Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.709
Revised: November 2, 2006
Accepted: November 29, 2006
Published online: February 7, 2007
AIM: To investigate the acupuncture-modulated gastric motility and its underlying neural mechanism.
METHODS: Intragastric pressure and/or waves of gastric contraction in rats were recorded by intrapyloric balloon and changes of gastric motility induced by acupuncture stimulation were compared with the background activity before any stimulation. Gastro-vagal or splanchnic-sympathetic nerves were recorded or cut respectively for investigating the involvement of autonomic nerve pathways. Spinalization experiment was also performed.
RESULTS: Acupuncture-stimulation by exciting Aδ and/or C afferent fibers, could only modulate gastric motility. Acupuncture-stimulation on fore- and hind-limbs evoked a moderate gastric motility followed by increased vagus discharges with unchanged sympathetic activity, while the same stimulus to the acupoints in abdomen resulted in reversed effects on gastric motility and autonomic nervous activities. The inhibitory gastric response was completely abolished by splanchnic denervation, but the facilitative gastric response to stimulation of acupoints in limbs was not influenced, which was opposite to the effect when vagotomy was performed. The similar depressive effects were produced by the stimulation at the acupoints homo-segmental to the gastric innervation in the animals with or without spinalization. However, the facilitation induced by the stimulation at the acupoints hetero-segmental to the gastric innervation was not observed in the spinalized animals.
CONCLUSION: Facilitative effects of stimulating hetero-segmental acupoints are involved in the intact preparation of vagal nerves and spinal cord, while the inhibitory response induced by stimulating homo-segmental acupoints is involved in the intact preparation of sympathetic nerves. Only the acupuncture-stimulation with intensity over the threshold of Aδ and/or C afferent fibers can markedly modulate gastrointestinal motility.
- Citation: Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol 2007; 13(5): 709-716
- URL: https://www.wjgnet.com/1007-9327/full/v13/i5/709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.709
Acupuncture, a commonly used neuromodulating technique, has been widely accepted in the treatment of pain syndromes[1]. It modulates the functions of visceral organs by inducing activation of the somato-visceral reflexes and change of the autonomic nervous system[2-4].
Electro-acupuncture therapy is increasingly adopted in Western countries. Acupuncture involves stimulating specific somatic points on the body by puncturing the skin with a needle. Heat, pressure or impulses of electrical energy can also stimulate the points. It was reported that the nervous system, neurotransmitters and endogenous substances respond to electro-acupuncture. Abundant information is now available concerning the neurobiological mechanisms of acupuncture related with the neural pathways and neurotransmitters/hormonal factors that mediate autonomic regulation, pain relief and other therapeutics[5]. Being a kind of traditional technique with a long history, acupuncture has been used to treat a variety of diseases including pain. Electrophysiological studies showed that acupuncture might inhibit the neuron discharge induced by pain of both somatic and visceral sources at different levels of the central nervous system, which gives a good explanation for the clinic phenomena in which acupuncture produces quick effect on somatic and visceral pain and relatively slow and long post-effect. Though there are data relating the modulation of somatic afferent inputs on visceral nociception or dysfunction, evidence for the role of acupuncture in this process is not sufficient.
The Committee of NIH published a report on the indications of acupuncture in November 1997. The summary of the consensus statement indicates that there is clear evidence that needle acupuncture treatment is effective on postoperative and chemotherapy-induced nausea and vomiting during pregnancy and post-operative dental pain[6]. Recent investigations suggest that acupuncture modulations on visceral functional activities can be mediated via the autonomic nervous system[2,4,7-9].
In the present study, therefore, we investigated the modulation of gastric motility by manual/electrical acupuncture at representative meridian-acupoints in different parts of the body and its underlying mechanism involved in different afferent fibers, autonomic nervous system and the supraspinal center.
