Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.754
Revised: October 13, 2006
Accepted: January 4, 2007
Published online: February 7, 2007
AIM: To investigate active cytomegalovirus (CMV) infection following the cyclosporine A (CyA) treatment of steroid-refractory ulcerative colitis (UC).
METHODS: Twenty-three patients with severe UC not responding to steroid therapy (male 14, and female 9) enrolled at Nagoya University Hospital from 1999 to 2005. They received continuous intravenous infusion of CyA (average 4 mg/kg per day) for 1 mo. Serum and colonic biopsy samples were collected before CyA treatment and 4 d, 10 d, 20 d, and 30 d after treatment. Patients were evaluated for CMV by using serology (IgM antibody by ELISA), quantitative real-time PCR for CMV DNA, and histopathological assessment of hematoxylin and eosin (HE)-stained colonic biopsies. CMV infection was indicated by positive results in any test.
RESULTS: No patients had active CMV infection before CyA treatment. Eighteen of 23 UC patients treated with CyA were infected with active CMV (IgM antibody in 16/23 patients, 69.6%; CMV DNA in 18/23 patients, 78.2%; and inclusion bodies in 4/23 patients, 17.3%). There was no difference in the active CMV-infection rate between males and females. Active CMV infection was observed after approximately 8 d of CyA treatment, leading to an exacerbation of colitis. Fifteen of these 18 patients with active CMV infection (83.3%) required surgical treatment because of severe deteriorating colitis. Treatment with ganciclovir rendered surgery avoidable in three patients.
CONCLUSION: Our results suggest that active CMV infection in severe UC patients treated with CyA is associated with poor outcome. Further, ganciclovir is useful for treatment of CMV-associated UC after immuno-suppressive therapy.
- Citation: Minami M, Ohta M, Ohkura T, Ando T, Ohmiya N, Niwa Y, Goto H. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J Gastroenterol 2007; 13(5): 754-760
- URL: https://www.wjgnet.com/1007-9327/full/v13/i5/754.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.754
Cytomegalovirus (CMV) infection is one of the most common infectious complications after immuno-suppressive therapy. It occurs mainly as a secondary infection in CMV-seropositive patients. CMV infection is a common viral infection in humans, occurring in 40%-100% of adults[1]. CMV infections are generally asymptomatic or are manifested as a mild mononucleosis-like syndrome[2]. Significant CMV disease may occur in various organs such as the retina, lung, and gastrointestinal tract, and the target organ is related to the etiology of immunosuppression[1]. Gastrointestinal (GI) CMV infection is rare in immunocompetent individuals. Clinically significant GI CMV infection generally occurs in immune-ocompromised patients[3]. In the gastrointestinal tract, CMV disease can occur in all locations, from the mouth to the rectum, and generally involves the formation of ulcers in the mucosa, often accompanied by hemorrhage[1].
Ulcerative colitis (UC) is common all over the world and is generally more frequent than Crohn’s disease (CD)[4]. UC is thought to result from the inappropriate and progressive activation of the mucosal immune system driven by the presence of normal luminal flora. The aberrant response is most likely facilitated by defects in both the barrier function of the intestinal epithelium and the mucosal immune system[4].
Cyclosporine A (CyA) selectively inhibits immune responses mediated by T lymphocytes by modulating the interaction of calcineurin-calmodulin[5]. This recognition has led to its use in patients with severe UC, with variable results. In an uncontrolled study, approximately 60%-80% of patients suffering from severe corticosteroid-refractory ulcerative colitis responded to cyclosporine therapy[6,7]. Since an intravenous infusion of CyA has clinical benefits in patients with steroid-resistant UC, it has been generally accepted that CyA selectively blocks the activation of helper and cytotoxic T cells and acts by inhibiting the nuclear factor of activated T cells and cytokine gene expression[8].
In Japan, patients with severe, corticosteroid-refractory or corticosteroid-dependent UC are frequently treated with strong immunosuppressive agents, including CyA. Therefore, patients with inflammatory bowel disease (IBD) are expected to be at an increased risk of infection with CMV. Despite the frequent use of immunosuppressive drugs in patients with UC, data on the frequency of CMV infection and its clinical significance in patients with UC are limited. The aim of this study was to describe our experience with active CMV infection following treatment with CyA for UC.
