene (Figure 5B). Quantitative analysis of islet viability was performed by using fluorescence microscopy to determine the proportion of living cells within islets. As shown in Figure 5C, islet viability decreased greatly after 7 d culture (92% ± 6% vs 52% ± 13%, P < 0.05), but HO-1 gene transfer could reverse the viability reduction (71% ± 15% vs 52% ± 13%, P < 0.05).
Since adenoviral transfection at a high transfecting dose may damage islets and interfere with their biologic function[16], glucose-stimulated insulin release was employed to evaluate the influence of adenoviral transfection at an MOI of 20 on insulin release. As shown in Figure 6, there was no significant difference in glucose-stimulated insulin release between Ad-EGFP transfected islets and control islets at 7 d post-transfection (4.30 ±0.40 vs 4.57 ± 0.40 mIU/L/30IEQ, 8.87 ± 0.65 vs 9.63 ±0.71 mIU/L/30IEQ, respectively). It demonstrated that adenoviral transfection at an MOI of 20 was safe for islets.
After 7 d culture post transfection, the insulin release upon low and high glucose of uninfected (control islets), Ad-EGFP transfected, and Ad-HO-1 transfected islets were 4.57 ± 0.40 vs 9.63 ± 0.71 mIU/L/30IEQ, 4.30 ± 0.40 vs 8.87 ± 0.65 mIU/L/30IEQ, 5.67 ± 0.99 vs 12.50 ± 2.17 mIU/L/30IEQ, respectively. SI of control group, Ad-EGFP group, and Ad-HO-1 group were 2.11 ± 0.03, 2.08 ± 0.05, 2.21 ± 0.02, respectively. As shown in Figures 6 and 7,the insulin release upon high level glucose stimulation and SI of Ad-HO-1 transfected islets were significantly higher than those of Ad-EGFP transfected islets and control islets(P < 0.05).Therefore, HO-1 gene transfer can improve the function of cultured islets.
With recent advances in techniques of islet isolation and the introduction of more potent and less diabetogenic immunosuppressive therapies, islet transplantation has progressed from research to clinical reality, and it has been regarded as a safe and viable route to achieve insulin independence in a population of patients with type I diabetes[3]. Many transplant protocols incorporate a period of short-term (24 to 72 h) islet culture before transplantation for the recipient to be treated with immunodepleting agents[4,5,17,18], and to provide time for in vitro assessment of islet quality. Short-term islet culture indeed has some benefits, such as purification of the islet preparation, immunomodulation[6], and possibly improved allograft survival. However, cultured islets are known to degrade rapidly[19,20], and lose viability and functional responsiveness to glucose stimulation with the extension of culturing time[7,8]. Islet loss as high as 30% to 50% has been reported after 48 hours of culture[21].
The islet, once removed from its natural surroundings within the pancreas and placed within the alien environment of the culture plate, becomes deprived of normal physiological organization and exposed to a number of hostile factors, such as hypoxia, activation of free radicals, apoptosis and necrosis that cause its premature demise. The quality of the transplanted islets is of the utmost importance to successfully achieve normal levels of glucose in transplant recipients, therefore, preservation of viability and function of islets post-isolation is a pre-requisite in islet transplantation. Pancreatic islets, as a cellular graft, are especially suited for gene therapy, as they can be infected efficiently ex vivo and then transplanted with minimal systemic exposure of the recipient to the vector.
HO-1 has been identified as a ubiquitous stress protein induced in many cell types by various stimulants, such as hemolysis, inflammatory cytokines, oxidative stress, heat shock, heavy metals, and endotoxin[22]. HO-1 is the rate-limiting enzyme of heme degradation into its byproducts biliverdin, CO, and iron[9,10]. Biliverdin is subsequently reduced into bilirubin, a powerful anti-oxidant, and it may inhibit the generation of reactive oxygen species[23].
CO has a cytoprotective role in different systems[24-26], including pancreatic β-cells[27]. Heme catabolism by HO-1 also releases free iron, which has the potential to exacerbate the cytotoxic effects of reactive oxygen species[28,29].
However, generation of intracellular free iron upregulates the expression of ferritin[28,29], which has a high capacity to store free iron[29]. Ferritin has been shown to protect endothelial cells against activated neutrophils as well as H2O2-mediated cytotoxicity[29], suggesting that some of the effects of HO-1 may be mediated by ferritin[28,29]. As HO-1 is not expressed constitutively, it has been demonstrated that overexpression of HO-1 by chemical induction can protect islet cells from apoptosis and improve in vivo function after transplantation[22]. Compared with chemical induction, gene transfer can provide effective, targeted, and relatively persistent expression of HO-1.
Adenoviral vectors are useful for efficient gene delivery to differentiated and non-proliferating cells, such as isolated pancreatic islets[30]. In addition, adenoviral vectors can be produced in high titers and there is no risk of insertional mutagenesis as their genomes are not integrated into chromosomes. In the present study, recombinant adenoviral vectors were generated by use of the AdEasy system, and employed to transfect rat islets at an MOI of 20. According to fluorescence photographs taken at 48 h post-transfection, the expression of EGFP was intense. Furthermore, human HO-1 protein was detected by Western blot in Ad-HO-1 transfected islets at the same time. This demonstrated that recombinant adenoviral vectors were efficient to deliver exogenous genes into rat islets in vitro. Insulin release upon glucose stimulation was a measure of islet function, which was a prerequisite to any gene therapy program for diabetes treatment by islet transplantation[31]. In our experiments, no significant difference in glucose-stimulated insulin release was detected between Ad-EGFP transfected islets and control islets at 7 d post-transfection. This is consistent with the results reported by others showing that adenoviral vectors at a low transfecting dose (MOI 20) provided effective transfer of a marker gene into islet cells without impairing cell function[16].
Even though many clinical islet transplant protocols culture islets for only 24 to 72 h, islets were cultured for seven days in our study. This culture period was selected to minimize the effects of isolation factors on islet function, while maximizing the effect of culture. After seven days culturing, the insulin release upon either low or high concentration glucose stimulation was significantly lower than that of freshly isolated islets (P < 0.05). This is consistent with previous reports showing that islet function degrades in in vitro culture over time. Nevertheless, the insulin release upon high concentration glucose stimulation and stimulation index (SI) of Ad-HO-1 transfected islets were significantly higher than those of Ad-EGFP transfected islets and control islets (P < 0.05). As glucose-stimulated insulin release was a favourable marker of the function of islets, our study demonstrated that HO-1 gene transfer conferred cytoprotection and improved islet function in in vitro culture. This was probably related to the effect of CO, which was not only a stimulator of insulin release but also a trigger of the transients of [Ca2+] i assumed to coordinate the secretory activity of the β-cells[32]. Furthermore, results of fluorescence microscopy studies with AO/PI staining indicated that HO-1 gene transfection significantly improved islet viability and survival during in vitro culture.
Despite the efficient transfection capacity of adenoviral vectors, the transgene cannot integrate into the host cell genome, leading to transient transgenic expression. In our study, we found that the EGFP expression time in cultured islets was more than 3 wk (data not shown). However, for some therapeutic strategies, such as islet cytoprotection in in vitro culture or early after transplantation, temporal expression of the cytoprotective transgene mediated by adenoviral vectors is still recommended because the factors related to early islet injury usually play a major role in the outcome of islet transplantation.
In summary, we demonstrated that adenoviral vectors could successfully transfer exogenous HO-1 gene into rat islet cells, and HO-1 gene transfer could improve rat islet viability and function in in vitro culture. Strategies using HO-1 gene transfer to islets might lead to better outcome in islet transplantation.
We thank the staff of the Diabetes Research Laboratory, the First Affiliated Hospital of Shanghai Jiao Tong University, for helping us to isolate rat islets.
With recent advances in methods of islet isolation and the introduction of more potent and less diabetogenic immunosuppressive therapies, islet transplantation has progressed from research to clinical reality. Islet culture for a brief period (24 to 72 h) has emerged as the current standard procedure prior to clinical transplantation. Short-term islet culture indeed have some benefits, such as purification of the islet preparation, immunomodulation, assessment of islet quality, and possibly improved allograft survival. But in vitro culture of islets has been shown to result in a loss of viability over time, and a decrease in glucose responsiveness has been observed for those islets which survive.
As a cellular graft, islets are especially suited for gene therapy applications. HO-1 has been described as a ubiquitous inducible stress protein capable of cytoprotection via radical scavenging and apoptosis prevention. Overexpression of HO-1 can be chemically induced, but compared with chemical induction, gene transfer can provide effective, targeted, and relatively persistent expression of HO-1. It’s an attractive strategy to protect islets in vitro culture by using gene therapy to transduce islets with cytoprotective gene that can make islets more resistant to injury.
Although advances in islet isolation and less diabetogenic immunosuppression have moved islet transplantation forward from research to clinical reality, many challenges have to be faced with, such as keeping islet viable, single donor grafts, limited donor supply, tolerance induction.
Overexpression of HO-1 by chemical induction can protect islet cells from apoptosis and improve islet function. We used gene technique to modify rat islets in vitro, and found that HO-1 gene transfer could protect islet viability and function after seven days culture. Compared with chemical induction, gene transfer can provide effective, targeted, and relatively persistent expression of HO-1.
HO-1 gene transfer can improve islet viability and function. It suggests a potential therapeutic application for HO-1 gene in improving islet survival/function in islet transplantation.
Islet equivalent (IEQ): islets with an average diameter of 150 μm.
In this manuscript, Chen et al. have analyzed whether adenovirus-mediated gene transfer of heme oxygenase-1 into rat isolated pancreatic islets, affect the long term viability of the cells. This manuscript is well written, experiments and analyses of data are adequately performed.
S- Editor Wang J L- Editor Zhu LH E- Editor Lu W
| 1. | Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24:1936-1940. [PubMed] |
| 2. | Stratta RJ. Mortality after vascularized pancreas transplantation. Surgery. 1998;124:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3836] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 4. | Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, Nakano M. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 269] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Kim SC, Han DJ, Kim IH, Woo KO, We YM, Kang SY, Back JH, Kim YH, Kim JH, Lim DG. Comparative study on biologic and immunologic characteristics of the pancreas islet cell between 24 degrees C and 37 degrees C culture in the rat. Transplant Proc. 2005;37:3472-3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schmied BM, Ulrich A, Matsuzaki H, Ding X, Ricordi C, Moyer MP, Batra SK, Adrian TE, Pour PM. Maintenance of human islets in long-term culture. Differentiation. 2000;66:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Cui YF, Ma M, Wang GY, Han DE, Vollmar B, Menger MD. Prevention of core cell damage in isolated islets of Langerhans by low temperature preconditioning. World J Gastroenterol. 2005;11:545-550. [PubMed] |
| 9. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1997] [Cited by in RCA: 1976] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 10. | Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Tobiasch E, Günther L, Bach FH. Heme oxygenase-1 protects pancreatic beta cells from apoptosis caused by various stimuli. J Investig Med. 2001;49:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tsuburai T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Hasiba T, Ueda A, Ikehara K, Matsuse T, Ishigatsubo Y. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther. 2002;13:1945-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | McCarter SD, Akyea TG, Lu X, Bihari A, Scott JR, Badhwar A, Dungey AA, Harris KA, Feng Q, Potter RF. Endogenous heme oxygenase induction is a critical mechanism attenuating apoptosis and restoring microvascular perfusion following limb ischemia/reperfusion. Surgery. 2004;136:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Wang XH, Wang K, Zhang F, Li XC, Li J, De W, Guo J, Qian XF, Fan Y. Heme oxygenase-1 alleviates ischemia/reperfusion injury in aged liver. World J Gastroenterol. 2005;11:690-694. [PubMed] |
| 15. | Sutton R, Peters M, McShane P, Gray DW, Morris PJ. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986;42:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Weber M, Deng S, Kucher T, Shaked A, Ketchum RJ, Brayman KL. Adenoviral transfection of isolated pancreatic islets: a study of programmed cell death (apoptosis) and islet function. J Surg Res. 1997;69:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Stock PG, Bluestone JA. Beta-cell replacement for type I diabetes. Annu Rev Med. 2004;55:133-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, Frank A, Markmann E, Palanjian M, Brayman K. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237:741-749; discussion 749-750;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Brendel MD, Kong SS, Alejandro R, Mintz DH. Improved functional survival of human islets of Langerhans in three-dimensional matrix culture. Cell Transplant. 1994;3:427-435. [PubMed] |
| 20. | Korbutt GS, Pipeleers DG. Cold-preservation of pancreatic beta cells. Cell Transplant. 1994;3:291-297. [PubMed] |
| 21. | London NJ, Swift SM, Clayton HA. Isolation, culture and functional evaluation of islets of Langerhans. Diabetes Metab. 1998;24:200-207. [PubMed] |
| 22. | Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2635] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 24. | Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 764] [Cited by in RCA: 786] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 25. | Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Schafer AI, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Maines MD. Heme oxygenase 1 transgenic mice as a model to study neuroprotection. Methods Enzymol. 2002;353:374-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Günther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, Bach FH, Tobiasch E. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] |
| 29. | Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148-18153. [PubMed] |
| 30. | Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 340] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Fernandes JR, Duvivier-Kali VF, Keegan M, Hollister-Lock J, Omer A, Su S, Bonner-Weir S, Feng S, Lee JS, Mulligan RC. Transplantation of islets transduced with CTLA4-Ig and TGFbeta using adenovirus and lentivirus vectors. Transpl Immunol. 2004;13:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Lundquist I, Alm P, Salehi A, Henningsson R, Grapengiesser E, Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2003;285:E1055-E1063. [PubMed] |