All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of China Academy of Chinese Medical Sciences. Experiments were performed on 48 adult male Sprague-Dawley rats weighing 250-300 g. The rats were fasted overnight with free access to water and anesthetized with an intraperitoneal injection of urethane (1.0-1.2 g/kg). The trachea was cannulated and a catheter was inserted into one of the jugular veins for infusion of necessary solutions or anesthetics. The abdomen was opened by midline, and then a small longitudinal incision was made in the duodenum about 2-3 cm from the pylorus. A small balloon made of condom was inserted via the incision into the pyloric area and fixed. Another catheter (1 mm in inner diameter) was also inserted into the same incision to drain digestive juices secreted from the stomach. The balloon was filled with about 0.2-0.5 mL warm water, which gave the pressure at about 80-150 mmH2O. Pressure in the balloon was measured by a transducer (TP-400T, Nihon Kohden) through a thin polyethylene tube (1.5 mm in outer diameter) and then input into a polygraph amplifier (RM-6000, Nihon Kohden) and led to a data acquisition system (Power-Lab/8 s, AD Instruments) for further analysis. Demi-fasting gastric motor activity was recorded as a control for at least 1 h before any stimulation was applied.
Changes of gastric motility induced by the stimulation were compared with the background activity in terms of intragastric pressure and/or waves of gastric contraction. If the changes of gastric motility during stimulation were 20% more or less than the background activity, the response was considered to have an excitatory or inhibitory regulation.
Blood pressure in a common carotid artery and heart rate were continuously monitored. The rectal temperature was kept constantly around 37°C by a feedback-controlled heating blanket.
To investigate whether vagal or sympathetic pathways are involved in the stimulatory or inhibitory effects of acupuncture on gastric motility, subdiaphragmatic gastro-vagal or sympathetic postganglionic nerves coming from a celiac ganglion and innervating the gut branch were dissected under microscope. Discharges of the vagal or sympathetic nerves were put into a polygraph (RM-6000, Nihon Kohden) amplifier through bipolar platinum wire electrodes and input into a data acquisition system (Power-Lab) for further analysis. In experiments, gastro-vagal nerves were cut in subdiaphragmatic bilateral truncal vagotomy (an additional cervical bilateral vagotomy was also performed in several cases) or postgangliotic nerves innervating the gut branch were cut in sympathetomy (an additional bilateral splanchnicotomy was also performed in several cases) in 24 rats.
In spinalization experiments, laminectomy was performed in C8-T1 of 10 animals. Frozen physiological saline was used to produce a reversible cold block (five rats), or transected with knife to determine the involvement of supraspinal circuit in acupuncture-induced effect on gastric motility.
A needle (0.3 mm in diameter) was inserted into the skin and its underlying muscles at different acupoints on the body. The needle was rotated clockwise and anti-clockwise at 2 Hz for 30 s. Based on the anatomical localization in rats as compared with that in human body, the stimulated acupoints included St-13 (acupoint of Stomach-Meridian in upper-chest), Li-11 (acupoint of Large intestine-Meridian in forelimb), Cv-6 (acupoint of conception-vessel) and St-21 (acupoint of Stomach-Meridian in abdomen), Bl-21 (T12 segment, acupoint of Bladder-Meridian in dorsum) and St-36 (acupoint of Stomach-Meridian in hindlimb).
In electroacupuncture experiments, acupoints were stimulated by a pair of needle-electrodes inserted 0.3 cm deep into the skin and electroacupuncture intensities were chosen as the multiples of the threshold for the activation of Aδ-fiber or C-fiber, including 0.8 (0.8-TAδ, non-noxious stimuli, 1.42 ± 0.36 mA) and 2-times of the Aδ-fiber reflex threshold (2-TAδ, slight nociceptive stimuli, 3.62 ± 0.44 mA), and 1.5 times (1.5-TC, strong nociceptive stimuli, 7.68 ± 0.53 mA ) of C-fiber reflex threshold.
The thresholds of Aδ-fiber and C-fiber reflexes were decided by electrical stimulation of the sural nerve territory, which could elicite a two-component reflex response in the ipsilateral biceps femoris muscle. The first-component had a low threshold (1.64 ± 0.33 mA) response with a short latency (13.2 ± 1.4 ms) and duration (24.2 ± 1.5 ms). The second-component had a longer latency (158.8 ± 10.7 ms) and duration (243.8 ± 31.5 ms) and a higher threshold (4.84 ± 0.67 mA). These electrophysiological features clearly suggested that the two-components were produced due to the different afferent volleys activated by sural nerve stimulation. According to our previous study, it seems that the first component with a low-threshold and a short-latency could be elicited by activating the Aδ-fibers, whereas the second with a higher threshold and a longer latency might result from the activation of the unmyelinated C-fiber afferents[10].