Twenty-three patients with severe UC enrolled at Nagoya University Hospital from 1999 to 2005. They did not respond to a minimum of 7 d of intravenous systemic steroid therapy (prednisolone, more than 30 mg/d). The diagnosis of UC was based on clinical, endoscopic, radiological, and histological parameters. The study was approved by the Ethical Committee of the Graduate School of Medicine, Nagoya University, and all samples were obtained with informed consent in accordance with the Helsinki Declaration.
Twenty-three patients received a continuous intravenous infusion of CyA in the form of Sandimmun solution (Novartis Pharma KK, Tokyo, Japan) at an average daily dose of 4 mg/kg for 1 mo. The CyA dose was adjusted to maintain a whole-blood CyA concentration of less than 500 pg/L. Complete blood cell counts, C-reactive protein (CRP), liver function tests (aspartate aminotransferase and alanine aminotransferase), renal function tests (creatinine and blood urea nitrogen), and clinical evaluation were performed before CyA treatment and then 4 d, 10 d, 20 d, and 30 d after treatment.
Serum samples and colonic biopsy specimens were obtained from all patients before CyA treatment and then 4 d, 10 d, 20 d, and 30 d after treatment. Multiple biopsy samples were obtained from the inflamed area during colonoscopy for histopathological examination of inflammatory activity and CMV inclusion bodies; these samples were fixed in buffered neutral formalin. EDTA-treated venous blood (5 mL) was obtained from each patient under aseptic conditions for serological studies. The plasma was separated by centrifugation.
The colonic biopsy samples were paraffinized, sectioned, and stained with hematoxylin and eosin (HE). These sections were microscopically evaluated for the presence of characteristic cytomegalic cells and “owl’s eye” nuclear inclusion bodies. Histologically, the activity of IBD was classified according to a standard system described previously [9].
For the PCR assays, DNA was extracted from 200 μL of plasma by using the QIAamp Blood Kit (QIAGEN Ltd, Tokyo, Japan), eluted in 100 μL of distilled water, and stored at -30°C until analysis.
The presence of anti-CMV IgM antibodies in all sera were tested by using the CMV IgM ELISA kit (Genesis Diagnosis Ltd., UK), a commercially available kit, by using the positive and negative controls provided with the kit. Virus-specific IgM antibodies were measured in the plasma regardless of the PCR results.
The PCR primers used were from the immediate early (IE) gene[10]. The upstream primer was 5′-GACTAGTGTGTGATGATGCTGGCCAAG-3′, and the downstream primer was 5′-GCTACAATAGCCTCTTCCTCATCTG-3′. A fluorogenic probe (5′-carboxyfluorescein-AGCCTGAGGTTATCAGTGTAATGAAGCGCC-3′) was located between the PCR primers[11]. PCR was carried out by using a TaqMan PCR kit (PE Applied Biosystems, Foster City, CA), as described previously[11]. Briefly, 10 μL of the DNA extraction solution from the samples was added to a PCR mixture containing 10 mmol/L of Tris (pH 8.3); 50 mmol/L of KCl; 10 mmol/L of EDTA; 5 mmol/L of MgCl2; 100 mmol/L of dATP, dCTP, dGTP, and dTTP; 0.2 mmol/L of each primer; 0.1 mmol/L of fluorogenic probe; and 1.25 U of AmpliTaq Gold (PE Applied Biosystems). After activation of the AmpliTaq Gold for 10 min at 95°C, 50 cycles each of 15 s at 95°C and 1 min at 62°C were carried out in a Model 7700 Sequence Detector (PE Applied Biosystems). Real-time fluorescent measurements were taken and a threshold cycle (CT) value for each sample was calculated by determining the point at which the fluorescence exceeded a threshold limit (10 × SD of the base line). For a positive control, a plasmid that contained the IE gene was constructed using the pGEM-T vector (Promega, Madison, WI) and was termed pGEM-IE. A standard graph was constructed using the CT values obtained from the serially diluted pGME-IE. The CT values from the clinical samples were plotted on the standard curve, and the copy number was calculated automatically by using Sequence Detector v1.6 (PE Applied Biosystems), a software package for data analysis. Samples were defined as negative when the CT value exceeded 50 cycles[12]. The DNA copy numbers in the plasma were expressed per milliliters. The minimum detection level was 100 copies/mL of plasma.
