Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1027
Revised: December 23, 2006
Accepted: January 26, 2007
Published online: February 21, 2007
AIM: To investigate the infection and replication of hepatitis B virus (HBV) in primarily cultured human fetal hepatocytes (HFHs).
METHODS: The human fetal hepatocytes were cultured in serum-free medium, HBV-positive serum was added into the medium to study the susceptibility of hepatocytes to HBV infection. The supernatant was collected for ELISA assay of HBsAg and HBeAg, and quantitative fluorescence PCR for HBV-DNA assay daily. Albumin and HBcAg, CK8 and CK18 expressions were detected by immunohistochemistry in cultured hepatocytes. Content of lactate dehydrogenate (LDH) was measured to find out the integrity of the cell membrane.
RESULTS: A stable hepatocyte culture system was established. HBV could infect the hepatocytes and replicate, and HBcAg expression could be detected by immunohistochemistry in hepatocyte-like cells. HBV-DNA in the supernatant could be detected from d 2 to d 18 and HBsAg and HBeAg were positive on d 3-d 18 after HBV infection. HBV in medium increased from d 0 to d 6 and subsequently decreased as the cells were progressively loosing their hepatocyte phenotypes.
CONCLUSION: HBV could infect human fetal hepato-cytes and replicate. This in vitro model allowed a detailed study on early events associated with human HBV entry into cells and subsequent replication.
- Citation: Lin M, Chen Q, Yang LY, Li WY, Cao XB, Wu JR, Peng YP, Chen MR. Hepatitis B virus infection and replication in primarily cultured human fetal hepatocytes. World J Gastroenterol 2007; 13(7): 1027-1031
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1027
Hepatitis B virus (HBV) infection is one of the commonest infections in the world. According to some reports, about 400 million people have been infected with HBV, and about 5% are chronically infected[1]. Chronic hepatitis B can cause cirrhosis and liver cancer[2-4]. It is estimated that there are more than one million deaths of this infection and 320 thousand deaths from liver cancer associated with HBV each year in the world[5]. There are about 400 million people with chronic hepatitis B worldwide[6]. HBV exhibits a very narrow host range and shows a strong tropism for liver parenchyma cells. It has therefore, been assumed that the susceptibility to HBV infection is restricted to differentiated cells. It was found by some authors that human primarily cultured hepatocytes were more susceptible than other kinds of cells to HBV infection[7,8]. Here we describe a system of experimental infection by HBV virus using primary human fetal hepatocytes. Infection was obtained by co-cultivation of human fetal hepatocytes with HBV-positive serum. The infected fetal hepatocytes in vitro were found to initiate viral DNA replication, and they produced infectious viral particles into medium.
Hepatocytes were prepared from 6 wk old human fetal liver. Embryos procurement was approved by the Ethics Committee of Chaozhou Central Hospital. The sera from the mothers were negative for hepatitis C virus (HCV), HBV and human immunodeficiency virus (HIV) by ELISA (Shanghai SIIC Ke-Hua Biotechnology). Firstly, we used Hank’s liquid to wash the aborted fetus 3 times, and liver tissues were taken out. Secondly, the liver tissues were cut with scissors into 0.1-0.5 mm3 pieces. At last, the little pieces were shattered with 5 mL syringe to single cells or cell aggregates. Viability, assessed by the trypan blue exclusion test, was between 70% and 90%. The cells were seeded into 12-well culture dishes (Orange Scientific) at about 2 × 105 cells per well and incubated with 1 mL of 10% FBS (Gibco) in DMEM (Dulbecco’s Modified Eagle's Medium)/F12 (Nutrient Mixture F-12 HAM) (1:1) (Sigma-Aldrich) supplemented with 0.1 U/L penicillin, 0.1 ng/L streptomycin, and 0.1 ng/L fluconazole at 37°C under 5% CO2 in air. The medium was changed after the first 48 h with serum-free medium. The serum-free medium was composed of DMEM/F12 (1:1) and 0.01 nmol/L nicotinamide, 0.02 ng/L epidermal growth factor (EGF), 0.02 ng/L basic fibroblast growth factor (bFGF), 0.365 ng/L glutamate, B27 (1:50) (Sigma), 0.1 U/L penicillin, 0.1 ng/L streptomycin, and 0.1 ng/L fluconazole.
A serum sample for infection test from HBV carriers was analyzed. The patient was anti-HbsAb positive as detected by the ELISA (Shanghai SIIC Ke-Hua Biotechnology), and HBV-DNA in the serum sample was quantified with fluorescence quantitative polymerase chain reaction (FQ-PCR) assay (Da-An Gene Corp). The patient had received no antiviral therapy prior to the study, and not infected with HCV or HIV. The sera were stored at -80°C until use. The number of serum HBV was 7.6 × 107 copy/mL as quantified by FQ-PCR.
After 24 h culture in serum-free medium as mentioned above, infection was obtained by incubation 1 mL serum-free culture medium with 5% dimethyl sulphoxide (DMSO) and 100 μL HBV serum. Following 24 h exposure, cells were washed 6 times with 3 mL Hank’s liquid and incubated in 1 mL fresh serum-free medium as described above. The medium was changed every day, and the supernatant was collected at various times during the culture period and stored at -80°C. We used one-well cells without HBV serum sample as negative control.
To qualify these DNA molecules, virus DNA was extracted from the culture medium using an alkaline lysis method for FQ-PCR analyses[9]. For detection of HBV-DNA in cultured cells, all of the cells were digested by trypsin-EDTA (0.25%-0.01 mmol/L) solution and centrifugated, then were washed six times with PBS. Cells were lysed with 0.05 mol/L Tris-HCl (pH 7.4)-1% sodium dodecyl sulfate (SDS)-0.02 mol/L NaCl-0.02 mol/L EDTA and incubated with 0.5 g/L of proteinase K at 37°C overnight[9]. Total DNA was extracted by an alkaline lysis method.
HBV-DNA was measured by FQ-PCR diagnostic kit from Da-An Gene Corp. with LightcyclerTM Roche. In this reaction, the nucleotide sequences of the primers were as follows: P1: 5’ATCCTGCTGCTATGCCTCATCTT3’ (23 bp), P2: 5’ACAGTGGGGGAAAGCCCTACGAA3’ (23 bp), FISH: 5’TGGCTAGTTTACTAGTGCCATTTG3’ (25 bp)[10].
QT-PCR amplification was performed using Roche QT-PCR system with 2 min initial denaturation at 93°C for 40 cycles of 5 s at 93°C and 45 s at 57°C, followed by 1 s of extension at 37°C.
HBsAg and HBeAg levels in the supernatant were detected using the monoclonal II enzyme-linked immunosorbent assay. ELISA kits were obtained from Shanghai SIIC Ke-Hua Biotechnology. Titers were expressed as the ratios against cut-off values (A450 of negative control + 0.05 for HBsAg and + 0.05 for HBeAg).
To discriminate liver cells from stromal cells for general observation of morphology, cells were stained with periodic acid-Schiff’s reagent (PAS) by standard methods[12]. The reagents were from Shanghai Zhu-Chun Biotechnology.
The cells were fixed in 0.4% formaldehyde in PBS at room temperature for 15 min, and washed 3 times by PBS. After that, they were incubated for 15 min with 0.25% Triton-X100 and washed 3 times by PBS. Cells were then incubated with the following dilutions of primary antibodies for 1 h at room temperature: antibody to cytokeratin-8 (CK-8) and cytokeratin-18 (CK-18) (Beijing Zhong-Shan Biotechnology), which were diluted at 1:100 in PBS; antibody to HBcAg (1:1) (Fuzhou Maxim Biotechnology); and antibody to human albumin diluted at 1:1000 in PBS (Sigma). Other steps were performed according to manufacturer’s instruction of SP-9000 kits, and AEC served as chromagens.
Content of LDH was measured using automatic blood biochemistry analysis(HITACHI 7060)with Roche reagents. LDH was expressed as U/L released into the medium to find out the integrity of the cell membrane every 24 h.
The statistical analysis was performed with SPSS 13.0 statistic software.
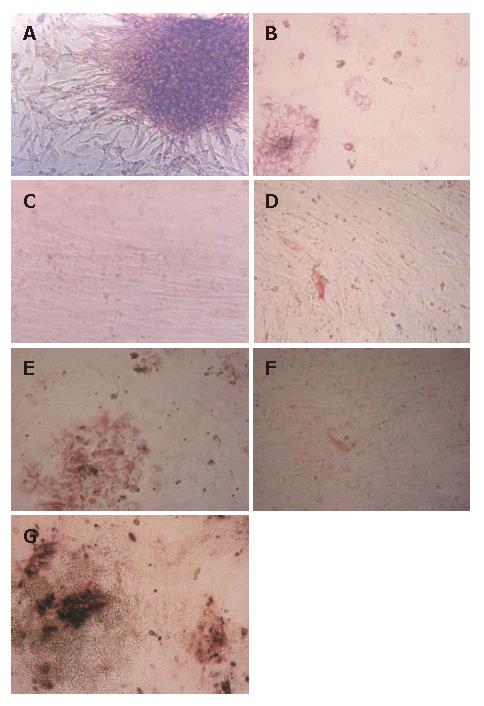
Single cells or cell aggregates were isolated from fetal human livers. The initial cell population was an obvious mixture of hematopoietic and epithelial cells. For instance, red blood cells were copious, although these were rapidly removed at the first medium change. They were plated onto plastic dishes with serum containing medium. After 12 h, the hepatocytes started to attach to the dishes but did not proliferate. After 48 h, the media became serum free, and the HFHs began to show two kinds of state, including cell aggregates or spheroid and scattered cells. The spheroid was made up of many epithelial cells, and fibroblast-like cells migrated from them (Figure 1A). With the elongation of culture time, the percentage of fibroblast-like cells gradually increased and the percentage of flat epithelial cells gradually decreased. After 3-5 wk, fibroblast-like cells proliferated to form a monolayer (Figure 1C).
To confirm the phenotypes of HFHs after 2 wk, cells were analyzed by PAS staining. The majority of cells were stained, and these cells were multiangular and flat, their morphology were similar to that of hepatocytes (Figure 1B), Furthermore, we performed S-P staining using primary antibodies against ALB, CK8 and CK18, the results showed clearly that these proteins were expressed in about 90% cells, confirming that the cells expressed hepatocyte phenotypes (Figure 1E and 1G). After 50 d, only less than 1% cells expressed CK8 and CK18 (Figure 1F). The above-mentioned results were also reported by HU et al[11]. CK8 and CK18 are cytoskeletal proteins characteristic of hepatocytes, they play a very important role in maintaining the structure of hepatocytes, and are expressed in the hepatocyte cytoplasm of the fetal hepatocytes in vivo. Thus, they are good markers for hepatocytes. As culture time extending, the cells that expressed hepatocyte phenotypes gradually decreased, while those cells that expressed fibroblast phenotypes gradually increased. In our opinion, there were two factors to explain this situation: (1) the fibroblast-like cells proliferated faster than hepatocyte-like cells. Therefore, after several weeks culture, fibroblast-like cells proliferated to form a monolayer and few hepatocyte-like cells could be observed; (2) HFHs could change their phenotypes from hepatocytes to mesenchymal cells, this phenomenon has also been reported by other studies[9].
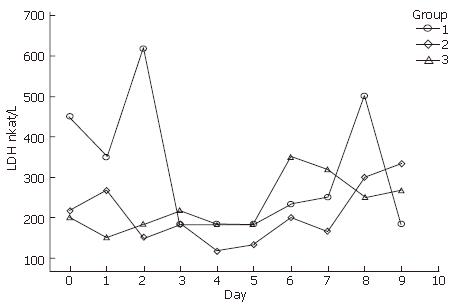
In the course of this study, it was visually apparent that the tendency of LDH increased with culture time in serum-free medium (Figure 2). The amount of LDH in the supernatant ranged from 620 nkat/L to 130 nkat/L.
The serum-free culture medium was collected periodically and the levels of HBsAg and HBeAg were analyzed. As shown in Figures 3A and 3B, the HBsAg and HBeAg were first detected at d 3 after the infection and continued to appear positive during the following 16 d. They must have been synthesized in liver cells because the wash liquid (time zero) and medium from d 1 to d 2 were negative for HBsAg and HBeAg. Thus, the human fetal hepatocytes should be infected by wild virus and replicated HBV.
Human fetal hepatocytes were cultured for 24 h with HBV serum, washed, and incubated with serum-free medium. The supernatant was taken every day and assayed for HBsAg and HbeAg.
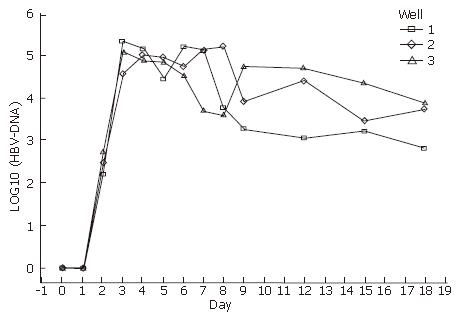
The culture media were collected from d 0 to d 18 (the day of human HBV serum deprivation was indicated as d 0), and were measured by FQ-PCR. HBV-DNA appeared at d 2, and reached a secretion peak from d 3 to d 6. The tendency began to decrease on d 6. Because there was no HBV-DNA in the medium at d 0-2 after infection, HBV-DNA was detected in the serum-free medium from d 3 to d 18 (Figure 4). Therefore, they must have been released from the infected hepatocytes, and the hepatocytes must release and replicate virus DNA. The results of HBV-DNA in liver cells are shown in Table 1.
| Well | Cell number | HBV-DNA (copy/L) |
| 1 | 1.0 × 104 | 2.092 × 109 |
| 2 | 1.5 × 104 | 8.506 × 109 |
| 3 | 1.1 × 105 | 1.371 × 1010 |
To estimate the population of infected cells, immuno-histochemical staining was used to assay HBcAg. HBcAg was detected in about 10% of hepatocyte-like cells 3 d after infection (Figure 5A), whereas no HBcAg was found in the negative control (Figure 5 B).
Replication of HBV has been achieved in human hepatoma cell lines using integrated or transfected HBV genomes as templates[13-16]. Duck cells and adult human liver cells were also successfully infected by HBV[17,18]. However, no signs of viral penetration, replication, or particle production have been observed except for a transient expression of some viral markers in hepatoma cell lines[14-15]. Duck cells and adult human liver cells could not perfectly analogue the process of the HBV infection in human fetal hepatocytes[17,18], and human fetal hepatocytes were used in a few experiments for HBV infection[19,20]. We demonstrated in this study that primary cultures of fetal human hepatocytes could maintain HBV infection in vitro and support the replication of HBV DNA.
In this study, an evidence of virus DNA replication in primary human fetal hepatocytes was testified from d 2 to d 18 after infection by FQ-PCR, another evidence was that HBsAg and HBeAg appeared positive from d 3 to d 18 by ELISA. The process of HBV replication and release continued for 16 d. The results were similar to other experiments with DMSO supplement[18]. But the HBV secretion time in our experiment was longer than the experiments without DMSO[19-21]. The data demonstrated that viral replication in vitro might be strongly enhanced by DMSO.
Previous studies of primary hepatocytes provided very little information about the quantity of HBV-DNA in the culture medium[21-23]. In their reports, DNA or RNA used to be detected by Southern blot or Northern blot[14,18,23]. The procedures of these methods were complicated, and the results were not stable. In our experiment, we used FQ-PCR to measure the HBV-DNA in the supernatant and cultured cells. FQ-PCR combines gene amplification and molecule hybridization with fluorescence physics, conducts the whole process of DNA amplification and PCR products analysis in an enclosed tube, and real-time detection as well as auto-analysis under computer control[10]. As a result, the cross-contamination of conventional PCR products and incapacity of quantification can be eliminated fundamentally[10]. Furthermore, the specificity and sensitivity increased remarkably. This method could provide reliable and precise data for HBV quantification.
The quantity of HBV in medium increased from d 0 to d 6 and decreased from d 7 to d 18 in the infection process. From d 0 to d 6, HBV replicated in infected cells and released to the medium, and then the free HBV infected other hepatocytes. With the increasing number of hepatocytes, it formed one infected cycle in cells and the quantity of HBV increased in the medium. From d 7 to d 18, a part of cells gradually lost their hepatocyte phenotypes and HBV infection susceptibility, and the cycle was broken. Meanwhile, a part of floating cells and cell spheroid died gradually, subsequently, the quantity of HBV decreased.
In this study, we found that the presence of HbeAg was more correlated with HBV-DNA in the medium than that of HBsAg. According to our knowledge, HBeAg was a marker of extensive viral replication in HBsAg-positive sera of patients with hepatitis B virus infection[24]. The presence of HBeAg in the serum correlated well with hepatitis B-DNA[24]. Therefore, our results were similar to the status in human body.
By immunohistochemical analysis, we were able to observe a part of infection of the hepatocytes in the cultures. Similar results have previously been reported[23]. Recently, Tuttleman et al[17] used primary duck hepatocytes to infect HBV and only 10% of the primary duck hepatocytes displayed HBcAg[25]. The events could be observed in duck hepatocytes and human fetal liver cells. According to some reports, the procedure of liver cell isolation could destroy the capacity for infection of all but some cells. As it is well known, human hepatocytes were hard to maintain in cultures, such as albumin expression. Such phenotypes are easily lost within one week culture when the cells are inoculated in serum-containing medium. In our observation, the shape of the hepatocytes was polygonal at 48 h. With elongation of culture time, some hepatocytes gradually changed and extended in shape and became fibroblast-like, meanwhile they lost hepatocyte phenotypes such as ALB, CK18 and CK8 expressions. So it is likely that some flat cells may lessen the susceptibility to HBV infection.
HBV infection in primary fetal hepatocyte cultures is suitable for cloning virus because of the limited infectivity of the cells. However, our system in vitro has been found very useful for studying the early events in viral entry into cells as well as viral replication.
S- Editor Liu Y L- Editor Ma JY E- Editor Lu W
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, Chen DS. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis. 2006;194:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Whitworth A. Ten years later: liver cancer treatment reevaluated. J Natl Cancer Inst. 2006;98:958-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823-3833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko GM, Lotze MT. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 321] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Yan JC, Ma JY, Pan BR, Ma LS. The study of chronic hepatitis B in China. Shijie Huaren Xiaohua Zazhi. 2001;9:611-616. |
| 7. | Guha C, Mohan S, Roy-Chowdhury N, Roy-Chowdhury J. Cell culture and animal models of viral hepatitis. Part I: hepatitis B. Lab Anim (NY). 2004;33:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Walter E, Keist R, Niederöst B, Pult I, Blum HE. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology. 1996;24:1-5. [PubMed] |
| 9. | Klintschar M, Neuhuber F. Evaluation of an alkaline lysis method for the extraction of DNA from whole blood and forensic stains for STR analysis. J Forensic Sci. 2000;45:669-673. [PubMed] |
| 10. | Cheng G, He YS, Zhou XY. Fluorescence quantitative PCR and its application in detection of hepatitis B virus. Zhonghua Jianyan Yixue Zazhi. 1999;22:135-136. |
| 11. | Roberts GP. Histochemical detection of sialic acid residues using periodate oxidation. Histochem J. 1977;9:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hu A, Cai J, Zheng Q, He X, Pan Y, Li L. Hepatic differentiation from embryonic stem cells in vitro. Chin Med J (Engl). 2003;116:1893-1897. [PubMed] |
| 13. | Lu X, Block TM, Gerlich WH. Protease-induced infectivity of hepatitis B virus for a human hepatoblastoma cell line. J Virol. 1996;70:2277-2285. [PubMed] |
| 14. | Tsurimoto T, Fujiyama A, Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci USA. 1987;84:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 939] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 16. | Sureau C, Romet-Lemonne JL, Mullins JI, Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986;47:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Tuttleman JS, Pugh JC, Summers JW. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17-25. [PubMed] |
| 18. | Gripon P, Diot C, Thézé N, Fourel I, Loreal O, Brechot C, Guguen-Guillouzo C. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J Virol. 1988;62:4136-4143. [PubMed] |
| 19. | Jiang YG, Li QF, Qing M, Wang YM, Primary human fetal hepatocytes with HBV infection in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:403-405. |
| 20. | Wang F, Wang YM, Tang B, Liu J, Liu GD, Wang XH. Assays for HBV infection in primary human fetal hepatocytes cultured in vitro. Disan Junyidaxue Xuebao. 2004;26:74-77. |
| 21. | Tang B, Wang YM, Wang F, Liu J, Zhang R. Susceptibility change to HBV in primary culture of first trimester human fetal hepatocytes. Zhonghua GanZan Bing ZaZhi. 2004;12:21-24. [PubMed] |
| 22. | Galle PR, Hagelstein J, Kommerell B, Volkmann M, Schranz P, Zentgraf H. In vitro experimental infection of primary human hepatocytes with hepatitis B virus. Gastroenterology. 1994;106:664-673. [PubMed] |
| 23. | Ochiya T, Tsurimoto T, Ueda K, Okubo K, Shiozawa M, Matsubara K. An in vitro system for infection with hepatitis B virus that uses primary human fetal hepatocytes. Proc Natl Acad Sci USA. 1989;86:1875-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Lu ZR, Lei H. Textbook of Diagnostics (Engl). 1st ed. Oxford: Chin Sci Pub 2006; 301. |
| 25. | Zhou GY, Jiang XC. Textbook of Pathology (Engl). 1st ed. Oxford: Chin Sci Pub 2006; 243. |