The rats were kept in supine position and gastric motor activity was first analyzed visually to detect the waves of contractions. A standard protocol was employed for the determination of effects of acupuncture-stimulation on gastric motility response. A background gastric activity was recorded for 5-10 min followed by a test of the responses to manual or electro-acupuncture at the acupoints for 30 s. The post-stimuli response was recorded for another 5-10 min.
After a series of experiments was finished, the rats were sacrificed under deep anesthesia (urethane, 2 g/kg ip).
The data obtained before and after intervention between the two groups were compared statistically by independent t-test and paired t-test respectively. P < 0.05 was considered statistically significant. All data were expressed as mean ± SE.
Gastric motor characteristics were observed in 42 rats. When the intrapyloric balloon pressure was increased to about 80-200 mmH2O, the rhythmic waves of contractions in pyloric area were observed. When the pressure was maintained at about 100 mmH2O by expanding the volume of the balloon with warm-water, rhythmic contractions occurred at a rate of four to six per minute and these rhythmically gastric contractions could be recorded in either the spinalized rats or the rats with intact central nervous system. The gastric contractions could also be recorded after denervation of gastric nerve branches of the vagal or splanchnic nerves.
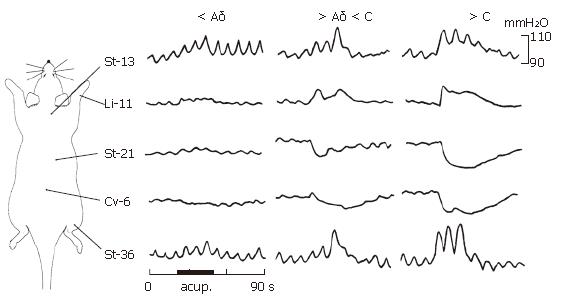
The response of gastric motility to the activation of Aδ-/C-fibers was elicited by stimulating the ipsilateral sural nerve. The effects of acupuncture stimulation to Li-11 in the forelimb, St-13 in upper-breast, St-21 and Cv-6 in the abdomen and St-36 in the hindlimb on gastric motility were studied. As illustrated by an representative example in Figure 1 in combination with data in Figure 2, the acupuncture stimulation with the intensity of 0.8-TAδ (1.42 ± 0.36 mA) at the above five acupoints did not produce any significant influences on the gastric motility. The acupuncture stimulation with the intensity of 2-TAδ (3.62 ± 0.44 mA) at the acupoints of Li-11, St-13 and St-36 elicited a mild-moderate facilitation on the gastric motility, whereas the acupuncture stimulation with the same intensity to the acupoints of St-21 and Cv-6 brought about a moderate depression of gastric motility. The acupuncture stimulation with the intensity of 1.5-TC (7.68 ± 0.53 mA) at the acupoints of Li-11, St-13 and St-36 resulted in a strong excitatory gastric motility. In contrast, a strong inhibition on the gastric motility was induced by the same acupuncture stimulation at the acupoints of St-21 and Cv-6.
Electro-acupuncture stimulation could induce regulatory effects on gastric motility. Moreover, facilitation/depression on the gastric motility induced by acupuncture stimulation was intensity-dependent. Only the stimulation with an intensity over the threshold for activation of Aδ- and/or C-fibers could modulate gastric motility (P < 0.05, P < 0.001) and the powerful effects were produced when the strongest electro-acupuncture stimulation, i.e. 1.5-TC, was given (P < 0.05, P < 0.001).