Positive results in any tests (IgM antibody, CMV DNA, or inclusion bodies in HE stained sections) were considered as evidence of CMV infection.
The results were expressed as mean ± SD. The P values below 0.05 were considered significant. The accuracy of each test was calculated considering a positive result in any test for CMV as evidence of infection.
Twenty-three patients were treated with intravenous CyA and they evidenced no severe side effects of the drug, such as renal failure or liver failure. The patient characteristics are listed in Table 1. All patients had received corticosteroids before the initiation of treatment with CyA. The degree of the severity of UC in all patients was pan colitis. Prior to treatment with CyA, active CMV infection was not observed in any of the patients. Eighteen of the 23 UC patients treated with CyA were infected with CMV (IgM antibodies in 16/23 patients, 69.6%; CMV DNA in 18/23 patients, 78.2%; and inclusion bodies in 4/23 patients, 17.3%). The rate of CMV detection by real-time PCR was significantly higher than other methods (P < 0.05). There was no difference in the CMV infection rate between males and females (males, 11/14; 78.57% and females, 7/9; 77.7%) (Table 2). With the exception of four of the tissue sections, all the sections from our UC patients who were treated with CyA were negative for CMV as demonstrated by histochemistry. After CyA treatment, none of the CMV-positive patients had pneumonia, hepatitis, retinitis, nephritis, or pancreatitis. Their only clinical symptoms were those of gastrointestinal disorder caused by colitis.
| Male | Female | Total | |
| Age, yr (median, range) | 30 (18-56) | 29 (8-56) | 27 (8-56) |
| Gender | 14 | 9 | 23 |
| CMV infection diagnosed | 11 | 7 | 18 |
| Recent corticosteroid therapy | 14 | 9 | 23 |
| Outcome: Surgery | 9 | 6 | 15 |
| Ganciclovir therapy | 2 | 1 | 3 |
| Parameter | Real-time PCR(%) | Serum IgMantibodies (%) | Inclusion bodies in HEstained biopsy (%) |
| Number | 18 | 16 | 4 |
| Sensitivity | 100 | 88.88 | 22.22 |
| Specificity | 100 | 100 | 100 |
| PPV | 100 | 100 | 100 |
| NPV | 100 | 71.4 | 26.3 |
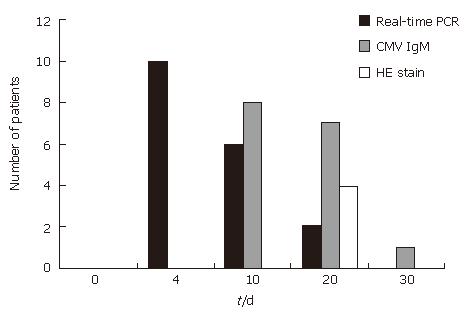
The median time of onset of the CMV infection after CyA treatment was 8.5 d (range: 4-20 d).
Real-time PCR detection is the most rapid of the three methods used (CMV IgM, HE biopsy, and real-time PCR results) (Figure 1). In particular, the HE biopsy method is slower and less sensitive than the other methods.
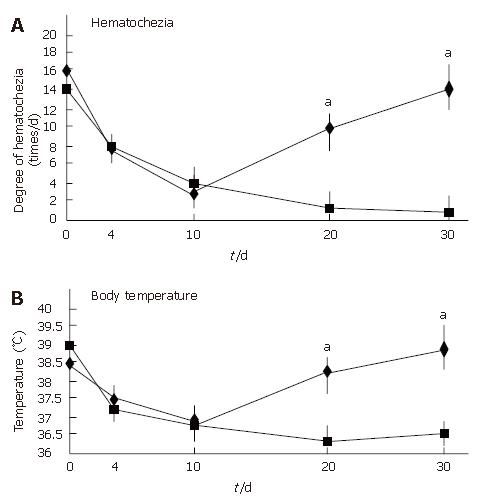
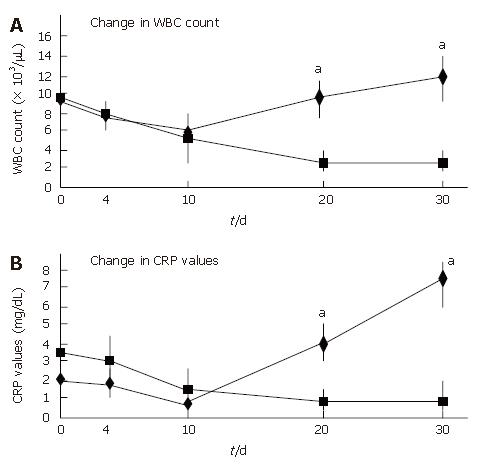
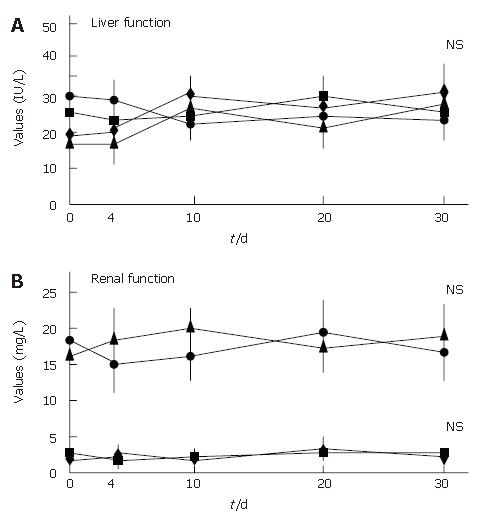
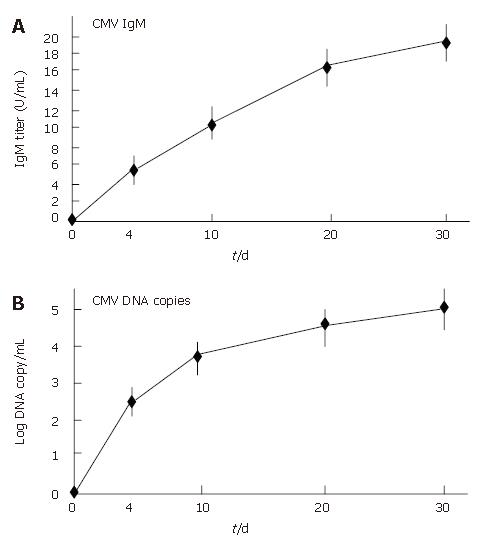
All patients showed an improvement in their symptoms after the initiation of the CyA treatment. Four CMV-negative patients treated with CyA showed a drastic improvement in their symptoms, and one CMV-negative patient who received CyA showed slight improvement. The degree of hematochezia progressively decreased after CyA treatment. CMV-negative patients continued to show an improvement in their symptoms during CyA treatment; further, even though CyA treatment was terminated, no deterioration in the degree of bowel symptoms was observed. On the other hand, all CMV-infected patients demonstrated an aggravation of the symptoms of colitis, i.e., an increase in the body temperature and the degree of hematochezia, after CMV infection (P < 0.05) (Figure 2). Further, the inflammatory values, i.e., the white blood cell (WBC) count and the CRP values worsened after CMV infection (P < 0.05) (Figure 3). However, an assessment of liver and renal functions after infection did not reveal any deterioration (Figure 4). After 10 d of CyA administration, CMV DNA was detected in 16 of the 18 UC patients, however, the clinical symptoms were improving. On the other hand, after 20 d, the clinical symptoms worsened in the CMV infected patients and CMV IgM and the histopathology results were positive. The values of the CMV IgM titer and the number of CMV DNA copies both increased after infection (Figure 5). The increased CMV load was followed by an increase in the severity of colitis.
Fifteen of these 18 patients with CMV infection (83.3%) required surgical treatment because of uncontrolled, severe deteriorating colitis. Perforation of the colon was observed in one of the patients after CMV infection; he underwent an emergency total colectomy. Nevertheless, in our study, there were no deaths among the UC patients after CMV infection.
Only three patients (males 2, and female 1) showed an improvement in the severity of colitis and did not require a colorectomy since they were treated with ganciclovir following the identification of the CMV infection. The CMV IgM values did not decrease after ganciclovir treatment. However, in these three patients, the CMV DNA copy number decreased and did not show any further increase. Further, CMV infection was detected in one of the patients by an HE histopathological study. After ganciclovir administration, the owl’s eye sign in the colon tissue was not observed. Thus, the three patients who were treated with ganciclovir were not reinfected with CMV although CyA treatment was continued.
We clarified a possible relationship between CMV infection and UC treated with CyA. We showed a significant deterioration in the clinical symptoms and the inflammatory response after active CMV infection. However, no impairment in hepatic or renal function was observed since we could not detect either CMV hepatitis or acute renal failure. Our results suggest that CMV infection in patients with severe UC treated with CyA is associated with a poor outcome because of severe deterioration in UC.
In general, a primary CMV infection, which is usually acquired early in life, is overcome by the humoral and cellular immunological response. Thereafter, the virus remains latent, particularly in endothelial cells and monocytes. Reactivation occurs during the use of immunosuppressive drugs. The viral load is the highest during active infection and the lowest during the latency stage in immunocompetent persons. We observed that CyA treatment may cause the reactivation of CMV and a subsequent deterioration in colitis. During a CMV infection, an increase in intestinal permeability has been shown to occur in kidney transplant recipients[14]. This defect in the barrier function may facilitate the exposure of the mucosal immune system to antigens from the luminal flora. CMV may also spread from the mucosa to the bloodstream because of such a defect in the barrier function, leading to CMV viremia in the bloodstream.
Steroid-resistant UC is defined as persistent active disease despite high-dose systemic corticosteroid therapy. A history of steroid resistance increases the possibility of complications such as CMV infection. CMV infections were detected in 4.6% to 13% of the patients diagnosed with UC[15,16]. Up to 33% of the patients with severe steroid-refractory UC were found to harbor the CMV virus. CMV was detected in 25% of the patients with steroid-refractory UC as compared to only 2.5% of patients with medically non-refractory UC. All the cases of UC with CMV infection in other investigations were clinically severe and steroid-resistant, and approximately 70% of the cases suffered from an acute exacerbation of symptoms and required immediate emergency surgeries. These results may suggest that CMV is not a coincidental occurrence but an exacerbating factor [17].
Although it is important to prevent the development of CMV disease[18], the availability of rapid, sensitive, and reliable methods for the early diagnosis of CMV infection is desirable[15]. As mentioned above, CMV disease can take many forms, depending upon the type of patient group under consideration. A consensus is available from the international CMV workshop for the definition of CMV disease[19]. The guidelines are purposefully rigorous and have aided the interpretation of clinical trial data and population based studies. However, they are likely to lead to an under appreciation of the contribution of CMV to patient morbidity. This is particularly true in the case of the histopathological diagnoses of CMV. Gastrointestinal symptoms along with histopathologically detected CMV are widely acceptable diagnostic criteria for CMV infections. In particular, the histopathological study of colon tissue is considered acceptable for CMV detection. Many CMV inclusion bodies were found in the surgical specimens of the UC cases in which CMV infection was detected, whereas CMV infection had not been detected by biopsy prior to surgery. CMV infection was observed in only 1 of the 55 cases investigated by endoscopic biopsy; however, it was detected in 8 of the 39 surgical cases[17]. Thus, it was rather difficult to detect CMV infection by biopsy, possibly because of sampling limitations. The characteristic inclusion bodies are not readily visible in routinely performed HE staining and CMV-infected cells are not always cytomegalic; therefore, the histopathological examination of the colonic biopsies had the lowest sensitivity. It has been suggested that CMV inclusion bodies are found more frequently in the right colon than in the left[16]; hence, multiple biopsies were taken from the colon, particularly from the inflamed and ulcerated areas, considering the fact that CMV exhibits tropism for the inflamed sites[20]. Therefore, it is likely that the false-negative results in the present study were related to sampling errors. Although our study did not confirm this, an immunohistochemical study of biopsy specimens may be more useful in diagnosis; however, immunohistochemistry is not a convenient tool for routine diagnosis.
The investigation of plasma CMV DNA is a simple, noninvasive, and nontraumatic method for evaluating and monitoring the CMV infection in patients. Procedures such as endoscopy and biopsies are traumatic and uncomfortable for a UC patient. The CMV-associated disease is generally the result of the reactivation of latent viruses rather than reinfection with the virus, and the measurement of CMV antibodies is often of no diagnostic use.
The real-time PCR assay was found to be as useful as the pp65 antigenemia assay because they were highly correlated[12]. Unlike the conventional qualitative PCR assay, which is not beneficial for determining the termination of antiviral therapy[21], this assay showed that the copy numbers of CMV DNA decreased and disappeared in response to anti-CMV therapy. Additionally, with this PCR method there is almost no room for bias due to subjective assessments by technicians. One investigator reported that real-time PCR is more sensitive (92%) than the pp65 test (88%) with regard to positive findings. Further, the correlations between real-time PCR and the pp65 antigenemia assay were statistically significant[22]. The advantage of quantitative techniques is that by defining a “threshold value” of CMV load, they may allow a distinction between the commonly occurring, clinically irrelevant CMV infection and the levels of active CMV replication that are likely to lead to clinical disease[23].
However, there is still disagreement with regard to the optimal type of sample material, for example, leukocyte fractions versus plasma versus whole blood, and to the desirable sensitivity, which depends on the initial sample volume.
We used a quantitative CMV PCR assay that was able to quantify CMV DNA in plasma over the 100 copies/mL. The assay is fully controlled for maximal efficiency in at all steps. This is achieved by employing the widely used principle of an “internal control” and taking it one step further as a control for extraction efficacy.
Although the quantitative results obtained with different assays are difficult to compare because of the absence of an international standard, the results obtained with quantitative PCR correlate well with those of a number of other assays, and its sensitivity is generally higher[24]. The high sensitivity of the quantitative PCR assay is partly due to the use of a relatively large sample volume and the concentration of the DNA contained therein. Using plasma appears to avoid the loss of sensitivity as reported previously as compared to using whole blood or leukocytes[25]. Using plasma may also avoid the possibility of amplifying “latent” CMV from leukocytes. The exclusion of CMV DNA of intracellular origin may increase the clinical relevance of latent CMV detection; this in turn may decrease the predictive value of the test for active CMV infection[26]. Plasma is also easier to handle than cellular fractions such as leukocyte preparations and is better standardized, particularly in leukopenic patients. In HIV-positive individuals, the CMV load in whole blood and plasma has also been shown to be an important indicator of pathogenesis[27]. The presence of CMV DNA in the blood of HIV-positive patients identifies a group of patients who are almost 20 times more likely to progress to CMV disease than those who remain negative for CMV DNA in their blood[28]. In addition, an increasing CMV load in the blood was associated with an increased risk of disease progression[28]. CMV load was correlated with tissue samples obtained at the postmortem examinations of the HIV patients with histological evidence of CMV inclusions, and it was found that a viral load of > 5 000 000 genomes/μg DNA is required before CMV inclusions are observed[29]. It should be noted that some of these manifestations do not satisfy the criteria outlined for the diagnosis of CMV disease. Thus, it appears likely that cases of CMV colitis may also be dismissed because they do not meet the definition of CMV infection, despite the presence of clinical symptoms and CMV DNA in the blood. It is important to appreciate that with the advent of more sensitive molecular-based assays, a reappraisal of many of these definitions may be required.
In conclusion, a CMV infection in patients with steroid-resistant UC should be ruled out prior to initiating aggressive immunosuppressive therapy such as CyA for steroid-resistant UC. Further, ganciclovir as an anti-viral agent is useful for the treatment of CMV-associated UC after immunosuppressive therapy.
We thank the members of the Departments of First Internal Medicine, Gastroenterology division, and Bacteriology, Nagoya University Graduate School of Medicine, for their technical advice.
Cytomegalovirus (CMV) infection is one of the most common infectious complications after immunosuppressive therapy. Ulcerative colitis (UC) is thought to result from the inappropriate and progressive activation of the mucosal immune system driven by the presence of normal luminal flora. Cyclosporine A (CyA) selectively inhibits immune responses mediated by T lymphocytes by modulating the interaction of calcineurin-calmodulin. This recognition has led to its use in patients with severe UC. In Japan, patients with severe UC are frequently treated with CyA and are expected to be at an increased risk of infection with CMV. Despite the frequent use of immunosuppressive drugs in patients with UC, data on the frequency of CMV infection and its clinical significance in patients with UC are limited.
The aim of this study was to describe our experience with active CMV infection following treatment with CyA for UC in Japan.
Twenty-three patients with severe UC not responding to steroid therapy at Nagoya University Hospital received continuous intravenous infusion of CyA (average 4 mg/kg per day) for 1 mo. Serum and colonic biopsy samples were collected from all patients before CyA treatment and 4 d, 10 d, 20 d, and 30 d after treatment and were evaluated for CMV by using serology (IgM antibody by ELISA), quantitative real-time PCR for CMV DNA, and histopathological assessment of hematoxylin and eosin -stained colonic biopsies.
No patients had active CMV infection before CyA treatment. Eighteen of 23 UC patients treated with CyA were infected with active CMV (IgM antibody in 16/23 patients, 69.6%; CMV DNA in 18/23 patients, 78.2%; and inclusion bodies in 4/23 patients, 17.3%). Fifteen of these 18 patients with active CMV infection (83.3%) required surgical treatment because of severe deteriorating colitis. Treatment with ganciclovir rendered surgery avoidable in three patients. Active CMV infection in severe UC patients treated with CyA is associated with poor outcome. Further, ganciclovir is useful for treatment of CMV-associated UC after immunosuppressive therapy.
CMV infection is one of the most common infectious complications in immune-compromised patients. UC is chronic inflammation colitis thought to result from the inappropriate and progressive activation of the mucosal immune system driven by the presence of normal luminal flora. CyA is a drug to inhibit immune responses selectively mediated by T lymphocytes by modulating the interaction of calcineurin-calmodulin.
Severe ulcerative colitis is a potentially life-threatening condition. Traditional treatment for such patients is high-dose intravenous corticosteroids but up to 40% of patients become refractory to this treatment. In these patients CyA therapy has been shown to have an initial positive clinical response in many patients. However, i.v. cyclosporine is recommended only as short term “bridging” therapy to induce remission followed by azathioprine or 6-mercaptopurine maintenance therapy. In the present study Minami and coworkers investigated the frequency of the development of a CMV infection during a continuous intravenous cyclosporine treatment in ulcerative colitis and the clinical outcome in CMV infected vs. non infected patients. The study is of interest.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 304] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Betts RF. Syndromes of cytomegalovirus infection. Adv Intern Med. 1980;26:447-466. [PubMed] |
| 3. | Surawicz CM, Myerson D. Self-limited cytomegalovirus colitis in immunocompetent individuals. Gastroenterology. 1988;94:194-199. [PubMed] |
| 4. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2746] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 5. | Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3063] [Cited by in RCA: 3161] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 6. | Sandborn WJ, Tremaine WJ. Cyclosporine treatment of inflammatory bowel disease. Mayo Clin Proc. 1992;67:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1172] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 8. | Ina K, Kusugami K, Shimada M, Tsuzuki T, Nishio Y, Binion DG, Imada A, Ando T. Suppressive effects of cyclosporine A on neutrophils and T cells may be related to therapeutic benefits in patients with steroid-resistant ulcerative colitis. Inflamm Bowel Dis. 2002;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Truelove SC, Richards WC. Biopsy studies in ulcerative colitis. Br Med J. 1956;1:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 271] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Akrigg A, Wilkinson GW, Oram JD. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985;2:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4152] [Cited by in RCA: 3919] [Article Influence: 135.1] [Reference Citation Analysis (1)] |
| 12. | Tanaka N, Kimura H, Iida K, Saito Y, Tsuge I, Yoshimi A, Matsuyama T, Morishima T. Quantitative analysis of cytomegalovirus load using a real-time PCR assay. J Med Virol. 2000;60:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Verdonk RC, Haagsma EB, Van Den Berg AP, Karrenbeld A, Slooff MJ, Kleibeuker JH, Dijkstra G. Inflammatory bowel disease after liver transplantation: a role for cytomegalovirus infection. Scand J Gastroenterol. 2006;41:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | de Maar EF, Kleibeuker JH, Boersma-van Ek W, The TH, van Son WJ. Increased intestinal permeability during cytomegalovirus infection in renal transplant recipients. Transpl Int. 1996;9:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Einsele H, Ehninger G, Hebart H, Wittkowski KM, Schuler U, Jahn G, Mackes P, Herter M, Klingebiel T, Löffler J. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood. 1995;86:2815-2820. [PubMed] |
| 16. | Hinnant KL, Rotterdam HZ, Bell ET, Tapper ML. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol. 1986;81:944-950. [PubMed] |
| 17. | Takahashi Y, Tange T. Prevalence of cytomegalovirus infection in inflammatory bowel disease patients. Dis Colon Rectum. 2004;47:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Goodrich JM, Mori M, Gleaves CA, Du Mond C, Cays M, Ebeling DF, Buhles WC, DeArmond B, Meyers JD. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 390] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Ljungman P, Plotkin SA. Workshop of CMV disease: definitions, clinical severity scores, and new syndromes. Scand J Infect Dis Suppl. 1995;99:87-89. |
| 20. | Goodman ZD, Boitnott JK, Yardley JH. Perforation of the colon associated with cytomegalovirus infection. Dig Dis Sci. 1979;24:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Gerna G, Zipeto D, Parea M, Revello MG, Silini E, Percivalle E, Zavattoni M, Grossi P, Milanesi G. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J Infect Dis. 1991;164:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 205] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Piiparinen H, Höckerstedt K, Grönhagen-Riska C, Lautenschlager I. Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J Clin Virol. 2004;30:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 391] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Preiser W, Bräuninger S, Schwerdtfeger R, Ayliffe U, Garson JA, Brink NS, Franck S, Doerr HW, Rabenau HF. Evaluation of diagnostic methods for the detection of cytomegalovirus in recipients of allogeneic stem cell transplants. J Clin Virol. 2001;20:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Boeckh M, Gallez-Hawkins GM, Myerson D, Zaia JA, Bowden RA. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation. 1997;64:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Hamprecht K, Steinmassl M, Einsele H, Jahn G. Discordant detection of human cytomegalovirus DNA from peripheral blood mononuclear cells, granulocytes and plasma: correlation to viremia and HCMV infection. J Clin Virol. 1998;11:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Bowen EF, Sabin CA, Wilson P, Griffiths PD, Davey CC, Johnson MA, Emery VC. Cytomegalovirus (CMV) viraemia detected by polymerase chain reaction identifies a group of HIV-positive patients at high risk of CMV disease. AIDS. 1997;11:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Shinkai M, Bozzette SA, Powderly W, Frame P, Spector SA. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of AIDS patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997;175:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Mattes FM, McLaughlin JE, Emery VC, Clark DA, Griffiths PD. Histopathological detection of owl's eye inclusions is still specific for cytomegalovirus in the era of human herpesviruses 6 and 7. J Clin Pathol. 2000;53:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |