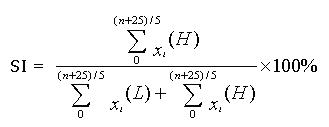Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.791
Revised: December 7, 2006
Accepted: January 8, 2007
Published online: February 7, 2007
AIM: To investigate potential roles of per rectal portal scintigraphy in diagnosis of esophageal varices and predicting the risk of bleeding.
METHODS: Fifteen normal subjects and fifty cirrhotic patients with endoscopically confirmed esophageal varices were included. Patients were categorized into bleeder and non-bleeder groups according to history of variceal bleeding. All had completed per rectal portal scintigraphy using 99mTechnetium pertechnetate. The shunt index was calculated from the ratio of 99mTechnetium pertechnetate in the heart and the liver. Data were analyzed using Student’s t-test and receiver operating characteristics.
RESULTS: Cirrhotic patients showed a higher shunt index than normal subjects (63.80 ± 25.21 vs 13.54 ± 6.46, P < 0.01). Patients with variceal bleeding showed a higher shunt index than those without bleeding (78.45 ± 9.40 vs 49.35 ± 27.72, P < 0.01). A shunt index of over 20% indicated the presence of varices and that of over 60% indicated the risk of variceal bleeding.
CONCLUSION: In cirrhotic patients, per rectal portal scintigraphy is a clinically useful test for identifying esophageal varices and risk of variceal bleeding.
- Citation: Chitapanarux T, Praisontarangkul OA, Thongsawat S, Pisespongsa P, Leerapun A. Per rectal portal scintigraphy as a useful tool for predicting esophageal variceal bleeding in cirrhotic patients. World J Gastroenterol 2007; 13(5): 791-795
- URL: https://www.wjgnet.com/1007-9327/full/v13/i5/791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.791
Esophageal variceal bleeding is a serious and potentially life-threatening complication of liver cirrhosis[1-4]. No simple noninvasive method which accurately predicts esophageal variceal bleeding is available so far and endoscopists have had to perform endoscopy every 3 to 6 mo to evaluate patients with previously bleeding esophageal varices[5,6]. However, this approach is costly and not all patients with liver cirrhosis and esophageal varices are good candidates for such procedures[7].
Per rectal portal scintigraphy is a noninvasive method for evaluation of portosystemic shunting using portal shunt index (SI) calculated from radioactivity curves of the liver and the heart. In humans, such an SI shows a good correlation with portal pressure measured by percutaneous transhepatic portography or intraoperative method[8]. Portal scintigraphy is also clinically useful, especially in establishing prognosis of cirrhotic patients with varying shunt indices[9]. Evaluating portal pressure using per rectal portal scintigraphy may be easily performed and might provide information on risk of variceal bleeding.
The aim of this study was to determine the benefit of per rectal portal scintigraphy for evaluation of non-bleeding esophageal varices, bleeding esophageal varices and normal control subjects. We also compared different portal shunt indices, Child-Pugh grades and endoscopic appearances in various groups and examined the correlation between the portal SI and risk of variceal bleeding.
Normal subjects: Normal adult volunteers of both sexes, with a minimum age of 18 years, were recruited. An attempt was made to obtain subjects in well distributed age groups, and equal in number for both sexes. All of the subjects were in good health, with no history of the liver and vascular diseases, or use of medication, alcohol or any substances that might affect the liver. The liver function test was normal, and tests for viral hepatitis (B, C) were negative or revealed immunized status. Hepatic ultrasonography was also performed to rule out structural liver disease or hepatocellular carcinoma.
Patients: Cirrhotic patients of either alcoholic or viral in etiology who had signs of portal hypertension, and a history or physical signs suggesting esophageal varices, or even esophageal variceal bleeding, were asked to participate in this study. Only patients with endoscopically confirmed esophageal varices were included. The esophageal varices were graded according to criteria developed by the Japanese Research Society for Portal Hypertension[10]. Thorough medical examination and interview for the history of gastrointestinal bleeding were performed. Only cirrhotic patients with esophageal varices were included in this study. The initial laboratory tests performed in this study included complete blood count, liver function test, viral hepatitis (B, C) profile, and renal function test. All patients were graded according to Child-Pugh classification. The etiology of bleeding was considered to be variceal if either actively bleeding varices were observed by endoscopy or if varices were endoscopically found without other sources of bleeding.
Informed consent were obtained from all patients and the protocol was in conformity with the ethical guidelines of the Declaration of Helsinki and the Clinical Research Committee at Chiang Mai University Hospital.
The procedure used for per rectal portal scintigraphy was the same for both normal subjects and cirrhotic patients[9]. After fasting overnight for at least 6 h, the rectum was emptied by administration of laxatives (unison enema 100 mL). A polyethylene device (Terumo® feeding tube Fr.8) was inserted deep into the rectum with the tip of the tube being placed in the upper rectum, 20 cm above the anal verge, to avoid absorption into the systemic circulation via the inferior rectal vein in the lower rectum. To generate time-activity curves, a gammascinti camera with a large field of view (Apex SP4, El-Scint Co., Israel), equipped with a low-energy, multipurpose, parallel-hold collimator was used. The collimator was positioned over the patient’s abdomen so that the field of view would always include the heart, the liver, and the spleen. Ten millicuries (2 mL) of 99mTechnetium pertechnetate, followed by 15 mL of air, was infused into the rectum through the tube. The sequential images were generated from counts in their areas of interest (AOI). For color display, the summed images were reconstructed by grouping of 20 s per image from the original 60 images. 99mTechnetium pertechnetate is commonly used in diagnostic scanning and imaging both in clinics and research. The procedure is safe and readily applicable because of the amount and short half life of radioactive substance used. Portal pressure increases shortly after an episode of variceal bleeding[11] and is stable over 72 h after bleeding[12]. Therefore, per rectal portal scintigraphy in patients of the bleeding group was performed 72 h after bleeding when the portal pressure returned to baseline level in order to determine the risk of that episode of bleeding.
To calculate the amount of blood that entered the portal system and went to the liver and the heart, we used the portal SI[8]. This index was derived from the ratio of 99mTechnetium pertechnetate in the heart and the liver at the exact time as shown in the following equation.
Math 1
n = time at which radionuclides appeared in the area of the liver (s).
n′ = time at which radionuclides appeared in the area of the heart (s).
Xi (L) = the count per 5 s over the AOI of the liver.
Xi (H) = the count per 5 s over the AOI of the heart.
In normal portal circulation, the transit time of blood circulation from the liver to the heart via hepatic veins and inferior vena cava was 18-26 s, and the scinti images were acquired in 5 s per image. The SI was calculated at the time of n + 25 s. The results were shown as the pattern of time-activity curves and summed-images.
Results were expressed as mean ± SD. The significance of difference between mean values was evaluated by Student’s t-test. Differences with probability values of less than 0.05 were considered to be significant. The selection of an appropriate cutoff point of portosystemic shunt for the presence of esophageal varices and the risk of variceal bleeding was analyzed by the receiver operating characteristic (ROC)[13].
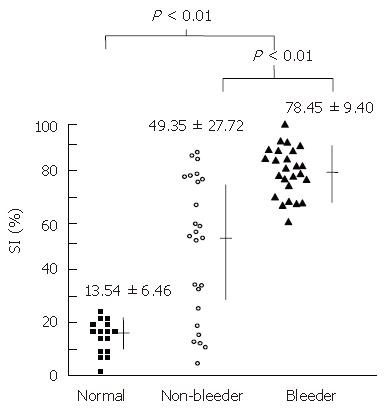
The normal control group consisted of eight male and seven female subjects (aged 28-59 years, mean 44.3 years). Fifty cirrhotic patients (45 males and 5 females), of various etiologies, aged between 30 and 70 years (mean 46.9 years) were recruited in this study. The baseline characteristics of all study patients are shown in Table 1. The patients were categorized into two groups according to their history of variceal bleeding, namely a non-bleeding group including 25 patients and a bleeding group with 25 patients. Bleeding was the first episode in all 25 patients in the bleeding group. All patients had received neither endoscopic variceal surveillance nor prophylactic treatment to prevent variceal bleeding. The two groups of patients showed similar baseline characteristics with the exception of alanine aminotransferase, total bilirubin, and prothrombin time. The severity of liver disease in the bleeding group was higher than that of the non-bleeding group. In the non-bleeding group, etiologies of liver disease were alcoholic in 9, hepatitis viral B in 6, hepatitis viral C in 5, and alcoholic and virus in 5 cases. In the bleeding group, etiologies of liver disease were alcoholic in 20, hepatitis virus B in 3, and alcoholic and virus in 2 cases. The mean SI in normal subjects was 13.54% ± 6.46%. For the cirrhotic patients, the mean SI was 63.80% ± 25.21%. Normal subjects were found to have significantly lower average SI than cirrhotic patients with esophageal varices (P < 0.01). If cirrhotic patients were grouped as non-bleeding and bleeding, the mean SIs were 49.35% ± 27.72% and 78.45% ± 9.40%, respectively. The average SI in bleeding group was significantly higher than in non-bleeding group (P < 0.01), as shown in Figure 1.
| Control(n =15) | Non bleeder(n = 25) | Bleeder(n = 25) | |||
| Age (yr) | 44.27 ± 8.48 | 49.32 ± 10.25 | 44.25 ± 8.86 | ||
| Sex (M/F) | 8/7 | 20/5 | 25/0 | ||
| Presence of ascites (%) | 0 | 10 (40) | 7 (28) | ||
| Hepatic encephalopathy (%) | 0 | 0 | 3 (12) | ||
| Serum albumin (g/dL) | 4.55 ± 0.26 | 3.16 ± 0.54 | 2.76 ± 0.83 | ||
| Alanine aminotransferase (U/L) | 18.93 ± 4.03 | 51.88 ± 45.9 | 77.12 ± 61.41b | ||
| Serum bilirubin (mg/dL) | 0.65 ± 0.19 | 2.49 ± 1.16 | 5.27 ± 6.55b | ||
| Prothrombin time (s) | - | 2.67 ± 1.02 | 3.27 ± 2.43b | ||
| Etiology of cirrhosis | |||||
| Alcohol | - | 9 | 20 | ||
| Hepatitis B virus | - | 6 | 3 | ||
| Hepatitis C virus | - | 5 | 0 | ||
| Alcohol and virus | - | 5 | 2 | ||
| Child-Pugh | A | - | 13 | 8 | |
| B | - | 9 | 9 | ||
| C | - | 3 | 8 | ||
Analysis was done to discover any difference in SIs when the patients were sub-grouped according to etiologies (i.e. alcoholic, viral, or both), presence of ascites, endoscopic appearances and Child-Pugh grade. Details are shown in Table 2. The mean SI was 78.87% ± 22.05% in cirrhosis from alcohol, 56.67% ± 24.08% in cirrhosis from virus, and 41.23% ± 23.47% in cirrhosis from alcohol plus viruses. The average SI in the etiology group of alcohol significantly differed from that of virus, and also that of alcohol plus virus (P < 0.05). The mean SI was 62.87% ± 28.62% in cirrhotic patients without ascites and 65.92% ± 23.64% in cirrhotic patients with ascites. The difference between both groups was not significant (P = 0.35). We used Child-Pugh classification to estimate the severity of liver disease in both bleeding and non-bleeding groups as a whole. The mean SI was 52.60% ± 25.31% in Child-Pugh grade A, 69.29% ± 22.34% in Child-Pugh grade B, and 76.64% ± 22.01% in Child-Pugh grade C. The SI in Child-Pugh grade A differed significantly (P < 0.05) from those in Child-Pugh grades B and C. Although the SI in Child-Pugh grade B seemed to be lower than that in Child-Pugh grade C, the difference was not statistically significant (P = 0.20).
| n | Portal shuntindex (%) | |||
| Etiology of cirrhosis | ||||
| Alcohol | 29 | 78.87 ± 22.05 | ||
| Virus1 | 14 | 56.67 ± 24.08 | ||
| Alcohol with virus1 | 7 | 41.23 ± 23.47 | ||
| Ascites | Absence | 33 | 62.87 ± 28.62 | |
| Presence | 17 | 65.92 ± 23.64 | ||
| Severity of liver diseases | Child A | 21 | 52.60 ± 25.31 | |
| Child B | 18 | 69.29 ± 22.34 | ||
| Child C | 11 | 76.64 ± 22.01 | ||
| Endoscopic appearances | F1 | 14 | 50.03 ± 27.63 | |
| F2 | 20 | 67.47 ± 24.99 | ||
| F3 | 16 | 72.65 ± 8.77 | ||
| RCS-positive | 27 | 68.16 ± 23.78 | ||
| RCS-negative | 23 | 52.04 ± 26.31 | ||
The esophageal varices were endoscopically graded according to the Japanese Research Society for Portal Hypertension by color, forms, location, diameters, and the red color sign (RCS). Variceal forms were classified into three types: straight (F1), enlarged tortuous (F2), and largest sized (F3). The mean SI was 57.03% ± 27.63% in F1 group, 67.47% ± 24.99% in F2 group, and 72.65% ± 8.77% in F3 group (Table 2). Mean SI in F3 group was higher than in F2 and F1 groups. The difference between the groups was statistically significant (P < 0.05). Mean SI in RCS-negative group was 52.94% ± 26.31% and in RCS-positive group was 68.16% ± 23.78%. The difference between both groups was not significant (P = 0.054).
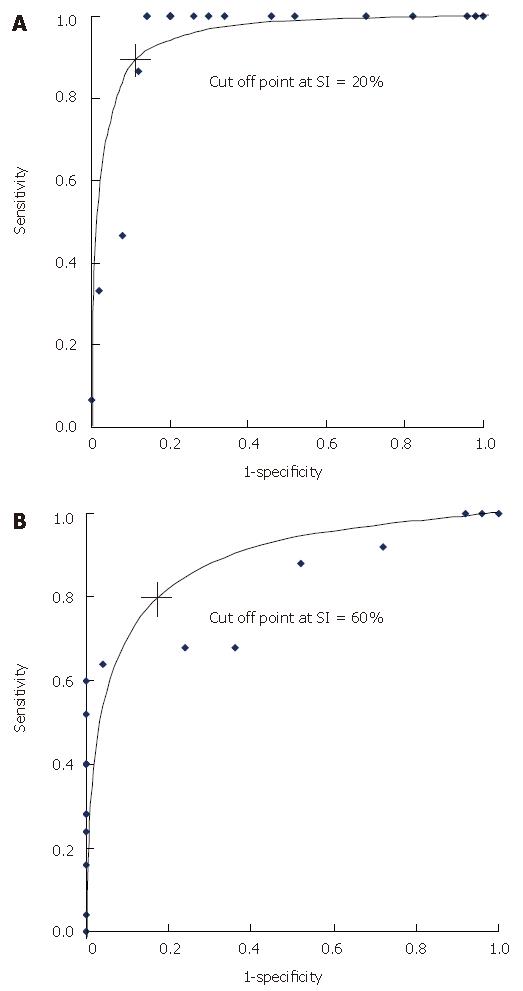
The receiver operating characteristic (ROC) showed the cutoff point of portal SI between normal subjects and cirrhotic patients at 20% with a 90.0% sensitivity and 53.3% specificity. The cutoff point of portal SI between the cirrhotic patients with and without variceal bleeding was 60% with a 96.0% sensitivity and 72.0% specificity (Figure 2). Basically, the cirrhotic patients with esophageal varices who had a portal SI of more than 60% might have high risk of variceal bleeding, and the patients who had a portal SI more than 20% were likely to develop esophageal varices.
In this study, we used per rectal portal scintigraphy to detect and investigate portosystemic shunting in order to determine the difference among the bleeding, the non-bleeding cirrhotic and the normal control groups. We found that the SI was higher in the bleeders compared with other groups. The SI correlated with the severity of liver disease and the size of esophageal varices. Bleeding from the varices was a major complication of portal hypertension that may vary according to the severity of portal hypertension. The cumulative survival rate of patients with esophageal varices was significantly lower than that of patients without varices[14]. The cumulative survival rate of patients with portosystemic shunting seen by scintillation splenoportography was also significantly lower than that of patients without such a shunt [15]. Many factors could potentially be used to predict variceal bleeding in cirrhotic patients with esophageal varices. These predictors include the following: (1) local factors such as variceal size, vessel radius, or red color sign; (2) hemodynamic factors: portal pressure > 12 mmHg, blood volume, and collateral blood flow; and (3) severity of liver disease[10]. In general, the direct measurement of portal pressure is performed invasively and not readily available in many clinical settings. In those circumstances, indirect measurement of portal pressure, such as portosystemic shunt evaluation may be used instead. Recently, various methods of measuring portosystemic shunt have been developed, and the relationships between the risk of variceal bleeding and the extent of portosystemic shunt have been studied.
Per rectal portal approach in the measurement of portal circulation using various radionuclides has been described as a relatively non-invasive method by many researchers[9,16-19]. In our present study, we chose to perform non-invasive per rectal portal scintigraphy using 99mTechnetium pertechnetate because this radionuclide is well absorbed by the rectum, has a short half life and large doses could be used[20]; it also generates images useful for diagnosis and is economical. The SI from per rectal scintigraphy also correlated well with the degree of portal pressure[9]. In this study, the mean SI in cirrhotic patients with esophageal varices was significantly higher than that in normal subjects. The cutoff level of SI indicating development of esophageal varices derived from the ROC was 20%. All six cirrhotic patients with esophageal varices whose SIs were less than 20% belonged to Child-Pugh grade A and had less severe variceal forms without red color signs than patients with esophageal varices and an SI more than 20%. None of these six patients with small varices bled.
We also studied the association between Child-Pugh class, etiology of cirrhosis and portal scintigraphy in cirrhotic patients with esophageal varices. The mean SI in Child-Pugh grade C was higher than that of Child-Pugh grade B and grade A, with a significant difference between grade C and A. The SI tended to be higher in patients with more severe liver disease. Because the Child-Pugh classification correlated well with the severity of liver disease, serial measurement of portal circulation by SI might be helpful in investigating the progress of cirrhosis. The SI in alcoholic cirrhosis was significantly higher than other etiologies because of more severe liver disease in alcoholic group. The SI in patients with alcohol and virus cirrhosis was lower compared with cirrhosis induced by alcohol or virus alone. This might possibly result from the small number of patients in alcohol with virus group. The SIs in cirrhotic patients with and without ascites were not significantly different. In comparison of the SI in different variceal forms, there were significant differences among the size of varices. Higher SIs were found in patients with higher variceal forms. There was a good relationship between SI and the size of esophageal varices.
The SI values in the present study were significantly different in cirrhotic patients with and without variceal bleeding. It was presumed that an increasing flow of blood through the collateral of inferior mesenteric veins measured by portal scintigraphy reflected the increasing pressure in the portal system, the development and the size of esophageal varices. This increasing pressure was at a certain level, adequately high for the varices to rupture. So the higher the SI, the higher was the risk of bleeding. In this study, the SI was clearly demonstrated to be a useful predictor of the risk of bleeding. By ROC, an SI of more than 60% correlated with esophageal variceal bleeding. We presumed that if cirrhotic patients had an SI of more than 20%, they would have adequate portal pressure to develop the esophageal varices, and if the SI was more than 60% they carried the risk of variceal bleeding. However, further prospective, longitudinal studies are needed to confirm these results.
In conclusion, this study suggests that portosystemic shunt, represented by the SI and evaluated by per rectal portal scintigraphy, is higher in cirrhotic patients with esophageal varices. The magnitude of blood shunting through these tributaries correlates well with the etiology and severity of cirrhosis and the risk of variceal bleeding. An SI of more than 60% in cirrhotic patients with esophageal varices reflects a high risk of variceal bleeding.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Van Ruiswyk J, Byrd JC. Efficacy of prophylactic sclerotherapy for prevention of a first variceal hemorrhage. Gastroenterology. 1992;102:587-597. [PubMed] |
| 2. | Thomopoulos KC, Labropoulou-Karatza C, Mimidis KP, Katsakoulis EC, Iconomou G, Nikolopoulou VN. Non-invasive predictors of the presence of large oesophageal varices in patients with cirrhosis. Dig Liver Dis. 2003;35:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | de Franchis R. Evaluation and follow-up of patients with cirrhosis and oesophageal varices. J Hepatol. 2003;38:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Mendez C, Marsano L, Wright R. Complications of cirrhosis. J Ky Med Assoc. 2003;101:403-414. [PubMed] |
| 5. | von Herbay A, Frieling T, Häussinger D. Color Doppler sonographic evaluation of spontaneous portosystemic shunts and inversion of portal venous flow in patients with cirrhosis. J Clin Ultrasound. 2000;28:332-339. [PubMed] [DOI] [Full Text] |
| 6. | Yin XY, Lu MD, Huang JF, Xie XY, Liang LJ. Color Doppler velocity profile assessment of portal hemodynamics in cirrhotic patients with portal hypertension: correlation with esophageal variceal bleeding. J Clin Ultrasound. 2001;29:7-13. [PubMed] [DOI] [Full Text] |
| 7. | Burkart DJ, Johnson CD, Ehman RL, Weaver AL, Ilstrup DM. Evaluation of portal venous hypertension with cine phase-contrast MR flow measurements: high association of hyperdynamic portal flow with variceal hemorrhage. Radiology. 1993;188:643-648. [PubMed] |
| 8. | Shiomi S, Kuroki T, Kurai O, Kobayashi K, Ikeoka N, Monna T, Ochi H. Portal circulation by technetium-99m pertechnetate per-rectal portal scintigraphy. J Nucl Med. 1988;29:460-465. [PubMed] |
| 9. | Shiomi S, Kuroki T, Ueda T, Takeda T, Ikeoka N, Nishiguchi S, Nakajima S, Kobayashi K, Ochi H. Clinical usefulness of evaluation of portal circulation by per rectal portal scintigraphy with technetium-99m pertechnetate. Am J Gastroenterol. 1995;90:460-465. [PubMed] |
| 10. | The general rules for recording endoscopic findings on esophageal varices. Jpn J Surg. 1980;10:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 235] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Pomier-Layrargues G, Villeneuve JP, Willems B, Huet PM, Marleau D. Systemic and hepatic hemodynamics after variceal hemorrhage: effects of propranolol and placebo. Gastroenterology. 1987;93:1218-1224. [PubMed] |
| 12. | Ready JB, Robertson AD, Goff JS, Rector WG. Assessment of the risk of bleeding from esophageal varices by continuous monitoring of portal pressure. Gastroenterology. 1991;100:1403-1410. [PubMed] |
| 13. | Dawson-Saunders B. Evaluating Diagnostic Procedures. Basic&Clinical Biostatistics. 2 nd ed. Connecticut: Appleton&Lange 1994; 243-246. |
| 14. | Baker LA, Smith C, Lieberman G. The natural history of esophageal varices; a study of 115 cirrhotic patients in whom varices were diagnosed prior to bleeding. Am J Med. 1959;26:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 80] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Syrota A, Paraf A, Gaudebout C, Desgrez A. Significance of intra- and extrahepatic portasystemic shunting in survival of cirrhotic patients. Dig Dis Sci. 1981;26:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Castell DO, Grace ND, Wennar MH, Chalmers TC, Moore EW. Evaluation of portal circulation in hepatic cirrhosis. A new method using xenon. Gastroenterology. 1969;57:533-541. [PubMed] |
| 17. | Tonami N, Nakajima K, Hisada K, Tanaka N, Kobayashi K. A noninvasive method for evaluating portal circulation by administration of Ti-201 per rectum. J Nucl Med. 1982;23:965-972. [PubMed] |
| 18. | Yen CK, Pollycove M, Crass R, Lin TH, Baldwin R, Lamb J. Portasystemic shunt fraction quantification with colonic iodine-123 iodoamphetamine. J Nucl Med. 1986;27:1321-1326. [PubMed] |
| 19. | Wang JY, Chen SL, Chen FZ, Xu WG, Hu DC, Chen XF, Jin G, Liu HY. A non-invasive method for evaluating cirrhotic portal hypertension by administration of 99mTc-MIBI per rectum. J Gastroenterol Hepatol. 1995;10:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Urbain D, Jeghers O, Ham HR. Per-rectal portal scintigraphy: comparison between technetium-99m, thallium-201, and iodine-123-HIPDM. J Nucl Med. 1988;29:2020-2021. [PubMed] |