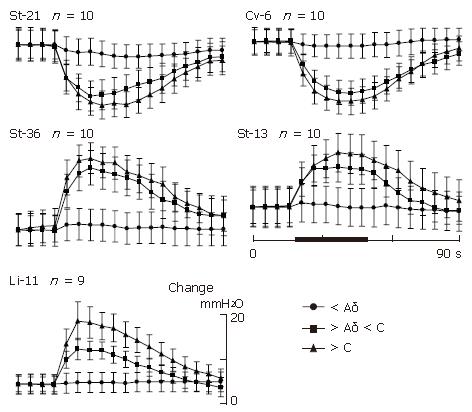
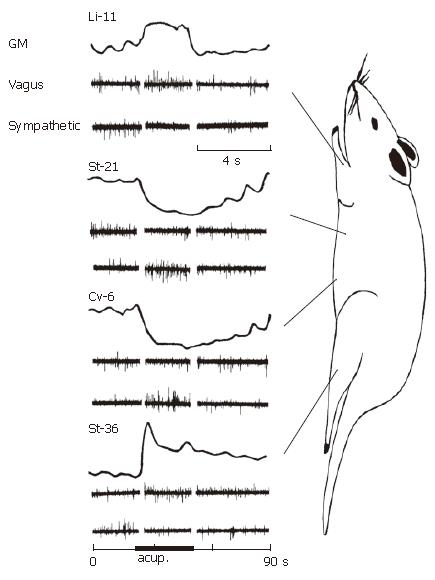
Gastric motility in the most rats showed various responses to acupuncture stimulation at some representative acupoints on several meridians and different parts of the body. Manual acupuncture stimulation at either Li-11 in forelimb or St-36 in hindlimb led to a moderate facilitation of gastric motility with a rapid enhancement at the beginning of stimulation, followed by a tonic gastric contraction lasting throughout the period of acupuncture stimulation. The typical effects of acupuncture stimulations at Li-11 or St-36 acupoints on discharges of the branches of both gastric vagal and postganglionic sympathetic nerves to the stomach are shown in Figure 3. Acupuncture stimulation at either Li-11 (n = 10) or St-36 (n = 16) acupoints markedly increased vagal discharges (339.42 ± 133.2 spikes/min vs 381.6 ± 135.42 spikes/min, P < 0.05). The latency of increased discharges was about 2-3 s after the onset of stimulation. The facilitated response lasted for 30 s with a maximum response at about 10 s after the onset of acupuncture stimulation. In contrast to vagus response, spontaneous activity of the sympathetic nerve branch was not clearly influenced by acupuncture stimulation at either Li-11 (n = 12) or St-36 acupoints (n = 12) (P > 0.05). Generally, in the animals with high spontaneous activity of sympathetic nerves, the same acupuncture stimulation could mildly inhibit the discharges. However, the stimulation could produce a slight excitation in the animals with lower spontaneous sympathetic activity.
The same stimulus to the acupoints of either St-21 or Cv-6 in abdomen, resulted in high suppression with a rapid onset on gastric tonic motility, followed by an obvious inhibition on the rhythmic wave of contraction. The suppression maintained throughout the period of acupuncture stimulation. In the autonomic nerve participation, acupuncture stimulation at either St-21 (n = 12) or Cv-6 (n = 10) acupoints markedly increased sympathetic discharges (476.4 ± 150.78 spikes/min vs 638.82 ± 171.78 spikes/min, P < 0.05). In most cases, spontaneous vagal activity could also be inhibited slightly (P > 0.05, n = 12) or remained unchanged (Figure 3).
In order to discover their contribution to the facilitation/inhibition of acupoint-visceral reflex responses, denervation of the vagal or sympathetic nerves was performed in the present study.
The effect of acu-stimulation on gastric motility was examined in the rats after amputation of bilateral splanchnic nerves just beneath the diaphragm.
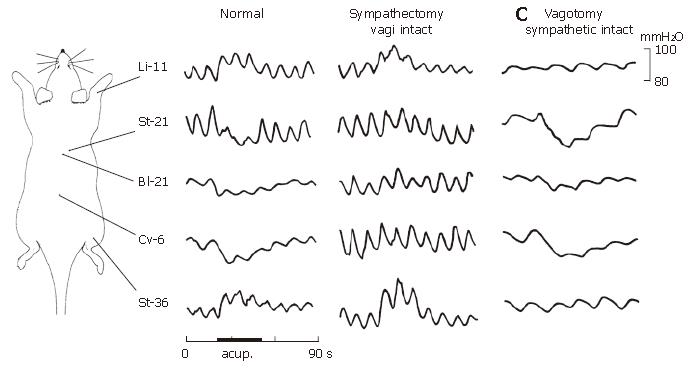
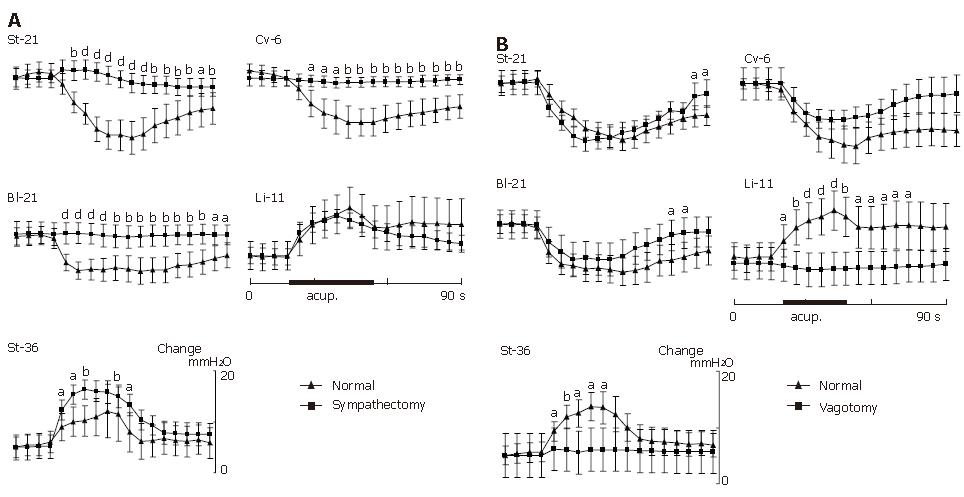
The inhibitory gastric response induced by acupuncture stimulation to acupoints of St-21 (n = 9), Cv-6 (n = 9) or Bl-21 (n = 9) was completely abolished after sympathectomy of the bilateral splanchnic nerves, but the facilitative gastric response induced by acupuncture stimulation to acupoint of Li-11 (n = 9) was not influenced significantly and St-36 (n = 9) was only less influenced by splanchnic denervation (Figure 4 and Figure 5A).
Bilateral subdiaphragmatic vagotomy was performed to determine the vagal involvement in the stimulatory or inhibitory effects of acupuncture on gastric motility (in some experiments, additional vagotomy of bilateral vagal nerves was also performed at the cervical level to observe the different results from those obtained in the rats after incomplete subdiaphragmatic vagotomy). As shown in Figure 4 and the cumulative results in Figure 5B, the bilateral vagotomy did not abolish the suppressive gastric response induced by acupuncture stimulation to the acupoints of either St-21 (n = 9), Cv-6 (n = 9) in abdomen, or Bl-21 in middle-dorsum (n = 9). However, the bilateral vagotomy completely depleted the facilitative gastric response provoked by acupuncture stimulation to the acupoints of Li-11 in forelimb (n = 9) or St-36 in hindlimb (n = 9).
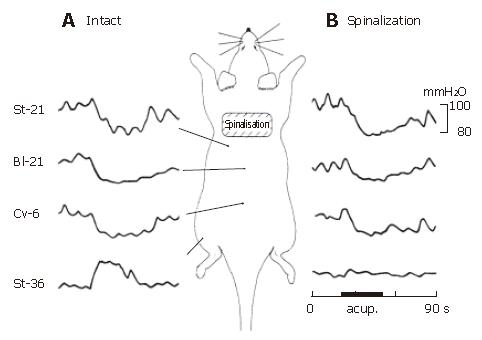
During acute spinalization at C8-T1 level, steady gastric motility could still be recorded. But for avoiding the spinal shock, 30-min period of rest was necessary.
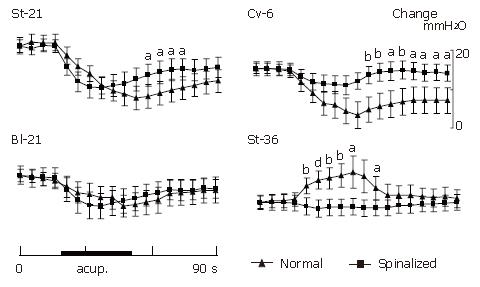
In pre-spinalized animals, as shown in Figures 6 and 7, the gastric motility was inhibited by acupoints of St-21, Cv-6 or Bl-21, and facilitated by St-36 acupoint. Acupuncture stimulation at the acupoints of St-21 (n = 10), Cv-6 (n = 10) or Bl-21(n = 10) could inhibit gastric motility in the spinalized rats. On the contrary, gastric facilitative response induced by St-36 (n = 10) acupuncture stimulation disappeared completely after spinalization.
Our previous study showed that acupuncture-stimulation had facilitative or inhibitory effect on gastric motility depending on the different stimulated acupoints on different parts of the body[10]. In this study, we investigated how strong the stimulation and what autonomic nervous system can effectively modulate the gastric motility.
The flexion reflex is usually described as an EMG discharge that mainly consists of two-components separated by periods of an EMG absence. The present results are very similar to those described by Falinower et al[11] and by our recent observation[12] in which sural nerve stimulation evoked a two-component reflex in the flexor muscles of the ipsilateral hindlimb. Based on accumulative electrophysiological and pharmacological evidence, the first-component is triggered by activities of small diameter Aδ-fibers, and the second-component is mediated by the unmyelinated C-fibers.
The present study demonstrated that electro-acupuncture stimulation with the strength below TAδ could not effectively trigger regulatory effects on gastric motility, regardless of the locations of acupoints. Only electro-acupuncture stimulation with the strength exceeding the thresholds for activation of Aδ and/or C-fibers could profoundly modulate gastric motility. Moreover, the effects were strength-dependent and the powerful effects were observed when the strongest stimulation was given.
Electro-acupuncture stimulation has been known to excite various afferent fibers of rats including groups II-III[13], groups III-IV[14] or groups II-IV[15]. Koizumi et al[16] performed a systematical analysis of the relationship between the magnitude of cutaneo-intestinal reflex response and groups of stimulated afferent fibers. Their results indicate that stimulation of groups II-III cutaneous fibers in the T10 spinal nerve could not produce any changes in jejunal motility, while stimulation of groups II-III-IV fibers could always induce inhibitory responses. Stimulation of the sural afferent nerves of the hindlimb could elicit facilitative jejunal reflex, which was obtained when the stimulus intensity could activate group III fibers and the maximal facilitation appeared when the stimulus intensity could activate group IV afferent fibers. Noguchi et al[17] reported that when electro-acupuncture stimulation intensity at hindlimb was less than 1.5-mA (only activation of group II fibers), equivalently to 0.8-TAδ (1.42 ± 0.36 mA) electro-acu puncyure-stimulation in our present study, there was no significant response of duodenal motility. When the intensity of electro-acupuncture stimulation exceeded 2.0-5.0-mA (activation of groups II-III and IV fibers), equivalently to 2-TAδ (3.62 ± 0.44 mA) electro-acu-stimulation in our study, a slight increase was observed in duodenal motility. The maximal excitatory response of duodenal motility to electro-acupuncture stimulation was observed in 10-mA (activation of various afferent fibers) at hindlimb, which was equivalent to1.5-TC (7.68 ± 0.53 mA) electro-acu puncyure-stimulation in our study. These studies demonstrated that stimulating groups III-IV, particularly group IV or C afferent fibers of hindlimb, could produce an excitatory intestinal reflex response. On the other hand, stimulation of only group IV abdominal nerves produces an inhibitory intestinal reflex response.
These data suggest that only acupuncture stimulation with an intensity strong enough to excite Aδ (or group III) and/or C (or group IV) afferent fibers, can lead to markedly excitatory/inhibitory modulation of gastro-intestinal motility.
The present study showed that acupuncture stimulation to the acupoints on face, neck, forelimbs, upper chest-dorsum and hindlinbs produced a facilitative response of gastric motility. These effects were involved in intact preparation of vagal nerves and the spinal cord, but did not require intact preparation of the splanchnic nerves.
Motor activity of the stomach, like other gastrointestinal organs, is modulated by the changes in vagal activity. It was reported that electro-acupuncture at acupoints of St-36 and Pc-6 (Neiguan, located in forelimb) enhances the gastric migrating myoelectrical complex in dogs by reducing the length of phase-I and increasing the length of phases II and III[18]. Ouyang et al[19] observed that electro-acupuncture at Pc-6 and St-36 substantially accelerates gastric emptying of liquid in dogs. The accelerating effect of electro-acupuncture on gastric emptying may be attributed to the improvement in gastric tone and antral contractile activity. Enhanced vagal activity by electro-acupuncture suggests a possible involvement of vagal pathways in its regulation of gastric motility. Xu[20] found that the regulatory effect of electro-acupuncture on gastric dysrhythmia in rabbits could be abolished after vagotomy. Sato et al[9] showed that acupuncture-like stimulation could elicit an excitatory gastric response when it is applied to the hindlimb. These findings suggest that acupuncture provokes a reflex that has cutaneous and muscle nerves as its afferent pathway, and the gastric vagus as its efferent pathway. In our study, acupuncture stimulation at St-36 could facilitate gastric motility by increasing the activity of gastric efferent nerves. However, this facilitation of gastric motility disappeared in spinalized rats. The facilitative duodenal response by electro-acupuncture stimulation to a hindpaw is a supraspinal reflex response involving vagal excitatory nerves, cutting the splanchnic nerve branches to the duodenal does not affect the enhanced duodenal response[17].
Facilitation in gastric motility and increase in parasympathetic activity by (electro-) acupuncture stimulation to all acupoints except for those of abdomen and middle-dorsum (the acupoints of homo-segmental to the innervation of stomach) are accompanied with a decrease in sympathetic activity or a breakage of possible “sympathetic dominance”[21,22], suggesting that these effects require the participation of supraspinal center.
The present study showed that acupuncture stimulation to the acupoints on lower-chest, middle-dorsum and whole abdomen could induce an inhibitory response of gastric motility with an increase in the activities of sympathetic and/or a slight inhibition in the activity of vagal nerves innervating the stomach, suggesting that these effects are involved in intact preparation of sympathetic (splanchnic) nerves, but do not require intact preparation of bilateral vagal nerves and high spinal cord.
Sato et al[9] showed that the abdominal acupuncture-like stimulation could elicit suppressive response of gastric motility, indicating that acupuncture provokes a reflex that has cutaneous and muscle nerves as its afferent pathway, and the sympathetic gastric branches as its efferent pathway. This inhibition is accompanied with an increase in the activity of efferent nerves of sympathetic gastric branches and can be observed in spinalized rats. The inhibitory duodenal response to electro-acupuncture stimulation in abdomen is a propriospinal reflex response involving splanchnic excitatory nerves, cutting the vagal nerve branches to the duodenal does not affect the suppressed duodenal response[17]. Using duplex Doppler sonography, Choi et al[23] found that the frequency of intestinal motility is increased during acupuncture stimulation at St-36 acupoint. Acupuncture on the lower abdomen causes a transient relaxation of the stomach, which can be abolished by splanchnic ganglionectomy but not by truncal vagotomy[24].
In conclusion, acupuncture stimulation to the acupoints on face, neck, forelimbs, upper chest-dorsum and hindlinbs, which are distant to the region of gastric innervation, produces a facilitative response of gastric motility with increased activities of vagus and/or a slight inhibition in the activity of sympathetic nerves. These effects are involved in the intact preparation of vagal nerves and spinal cord, but do not require intact preparation of the splanchnic nerves. Acupuncture stimulation to the acupoints on lower-chest, middle-dorsum and whole abdomen, which are homo-segmental to the region of gastric innervation, induces an inhibitory response of gastric motility with increased activities of sympathetic nerves and/or a slight inhibition in the activity of vagal nerves. These effects are involved in intact preparation of sympathetic nerves, but do not require intact preparation of bilateral vagal nerves and high spinal cord. Only the acupuncture stimulation with its intensity greater than the threshold for activation of Aδ and/or C afferent fibers can induce markedly excitatory/inhibitory modulation of gastrointestinal motility.
We gratefully acknowledge Professor XC Yu for the help in preparation of this manuscript.
Co-correspondence: Pei-Jing Rong
S- Editor Liu Y L- Editor Wang XL E- Editor Liu WF
| 1. | Ma SX. Neurobiology of Acupuncture: Toward CAM. Evid Based Complement Alternat Med. 2004;1:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Knardahl S, Elam M, Olausson B, Wallin BG. Sympathetic nerve activity after acupuncture in humans. Pain. 1998;75:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Haker E, Egekvist H, Bjerring P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst. 2000;79:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Sato A. Neural mechanisms of autonomic responses elicited by somatic sensory stimulation. Neurosci Behav Physiol. 1997;27:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res. 2000;122:457-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Consensus Development Panel Program and Abstracts. NIH Consensus Statement Online. 1997;15. |
| 7. | Chang CS, Chou JW, Wu CY, Chang YH, Ko CW, Chen GH. Atropine-induced gastric dysrhythmia is not normalized by electroacupuncture. Dig Dis Sci. 2002;47:2466-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Chang CS, Ko CW, Wu CY, Chen GH. Effect of electrical stimulation on acupuncture points in diabetic patients with gastric dysrhythmia: a pilot study. Digestion. 2001;64:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Effective regularity in modulation on gastric motility induced by different acupoint stimulation. World J Gastroenterol. 2006;12:7642-7648. [PubMed] |
| 11. | Falinower S, Willer JC, Junien JL, Le Bars D. A C-fiber reflex modulated by heterotopic noxious somatic stimuli in the rat. J Neurophysiol. 1994;72:194-213. [PubMed] |
| 12. | Zhu B, Xu WD, Rong PJ, Ben H, Gao XY. A C-fiber reflex inhibition induced by electroacupuncture with different intensities applied at homotopic and heterotopic acupoints in rats selectively destructive effects on myelinated and unmyelinated afferent fibers. Brain Res. 2004;1011:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kawakita K, Funakoshi M. Suppression of the jaw-opening reflex by conditioning a-delta fiber stimulation and electroacupuncture in the rat. Exp Neurol. 1982;78:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Noguchi E, Ohsawa H, Kobayashi S, Shimura M, Uchida S, Sato Y. The effect of electro-acupuncture stimulation on the muscle blood flow of the hindlimb in anesthetized rats. J Auton Nerv Syst. 1999;75:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Ohsawa H, Yamaguchi S, Ishimaru H, Shimura M, Sato Y. Neural mechanism of pupillary dilation elicited by electro-acupuncture stimulation in anesthetized rats. J Auton Nerv Syst. 1997;64:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Koizumi K, Sato A, Terui N. Role of somatic afferents in autonomic system control of the intestinal motility. Brain Res. 1980;182:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Noguchi E, Ohsawa H, Tanaka H, Ikeda H, Aikawa Y. Electro-acupuncture stimulation effects on duodenal motility in anesthetized rats. Jpn J Physiol. 2003;53:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Qian L, Peters LJ, Chen JD. Effects of electroacupuncture on gastric migrating myoelectrical complex in dogs. Dig Dis Sci. 1999;44:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JD. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol. 2002;282:G390-G396. [PubMed] |
| 20. | Xu G. Regulating effect of electro-acupuncture on dysrythmia of gastro-colonic electric activity induced by erythromycine in rabbits. ZhenCi YanJiu. 1994;19:71-74. [PubMed] |
| 21. | Daniel EE. Electrical and contractile responses of the pyloric region to adrenergic and cholinergic drugs. Can J Physiol Pharmacol. 1966;44:951-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kim CH, Zinsmeister AR, Malagelada JR. Mechanisms of canine gastric dysrhythmia. Gastroenterology. 1987;92:993-999. [PubMed] |
| 23. | Choi M, Jung J, Seo M, Lee K, Nam T, Yang I, Yoon Y, Yoon J. Ultrasonographic observation of intestinal mobility of dogs after acupunctural stimulation on acupoints ST-36 and BL-27. J Vet Sci. 2001;2:221-226. [PubMed] |
| 24. | Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci. 2003;48:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |