Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.657
Revised: December 3, 2006
Accepted: December 21, 2006
Published online: February 7, 2007
Liver ischaemic preconditioning (IPC) is known to protect the liver from the detrimental effects of ischaemic-reperfusion injury (IRI), which contributes significantly to the morbidity and mortality following major liver surgery. Recent studies have focused on the role of IPC in liver regeneration, the precise mechanism of which are not completely understood. This review discusses the current understanding of the mechanism of liver regeneration and the role of IPC in this setting. Relevant articles were reviewed from the published literature using the Medline database. The search was performed using the keywords “liver”, “ischaemic reperfusion”, “ischaemic preconditioning”, “regeneration”, “hepatectomy” and “transplantation”. The underlying mechanism of liver regeneration is a complex process involving the interaction of cytokines, growth factors and the metabolic demand of the liver. IPC, through various mediators, promotes liver regeneration by up-regulating growth-promoting factors and suppresses growth-inhibiting factors as well as damaging stresses. The increased understanding of the cellular mechanisms involved in IPC will enable the development of alternative treatment modalities aimed at promoting liver regeneration following major liver resection and transplantation.
- Citation: Gomez D, Homer-Vanniasinkam S, Graham A, Prasad K. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol 2007; 13(5): 657-670
- URL: https://www.wjgnet.com/1007-9327/full/v13/i5/657.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.657
Ischaemic-reperfusion injury (IRI) is an inevitable phenomenon that results following major liver surgery, including partial hepatectomy and liver transplantation. As a consequence, parenchymal cell injury and liver dysfunction[1,2] of varied severity leads to significant morbidity and mortality post-surgery[3-5], in particular, in patients with liver cirrhosis and steatosis[6-9]. In addition, IRI significantly impairs liver regeneration following hepatectomy[10,11].
Due to the inevitability of ischaemia and reperfusion in liver surgery, various investigators have attempted to elucidate methods to limit the detrimental effects of IRI and improve liver function and regeneration of the remnant liver[12,13]. These include hypothermic perfusion of the liver[14], intermittent liver inflow occlusion[15,16] and ischaemic preconditioning (IPC)[17]. Liver IPC is an endogenous mechanism consisting of a short period of vascular occlusion followed by reperfusion that renders the liver more tolerant to subsequent prolonged episodes of ischaemia. Besides having protective effects on IRI following major liver resection and transplantation[17-19], it has been suggested that IPC is also beneficial in liver regeneration[20]. This article describes the current understanding of the liver regeneration cascade as published and gives a balanced review on the mechanisms by which IPC influences liver regeneration.
Liver regeneration is a complex and multi-factorial process that is mediated by interactions between regenerative cytokines, growth factors and metabolic demand of the liver following surgery and IRI.
During the first few hours following IRI, regenerative cytokines are produced that render the resting hepatocytes responsive to growth factors required for cellular division and proliferation[21,22]. This period is known as the “priming phase”. Various mediators have been implicated as possible triggers of the regenerative cytokine network including gastrointestinal lipopolysaccharide (LPS)[23,24], Toll-like receptor-myeloid differentiation factor 88 (Myd88) signaling pathways[25-27], components from the complement cascade[28], nitric oxide (NO)[29-31] and prostaglandins[32].
Studies have identified tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) as important regenerative cytokines[21,33,34]. Akerman and co-workers showed that anti-TNF antibodies led to delayed DNA synthesis in the regenerating rat liver and inhibited the increase in IL-6 levels following partial hepatectomy[35]. The initiation of liver growth by TNF-α was shown to be dependent on its binding to TNF-receptor type 1 (TNF-R1)[36]. Mice deficient of TNF-R1 exhibited a delay in liver regeneration and increased mortality following liver resection, which was subsequently reversed by recombinant IL-6 injection[37]. However, IL-6 lacking mice demonstrated impaired liver regeneration despite the presence of TNF-α, suggesting that TNF-R1 signaling results in the release of IL-6[38]. IL-6 deficient mice not only showed impaired ability to regenerate, but also had increased IRI following liver resection[39]. However, Wuestefeld et al[40] reported that mice deficient in IL-6 and its common signal transducer, glycoprotein 130 (gp130) had no defects in DNA replication following partial hepatectomy. The groups of Zimmers and Blindenbacher have suggested that the levels of serum IL-6 present following liver resection in mice are critical in modulating its regenerative effects[41,42]. This may account for the difference in results observed in studies attempting to determine the effect of IL-6 in liver regeneration. It has been suggested that other mediators such as stem cell factor and oncostatin M may play a role in enhancing the effects of IL-6 on hepatocyte regeneration[43-45].
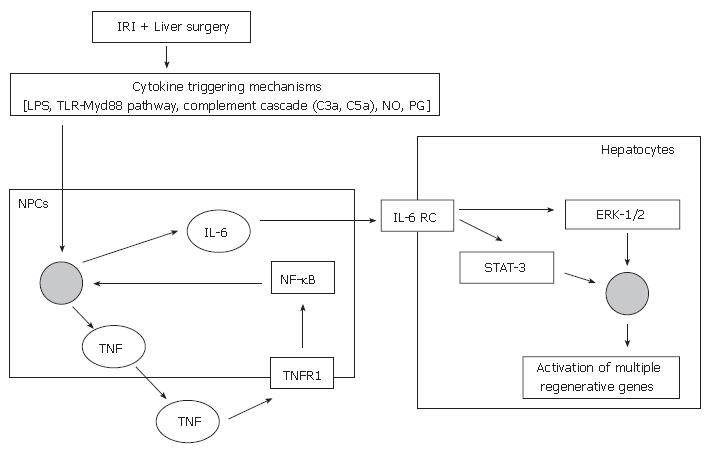
Non-parenchymal liver cells [Kupffer cells and sinusoidal endothelial cells (SECs)] are involved in the priming phase (Figure 1)[46]. Following stimulation from cytokine triggering mechanisms, TNF-α binds to TNF-R1 on non-parenchymal liver cells and stimulates the production of IL-6[47], via the activation of the transcription factor nuclear factor-kappa B (NF-κB)[48-50]. Ping and colleagues demonstrated that the secretion of regenerative cytokines, such as IL-6 by rat liver SECs was mediated by the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt signaling pathway[51], via NF-κB activation[50]. The transcription factor NF-κB is also known to be an important component of pro-survival cellular signaling responses, and hence its activation will not only stimulate IL-6 production but also activate survival genes[52,53].
IL-6 acts directly on hepatocytes by binding to the IL-6 receptor complex and induces the translocation of signal transducer and activator of transcription-3 (STAT-3) to the nucleus. This initiates a cascade of events that leads to progression of the cell cycle, culminating in the synthesis of DNA and subsequent cellular mitosis[54,55].
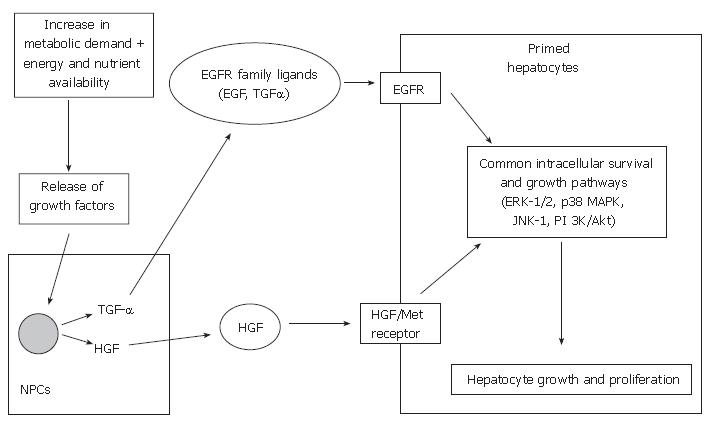
Following this priming phase, cell cycle progression is then dependent on growth factors, such as hepatocyte growth factor (HGF), transforming growth factor-alpha (TGF-α) and epidermal growth factor (EGF)[56,57]. The two main growth-promoting signaling systems involved in liver regeneration are the HGF and its receptor (Met) and the epidermal growth factor receptor (EGFR) and its relatively large family of ligands. The effect of growth factors and their corresponding signaling systems may be dependent on the metabolic state of the hepatocytes and the presence or absence of other effectors[58].
HGF is produced by non-parenchymal cells of the rat liver following liver injury[59-61] and partial hepatectomy[62], and acts on its receptor on hepatocytes. Several authors have demonstrated that HGF is crucial in promoting liver regeneration following partial hepatectomy and transplantation in animal models[63-67]. HGF administration to recipients of reduced-size liver grafts in rats illustrated early regeneration and provided hepatoprotection against rejection-related injuries[64,68,69]. These essential signals are regulated by HGF, via c-met, the gene on the HGF-receptor.
EGF is mainly produced in the salivary glands in rodents and plays an important role in hepatocyte proliferation by binding to the EGFR on hepatocytes[57]. Sialoadenectomy-induced decrease in circulating EGF in mice and rat models resulted in impaired liver regeneration following partial hepatectomy, which was reversed by the administration of EGF[70,71]. In addition, combined administration of EGF and insulin increases the DNA synthesis following liver resection in cirrhotic rats[72]. Results from these studies suggest that EGF has a direct effect on hepatocyte proliferation.
TGF-α is produced in non-parenchymal cells, mainly the Kupffer cells. Mead and Fausto demonstrated that TGF-α may function as a physiological inducer of hepato-cyte DNA synthesis during liver regeneration by an autocrine mechanism by binding to EGFR in both rat and culture models[73]. Besides TGF-α, there are many ligands for EGFR, including EGF, amphiregulin, heparin-binding EGF-like growth factor (HB-EGF) and epiregulin[57]. Although TGF-α expression increases following partial hepatectomy in mice, TGF-α lacking mice do not display diminished liver regeneration[74,75]. This may be due to the fact that ligands such as EGF can also stimulate EGFR and activate common intracellular growth signaling pathways. Studies have shown that other growth factors, such as HB-EGF[50,76,77], amphiregulin[78], insulin and glucagon[79,80] may also play a role in liver regeneration.
Although these two growth-promoting signaling systems are largely independent, some integration may exist. Scheving et al[81] demonstrated that EGFR kinase inhibition by PKI166 (selective, potent inhibitor of EGFR kinase) blocked the mitogenic effects of HGF in cultured rat hepatocytes, suggesting EGFR may regulate HGF-mediated hepatocyte proliferation. Nevertheless, various regenerative pathways are initiated by HGF/c-met and the EGFR signaling mechanisms (Figure 2), which include the activation of mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinases 1 and 2 (ERK-1/2; aka p42/44 MAPKs), c-jun-NH2-terminal kinases 1 and 2 (JNK-1/2; aka p46/p54 SAPK) and p38 MAPKs. Collectively, these MAPKs have been shown to play essential roles in cell growth, transformation differentiation and apoptosis[82-84].
ERK-1/2 activation has been shown to correlate with hepatocyte proliferation in animal studies and in vitro models[85-87]. Besides being responsive to growth factor signals, studies have demonstrated that ERK-1/2 activity can also be induced by cytokines such as TNF-α[88]. Hence, the ERK-1/2 may be a signaling pathway that integrates both growth factor and cytokine signaling. Serandour et al[89] demonstrated that a combination of EGF and TNF-α induced hepatocyte proliferation by 30%, compared to EGF alone in a hepatocyte-liver epithelial cell co-culture model. This study suggested that TNF-α mediated extracellular matrix remodeling was required for continued hepatocyte replication and proliferation. Hence, this study re-iterates the importance of the interaction of cytokines and growth factors in the liver regeneration cascade. Although ERK-1/2 has been identified to have a key role in hepatocyte growth, inhibition of ERK-1/2 does not significantly alter proliferation of regenerating rat hepatocytes. However, inhibition of p38 MAPKs results in decreased DNA synthesis, suggesting that p38 MAPKs activation is prerequisite for hepatocyte proliferation[90]. JNK-1 and p38 MAPKs are involved in the regulatory control of the induction of nuclear proteins, such as cyclin D1. The activation of cyclin D1 is one of the earliest steps in the pathway of resting cells to enter the pre-replicative phase of the cell cycle.
The increased metabolic demand imposed on the remnant liver following partial hepatectomy are likely to be inter-connected with the activation of the mechanisms involved in DNA replication to sustain liver function. This is likely to be dependent on energy levels and nutrient availability.
Mitochondria are the predominant source of the high energy phosphates that are essential for energy-dependent processes in cells. Mitochondrial activity has been shown to be correlated with the recovery of liver function and subsequent regeneration[91,92]. Maruyama et al[93] showed the recovery of liver weight following hepatectomy was proportional to the energy (ATP) levels of the remnant liver in rats.
Amino acid deprivation has been shown to inhibit the regeneration process in rat livers following liver resection[94]. Nelsen et al[95] showed that selective amino acid deprivation in culture and protein deprivation in mice impaired hepatocyte cyclin D1 expression and that transfection of cyclin D1 promoted cell cycle progression under these conditions. Hence, amino acids regulate hepatocyte proliferation via cyclin D1.
The absence of bile acids in the intestine has been shown to delay liver regeneration following partial hepatectomy in rats[96]. Following liver surgery or injury, bile flow is stimulated[97] which results in the release of bile from the gallbladder and its return through the entero-hepatic circulation exposes the remnant hepatocytes to an increase in relative bile acid flux. This early phase of bile acid overload and subsequent bile acid signaling is necessary for normal liver regeneration. However, liver-specific functions such as synthesis of clotting factors and albumin and the continuous formation of bile are impaired following partial hepatectomy and results in transient cholestasis[98]. The formation of bile is dependent on the active secretion of bile salts and other biliary constituents into the bile canaliculus by specific bile acid and organic anion transporters[99]. Gerloff et al[100] demonstrated that the expression of two ATP-dependent transporters [bile salt acid pump (Bsep) and multi-organic anion transporter (Mrp2)] was unchanged or slightly increased following partial hepatectomy in rats and this provided a potential mechanism by which the regenerating liver cells maintained or increased bile secretion. The authors in this study also suggested that the down-regulation of certain transporters [sodium-taurocholate cotransporter (Ntcp) and organic anion transporting polypeptides (Oatp1 and Oatp2)] could be a protective mechanism against the potentially hepatotoxic bile salts[100]. The differential regulation of hepatobiliary transporters during the regeneration process are likely to be mediated by cytokines such as TNF-α[101]. Recently, Huang et al[102] showed that bile acid activation of nuclear receptor-dependent signaling pathways regulated the regeneration process by sensing the liver’s functional capacity following partial hepatectomy in mice. When inadequate function causes bile acids to build up, the resultant nuclear receptors activation not only induces negative feedback pathways that protect hepatocytes from bile acid toxicity but also increases the capacity of the liver to manage the overload by promoting liver growth[102].
Results from recent studies have implicated the mammalian target of rapamycin (mTOR) complex as a sensor of nutrient-energy levels and its downstream mediators, such as p70 S6 kinase, are thought to regulate protein translation and cell growth[103,104]. Inhibition of the mTOR complex leads to diminish DNA replication following partial hepatectomy in mice and rat models[105,106]. The activity of p70 S6 kinase has been shown to increase following partial hepatectomy[106] and mice lacking the S6 kinase demonstrated diminished hepatocyte proliferation[107]. Hence, energy is required for energy-dependent signaling pathways and processing the available nutrients for the regeneration process to proceed.
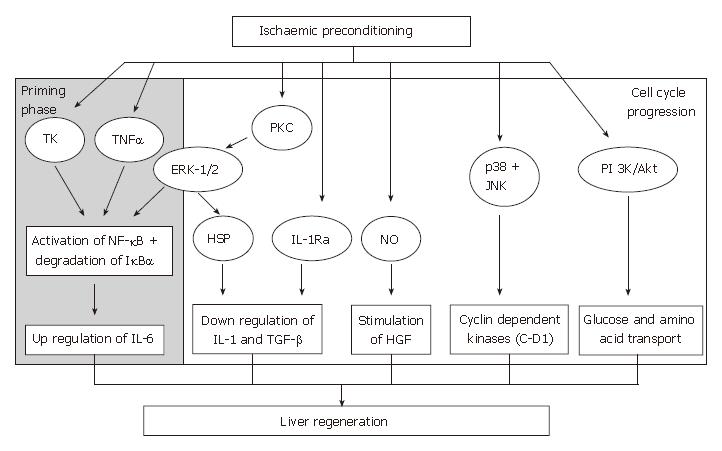
Since IPC was first described by Murry et al[108], this strategy has been developed more widely and is currently practiced in major liver surgery in several centers. The mechanism of IPC is thought to be divided into two phases; early (classical/acute) pre-conditioning and delayed pre-conditioning. The hepatoprotective effects of early preconditioning occur within minutes after reperfusion and are maintained for 1 to 2 h[109]. This phase is thought to be mediated by pre-existing substances. The “second window of protection”, delayed pre-conditioning, re-appears 24 to 72 h following IPC. The underlying mechanism is thought to rely on the modification of gene expression resulting in protein production as its effectors[110]. Various substances have been implicated as key effectors in liver IPC including adenosine[111-114], protein kinase C[115-117], NO[118-121], heat-shock proteins[122,123], tyrosine kinases[124], MAPKs[117], oxidative stress[125,126] and NF-κB[127,128].
The beneficial effects of IPC on the liver following IRI include decrease in severity of liver necrosis[129], anti-apoptotic effects[130], preservation of liver microcirculati-on[131,132] and improvement in survival rate[119], and more recently, its role in liver regeneration is currently being evaluated. For IPC to influence liver regeneration, its key effectors must be involved in either promoting or up-regulating mediators that are involved in the regeneration cascade or exert an inhibitory effect on growth-inhibitory factors of liver regeneration or vice versa (Figure 3).
Tumour necrosis factor-alpha and interleukin-6: The initiation of the regenerative response is dependent on the early activation of TNF-α and IL-6 responsive transcription factors[38,54]. Studies evaluating the effect of IPC in stimulating the release of TNF-α and IL-6 have produced conflicting findings. Tsuyama et al[133] reported that IPC prolonged survival, suppressed liver necrosis induced by IRI and inhibited the release of TNF-α at 1 h, and IL-6 at 2 and 5 h of the reperfusion phase in mice. IPC has also been shown to reduce TNF-α production in normothermic and cold ischaemic conditions[125,134,135].
In contrast, Bedirli and others demonstrated that IPC inhibited the production of TNF-α, but not IL-6 during the late (24 and 48 h) phases of reperfusion in rats following partial hepatectomy[136]. This study suggested that IL-6 is an important mediator in IPC-treated rats in promoting hepatocyte proliferation following liver resection[136]. Using a TNF gene-deleted mice model, Teoh et al[137] demonstrated that pre-treatment with low dose IL-6 prior to hepatic ischaemia conferred equivalent hepatoprotection and earlier cell cycle entry as IPC compared to non-IPC-treated mice at 2 h of reperfusion. Taken together, these studies emphasize the importance of IL-6 as a hepatoprotective and pro-proliferative mediator during the early and late phases of reperfusion following IRI. Although IPC attenuates the late onset prolonged release of TNF-α (up to 44 h) that mediates liver IRI in rats[138,139] and mice[140], IPC itself is associated with the early increase (10 min) in liver and serum TNF-α following hepatic ischaemia[140]. Injection of low dose TNF-α 30 min prior to liver ischaemia conferred similar hepatoprotection as IPC[140]. Both IPC and pre-treatment with low dose TNF-α injection were shown to stimulate earlier and more vigorous cell cycle entry following liver IRI compared to naïve mice during the first 24 h of the reperfusion period[140].
The differences reported among studies investigating the influence of IPC on the regenerative cytokine network may be related to the differences in experimental models and protocols employed. In addition, both TNF-α and IL-6 are known to be important components in other cellular signaling responses. For example, high levels of serum TNF-α following liver ischaemia in rats is thought to mediate liver IRI[138,139]. Investigators have suggested that the mechanism of action of IL-6 in promoting liver regeneration appear to be separate from those involved in the modulation of IRI[39,141]. The hepatoprotective mechanisms against IRI may involve the anti-inflammatory properties of IL-6 and the down regulation of TNF-α production[39,142,143]. This could potentially explain the differences in results obtained.
Nevertheless, the influence of IPC in promoting the early release of TNF-α and the up regulation of IL-6 during the reperfusion phase, and subsequent modulation of the hepatocellular proliferation process should not be discounted. In addition, these conflicting results may indicate that IPC can potentiate hepatocyte proliferation via at least two different pathways; liver regeneration via a TNF-α/IL-6-dependent pathway and a mitogen-induced proliferative pathway that does not require TNF-α or IL-6[144,145].
Hepatocyte growth is controlled by a balance of both growth-promoting and growth-inhibiting factors[65,146]. While those cytokines and pathways previously described promote liver regeneration following liver resection, there are various mediators that are involved directly or indirectly in inhibiting the liver regeneration process.
Interleukin-1 and transforming growth factor-beta as inhibitors of liver regeneration: IL-1 is a mediator of acute inflammation[147] and is a significant down-regulator of hepatocyte proliferation[148]. IL-1β delays and inhibits hepatocyte proliferation in both culture models[149] and following partial hepatectomy in rats[148]. Other investigators have implicated IL-1α as a mediator of IRI and an inhibitor of liver regeneration[150]. Besides antagonizing the stimulatory effects of growth factors such as HGF[150], IL-1 strongly inhibits hepatocyte DNA synthesis leading to impaired liver regeneration in primary culture models[149]. TGF-β is another inhibitor of hepatocyte DNA synthesis[151-153] and antagonizes the stimulatory effects of HGF during liver regeneration in both in vitro and experimental hepatectomy models[152,154]. This anti-regenerative effect of TGF-β is thought to be modulated by the induction of oxidative stress in hepatocytes[155,156].
Results from several studies have suggested that IPC antagonizes the effects of these inhibitory cytokines. Previous studies have shown that the release of IL-1 is potentially influenced by NO during IRI in a variety of cell types[157-159]. In IPC-treated rats that underwent reduced size liver transplantation, an increase in IL-1α levels were noted following NO synthesis inhibition[150]. This practice abolished the benefits of IPC on hepatic IRI, oxidative stress and liver regeneration[3,150]. Furthermore, the detrimental effects of NO inhibition were not observed when rats subjected to this treatment were subsequently treated with an IL-1 receptor antagonist (IL-1-RA)[150]. IL-1α has also been shown to be involved in pulmonary injury following liver IRI. IPC mediated by NO, reduced IL-1α release and protected against pulmonary damage[160]. Data from these studies suggests that IPC, through increased NO availability, inhibits the release of IL-1, thereby protecting the liver graft and the lungs against liver IRI and preventing inhibition of liver regeneration. Hence, one proposed mechanism by which IPC promotes liver regeneration is by inhibiting the release of growth-inhibitory cytokines from Kupffer cells, such as IL-1, which is dependent on up-regulation of the NO pathway.
The mechanism by which IPC inhibits IL-1 production may also be related to the induction of intracellular stress proteins[161,162], such as heat shock protein 70 (HSP 70). IPC induces over-expression of HSP 70 in isolated hepatocytes[163], and reduces liver IL-1 synthesis under normothermic conditions[164]. Besides expressing cytoprotective effects[165,166], HSP induction leads to the down-regulation of IL-1 synthesis in pancreatic[161,167] and lung[162,168] cell studies. The induction of HSP mediated by IPC may be independent of the NO pathway as the increase in HSP by IPC was not modified when NO synthesis was inhibited[150]. These results suggest that IPC potentiates the overexpression of HSP, via an NO independent pathway, leading to hepato-protection against IRI and ameliorates liver regeneration due to decrease inhibitor production.
Another proposed mechanism by which IPC promotes liver regeneration could be due to the stimulation of IL-1-RA. IL-1-RA is an acute phase protein that has been shown to inhibit the effects of IL-1α and IL-1β by competing for typeIand type II IL-1 receptors[169]. This leads to a decrease in the inflammatory response[169] and abrogates liver IRI in vivo[170]. A recent study on gene expression profiling on patients undergoing partial hepatectomy revealed that IPC stimulated the expression of the IL-1-RA gene[171]. Hence, liver over-expression of IL-1-RA following IPC directly inhibits the effect of high IL-1 concentrations induced by IRI and results in reduced liver injury and necrosis[171]. Although no study has formally assessed the effect of IL-1-RA on markers of liver regeneration, IL-1-RA could be indirectly involved in the regeneration process by antagonizing the inhibitory effects of IL-1 on hepatocyte proliferation.
Inhibitory binding protein for NF-κB (IκB-α) and NF-κB activity: The transcriptional activities of NF-κB are tightly controlled by its inhibitory proteins, especially IκB-α[172]. The phosphophorylation and subsequent degradation of IκB-α leads to the liberation of NF-κB proteins allowing binding to a variety of promoters and triggers gene expression. Data from studies examining the role of NF-κB as an effector of IPC have demonstrated conflicting results. IPC facilitated the activation of transcription factor NF-κB in an in vivo murine model, and this was parallel to the degradation of its inhibitory protein, IκB-α[173]. Ricciardi and co-workers found that IPC increased IκB-α phosphorylation and NF-κB concentration prior to cold ischemia in pig liver grafts. Data from this study suggested that the underlying mechanism involved was related to the activation of second messengers of tyrosine kinase[127]. Another proposed mechanism for NF-κB activation and degradation of IκB-α could be mediated by TNF-α. The release of TNF-α by IPC and pre-treatment with low-dose TNF-α was shown to increase IκB-α degradation and increase NF-κB DNA binding in a mouse model[140]. However, Li et al[174] observed that IPC inhibited the activity of the transcription factor NF-κB during the early reperfusion phase (1 and 2 h), and this was accompanied by diminished TNF-α expression and reduced IRI in liver transplantation rat model.
One explanation for this contradictory evidence might be the different experimental models and methodology used. Nevertheless, these results indicate that IPC does attenuate the nuclear levels of the transcription factor NF-κB. It is possible that other mediators may have an effect in determining the increase or inhibition of NF-κB activity and its corresponding cellular signaling responses.
Hepatocyte growth factor (HGF): Franco-Gou et al[175] demonstrated an increase in both liver and plasma HGF levels following IPC in reduced size liver transplantation rat model, and this was associated with an increase in hepatocyte proliferation. The modulation of HGF levels by IPC could be mediated by the generation of NO and its effect on TGF-β and HGF concentrations. IPC reduced the levels of TGF-β with an associated increase in HGF levels[150]. Similar results were seen with NO preconditioning treatment[150]. This suggests that IPC reduced TGF-β levels with a parallel increase in HGF and subsequent hepatocyte proliferation, possibly mediated by NO.
HGF may also exhibit the ability to promote hepatocyte survival. In a liver IRI rat model, pre-treatment with HGF inhibited the production of reactive oxygen species and its damaging effects[176]. Similar results were observed in a hypoxia-reoxygenation-induced oxidative stress model in hepatocytes[177]. These results suggest that the anti-apoptotic effect of HGF could pave the way for hepatocyte proliferation following IRI.
Extracellular signal-regulated kinases 1 and 2 (ERK-1/2): ERK-1/2 is predominantly activated by growth-promoting factors. Studies have shown that protein kinase C plays a pivotal role in the activation of ERK-1/2 signaling pathway[163,178] that participates in the preservation of hepatocytes[163]. A number of reports have indicated that protein kinase C was critical for the development of IPC in rat, rabbit and human myocardiocytes[179,180]. Gao and associates showed that the activation of protein kinase C and its downstream ERK-1/2 mediators were increased in IPC-treated in vivo and in vitro models[163]. Data from this study also suggested that the expression of HSP70 was reduced and the protective effect of IPC was diminished when ERK-1/2 activity was reduced by a MAPK inhibitor (PD-98059). HSP70 expression and the cytoprotective effect of IPC were also reduced by a protein kinase C inhibitor (chelerythrine) in both in vitro and in vivo settings. Hence, this suggests that IPC increased the activation of ERK-1/2, via protein kinase C. The increased activation of protein kinase C-dependent ERK-1/2 by IPC may also increase the expression of HSP70. The up-regulation of ERK-1/2 by IPC may help in promoting liver regeneration in both the priming phase and growth factor signaling pathways.
p38 mitogen-activated protein kinases and c-jun-NH2-terminal kinase 1: Both p38 MAPKs and JNK-1 are known to modulate proliferative or apoptotic signaling pathways[181]. The activation of MAPKs and its corresponding downstream signaling pathway is regulated by specific stimuli and is also dependent on cell type. The co-activation of NF-κB protects the hepatocyte from apoptosis and is involved in the priming of hepatocytes to enter the cell cycle[22,182].
Carini and co-investigators demonstrated that hypoxic preconditioning activated the p38 signaling pathway in rat hepatocytes subjected to hypoxia-re-oxygenation injury in vitro[117]. Teoh et al[173] demonstrated that proliferating hepatocytes were identified earlier in IPC-treated livers in a murine model of partial liver ischaemia, and this corresponded with the earlier activation and sustained maintenance of p38 and JNK-1. This suggests that IPC stimulus could prime quiescent hepatocytes to enter the cell cycle early, hence, setting up a regenerative response to compensate for hepatocyte injury by IRI.
Phosphatidylinositol 3-kinase (PI 3-kinase)/Akt signaling pathway: The activation of the PI 3-kinase/Akt cascade has been shown to have a positive impact on cell survival and proliferation[183-186], inhibition of apoptosis and encourage the uptake of glucose and amino acids following stimulation by various growth-promoting factors in certain cells[187-189]. Data from the Izuishi group showed significant Akt activation following IPC in an in vivo model and suggested that this might contribute to the up regulation of glucose and amino acid transport after IRI required for liver regeneration[190].
Cyclin D1: Cyclin and cyclin-dependent kinases are involved in cell cycle regulation[191], in particular, the D group cyclins[192]. Cyclin D1 is a nuclear protein required for cell cycle progression in the G1 phase[192-194], and controls hepatocyte proliferation[195]. IPC-treated livers showed earlier expression of cyclin D1 protein that corresponded with enhanced entry of hepatocytes into the cell cycle[173]. Cai and co-workers demonstrated that IPC stimulated cyclin D1 mRNA and protein expression during the early reperfusion phase in IPC-treated rat livers[196]. The early production of cyclin D1 could be mediated by the activation of the p38 MAPK pathway[182]. The cyclin D1 promoter region includes binding sites for NF-κB[197] and NF-κB activation is evident in hepatocytes during the early phase of regeneration following partial hepatectomy[48,198]. There is a close link between the activation of NF-κB by degradation of IκB-α cyclin D1 activation and cell cycle progression. IPC can modulate these transcription factors and increase cell proliferation, which is possibly one of the protective mechanisms against IRI.
Results obtained from an experimental model of 70% hepatectomy indicated that liver regeneration was closely correlated to the ATP levels of the liver remnant[93]. Studies have shown that IPC in normothermic conditions preserved the adenine nucleotide pool[199,200] and this is thought to be a consequence of the down regulation of cellular metabolism[199,201]. In a rat model of hypothermic transplant preservation injury, hepatocytes exposed to IPC had higher ATP concentrations and increased protein synthesis[202]. This improvement in energy metabolism is thought to contribute to hepatocyte viability following IRI[202]. Besides improvement in energy metabolism, Yoshizumi et al[203] also demonstrated an increase in bile production in IPC-treated rats. However, Franco-Gou and colleagues demonstrated similar energy metabolism (ATP, adenine nucleotides, ATP/ADP ratio and energy charge) in the IPC- and non-IPC-treated rat livers[175]. The difference in results obtained could be related to the difference in experimental models used. However, the role of IPC in modulating liver energy metabolism, which involves the preservation of ATP should not be discounted.
The use of IPC as a surgical strategy to limit the detrimental effects of IRI during liver surgery has been extensively researched. Encouraging findings in animal studies in both warm ischaemia[203] and transplantation models[119], led to the first human trial which demonstrated that IPC reduced the severity of post-operative liver injury as well as alleviating endothelial cell injury[17]. Further human liver resection studies have shown that IPC reduces post-operative serum aminotransferase levels in both steatotic[204] and cirrhotic[205] livers and improves post-reperfusion haemodynamic stability[206] (Table 1). Although IPC is protective against IRI[207,208], it did not influence the morbidity and mortality rates in human studies[204,209,210]. In the transplant setting, Jassem et al[211] reported lower serum aminotransferase levels and shorter intensive care stay in IPC-treated cadaveric donor allografts. Other studies have not shown IPC to be beneficial in terms of graft function. Azoulay et al[212] found that although IPC protected cadaveric liver grafts against IRI, this beneficial effect was counter-balanced by decreased early graft function. Cescon and colleagues demonstrated similar protective effects against IRI, but IPC showed no clinical benefit (primary graft function and survival rates) in liver transplantation from deceased donors[213]. Although gaining popularity, the incongruous evidence of the clinical effects of IPC has precluded its widespread adoption in liver transplantation units.
| Study group | Sample1 | Surgery | IPC2 | Ischaemia and reperfusion time (min)3 | Parameters assessed | Outcome of IPC |
| Clavien et al[17] (2000) | 24 (12) | Liver resection | 10I + 10R | IPC and control (TI: 30) | PT, Bilirubin, ALT, AST, Histology, Caspase-3 and 8 activity, SEC apoptosis, Blood loss, Transfusion, ITU stay, LOS | Protective against IRI Beneficial in patients with steatosis |
| Clavien et al[204] (2003) | 100 (50) | Liver resection | 10I + 10R | IPC (TI: 36 ± 5.9, Op: 225 ± 73), Control (TI: 35 ± 6.8, Op: 240 ± 92) | PT, Bilirubin, ALT, AST, Histology, Hepatic ATP, Blood loss, Transfusion, ITU stay, LOS | Protective against IRI Beneficial in younger patients, those with steatosis and longer periods of occlusion |
| Li et al[205] (2004) | 29 (14) | Liver resection | 5I + 5R | IPC (TI: 18 ± 3.6, Op: 191.3 ± 74.9), Control (TI: 17.4 ± 2.3, Op: 208.2 ± 45.3) | Bilirubin, ALT, AST, Histology, Caspase-3 activity, SEC apoptosis, LOS | Protective against IRI, mainly HCC patients with cirrhosis Shorter hospital stay |
| Nuzzo et al[209] (2004) | 42 (21) | Liver resection | 10I + 10R | IPC (TI: 54 ± 19, Op: 321 ± 92), Control (TI: 36 ± 14, Op: 339 ± 112) | PT, Bilirubin, ALT, AST, Transfusion, Morbidity, Mortality | Reduces operative bleeding Protective against IRI |
| Chouker et al[206] (2004) | 68 (22) | Liver resection | 10I + 10R | IPC (TI: 32 ± 6.3, Op: 251 ± 46), Control without PR (TI: NA, Op: 52 ± 30), Control with PR (TI: 35 ± 11, Op: 257 ± 83) | ALT, AST, Fluid loss, Transfusion, 4Cardiovascular status | Protective against IRI Improves haemodynamic stability |
| Chouker et al[207] (2005) | 75 (25) | Liver resection | 10I + 10R | IPC (TI: 35.5 ± 2.7, LR: 32.2 ± 2.0), Control without PR (TI: NA, LR: 39 ± 4.5), Control with PR (TI: 35.6 ± 2.6, LR: 33.2 ± 2.3) | IL-6, IL-8, Cytochrome c, Adhesion molecules [B2-integrins (CD18)], Histology (neutrophil infiltration) | Protective against IRI by attenuating neutrophil activation and IL-8 release |
| Chouker et al[208] (2005) | 73 (25) | Liver resection | 10I + 10R | IPC (TI: 35.12 ± 13.6, LR: 31.50 ± 9.1), Control without PR (TI: NA, LR: 34.77 ± 16.5), Control with PR (TI: 34.2 ± 10.9, LR: 32.13 ± 10) | PT, ALT, AST, α-GST | Protective against IRI Prevented early rise of α-GST |
| Koneru et al[210] (2005) | 62 (34) | Transplant | 5I + 5R | IPC (CI: 384 ± 92, WI: 41 ± 5.8), Control (CI: 415 ± 87, WI: 37 ± 5.6) | INR, Bilirubin, ALT, AST, Histology (apoptosis, hepatocyte swelling), LOS, Survival (6 mo) | No beneficial effect |
| Azoulay et al[212] (2005) | 91 (46) | Transplant | 10I + 10R | IPC (CI: 436 ± 116, Op: 441 ± 119), Control (CI: 461 ± 96, Op: 462 ± 98) | PT, Bilirubin, ALT, AST, Histology, Graft function, Morbidity, Mortality | Better ischaemic tolerance Decreased early graft function |
| Jassem et al[211] (2005) | 23 (9) | Transplant | 10I + 10R | IPC (CI: 620 ± 190, WI: 43.9 ± 13), Control (CI: 665 ± 280, WI: 40.4 ± 9) | AST, INR, Lactate, ITU stay, Histology (neutrophil infiltration, platelet deposition), Graft function | Protective against IRI Reduces inflammatory response Shorter ITU stay |
| Cescon et al[213] (2006) | 47 (23) | Transplant | 10I + 15R | 5IPC [TI: 388 (259-830), Op: 440 (225-725)], Control [TI: 383 (279-695), Op: 465 (280-1015)] | PT, Bilirubin, ALT, AST, Histology (neutrophil, lymphocyte infiltration, iNOS, apoptosis), Graft function, Survival (1 yr) | Protective against IRI No clinical benefit |
Following major liver resection and IRI, the ability of the liver to regenerate is crucial to maintain liver function. This also has implication in live donor orthotopic liver transplantation and transplantation of segmental liver grafts. Since Yamada and co-workers first demonstrated that IPC significantly increased the regenerative capacity of the remaining hepatocytes in a rat model of IRI[20], various investigators have attempted to elucidate the role of IPC on liver regeneration using both culture and animal model studies as described above. At present, although there is no clinical trial published on the effect of IPC on liver regeneration, there are studies evaluating methods of monitoring liver regeneration. Special radiological imaging techniques currently available not only show volume of regeneration, but also determine functional ability of the remnant and regenerated liver[214,215]. This is another step towards assessing the role of IPC in liver regeneration in the clinical scenario.
With the increasing laboratory evidence of protection against IRI and improved liver regeneration by IPC, several aspects of this strategy could be developed pharmacologically that may be more clinical applicable than IPC itself. This is especially in cases with a background of chemotherapy-induced steatohepatitis[216] and cirrhosis[217,218]. Pharmacologic agents targeting mediators of IPC that can be potentially developed include HGF, IL-6 and IL-1-RA. However, several issues such as the timing of administration of these agents, therapeutic doses and immunological response of the recipient need to be determined. Further understanding of the mechanistic pathways of IPC may pave the way for the development of these agents that are capable of conferring protection against IRI and promote liver regeneration.
Liver regeneration is of clinical significance in view of the increasing number of major liver resections and the increasing use of marginal donor liver and split-liver allografts for transplantation. Successful patient outcome often depends on liver regeneration, particularly in patients with cirrhotic and steatotic livers. Regeneration of the liver following IRI and major liver surgery is a complex process that involves the integration of a network of cytokines, growth factors, kinases, transcription factors and metabolic demands of the liver.
In comparison with the evidence available on the effect of IPC on IRI, its role in liver regeneration is still undetermined. However, current research has demonstrated that the beneficial effects of IPC on liver regeneration is mediated by up regulating growth-promoting factors, suppressing growth-inhibitory factors and preserving energy levels for regeneration. Nevertheless, more studies are still required to further delineate the underlying pathophysiology of IPC and impact on mediators of liver regeneration. It is also important to determine whether the beneficial effect of IPC in the laboratory setting is reproducible in clinical practice. By understanding the underlying mechanisms by which IPC influences liver regeneration, other strategies as alternatives to IPC, could be developed to modulate the regenerative pathways in the clinical setting and improve outcomes of patients following major liver resection and transplantation. The assessment of IPC on liver regeneration in human studies is clearly the next step.
S- Editor Liu Y L- Editor Negro F E- Editor Bi L
| 1. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 431] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 368] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454-458. [PubMed] |
| 5. | Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 236] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Ezaki T, Seo Y, Tomoda H, Furusawa M, Kanematsu T, Sugimachi K. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg. 1992;79:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 333] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Glanemann M, Langrehr JM, Stange BJ, Neumann U, Settmacher U, Steinmüller T, Neuhaus P. Clinical implications of hepatic preservation injury after adult liver transplantation. Am J Transplant. 2003;3:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 305] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Foschi D, Castoldi L, Lesma A, Musazzi M, Benevento A, Trabucchi E. Effects of ischaemia and reperfusion on liver regeneration in rats. Eur J Surg. 1993;159:393-398. [PubMed] |
| 11. | Watanabe M, Chijiiwa K, Kameoka N, Yamaguchi K, Kuroki S, Tanaka M. Gadolinium pretreatment decreases survival and impairs liver regeneration after partial hepatectomy under ischemia/reperfusion in rats. Surgery. 2000;127:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, Naili S, Vinet E, Saliba F, Lemoine A. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Uchinami M, Muraoka R, Horiuchi T, Tabo T, Kimura N, Naito Y, Yoshikawa T. Effect of intermittent hepatic pedicle clamping on free radical generation in the rat liver. Surgery. 1998;124:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Smyrniotis V, Kostopanagiotou G, Theodoraki K, Farantos C, Arkadopoulos N, Gamaletsos E, Condi-Paphitis A, Fotopoulos A, Dimakakos P. Ischemic preconditioning versus intermittent vascular inflow control during major liver resection in pigs. World J Surg. 2005;29:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [PubMed] |
| 20. | Yamada F, Abe T, Saito T, Tsuciya T, Ishii S, Gotoh M. Ischemic preconditioning enhances regenerative capacity of hepatocytes after prolonged ischemia. Transplant Proc. 2001;33:956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 910] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 23. | Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5767] [Cited by in RCA: 5775] [Article Influence: 213.9] [Reference Citation Analysis (0)] |
| 25. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3474] [Cited by in RCA: 3502] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 26. | Seki E, Tsutsui H, Iimuro Y, Naka T, Son G, Akira S, Kishimoto T, Nakanishi K, Fujimoto J. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Su GL, Wang SC, Aminlari A, Tipoe GL, Steinstraesser L, Nanji A. Impaired hepatocyte regeneration in toll-like receptor 4 mutant mice. Dig Dis Sci. 2004;49:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem. 2005;280:12305-12309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Wang HH, Lautt WW. Does nitric oxide (NO) trigger liver regeneration? Proc West Pharmacol Soc. 1997;40:17-18. [PubMed] |
| 32. | Schoen Smith JM, Lautt WW. The role of prostaglandins in triggering the liver regeneration cascade. Nitric Oxide. 2005;13:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Trautwein C, Rakemann T, Niehof M, Rose-John S, Manns MP. Acute-phase response factor, increased binding, and target gene transcription during liver regeneration. Gastroenterology. 1996;110:1854-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Iwai M, Cui TX, Kitamura H, Saito M, Shimazu T. Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine. 2001;13:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579-G585. [PubMed] |
| 36. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1394] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 37. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 38. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1212] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 39. | Camargo CA, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 321] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281-11288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest. 2003;112:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Kamiya A, Kojima N, Kinoshita T, Sakai Y, Miyaijma A. Maturation of fetal hepatocytes in vitro by extracellular matrices and oncostatin M: induction of tryptophan oxygenase. Hepatology. 2002;35:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417-427. [PubMed] |
| 49. | Kirillova I, Chaisson M, Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activation. Cell Growth Differ. 1999;10:819-828. [PubMed] |
| 50. | Sakuda S, Tamura S, Yamada A, Miyagawa J, Yamamoto K, Kiso S, Ito N, Higashiyama S, Taniguchi N, Kawata S. NF-kappaB activation in non-parenchymal liver cells after partial hepatectomy in rats: possible involvement in expression of heparin-binding epidermal growth factor-like growth factor. J Hepatol. 2002;36:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Ping C, Lin Z, Jiming D, Jin Z, Ying L, Shigang D, Hongtao Y, Yongwei H, Jiahong D. The phosphoinositide 3-kinase/Akt-signal pathway mediates proliferation and secretory function of hepatic sinusoidal endothelial cells in rats after partial hepatectomy. Biochem Biophys Res Commun. 2006;342:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2159] [Cited by in RCA: 2187] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 53. | De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 609] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 54. | Debonera F, Aldeguer X, Shen X, Gelman AE, Gao F, Que X, Greenbaum LE, Furth EE, Taub R, Olthoff KM. Activation of interleukin-6/STAT3 and liver regeneration following transplantation. J Surg Res. 2001;96:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411-28417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 56. | Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27-54. [PubMed] |
| 57. | Michalopoulos GK, Khan Z. Liver regeneration, growth factors, and amphiregulin. Gastroenterology. 2005;128:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Bucher NL. Liver regeneration: an overview. J Gastroenterol Hepatol. 1991;6:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Kinoshita T, Tashiro K, Nakamura T. Marked increase of HGF mRNA in non-parenchymal liver cells of rats treated with hepatotoxins. Biochem Biophys Res Commun. 1989;165:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Noji S, Tashiro K, Koyama E, Nohno T, Ohyama K, Taniguchi S, Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990;173:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Hu Z, Evarts RP, Fujio K, Marsden ER, Thorgeirsson SS. Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol. 1993;142:1823-1830. [PubMed] |
| 62. | Ping C, Xiaoling D, Jin Z, Jiahong D, Jiming D, Lin Z. Hepatic sinusoidal endothelial cells promote hepatocyte proliferation early after partial hepatectomy in rats. Arch Med Res. 2006;37:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Burr AW, Toole K, Chapman C, Hines JE, Burt AD. Anti-hepatocyte growth factor antibody inhibits hepatocyte proliferation during liver regeneration. J Pathol. 1998;185:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 64. | Uchiyama H, Yanaga K, Nishizaki T, Soejima Y, Yoshizumi T, Sugimachi K. Effects of deletion variant of hepatocyte growth factor on reduced-size liver transplantation in rats. Transplantation. 1999;68:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Masson S, Daveau M, Hiron M, Lyoumi S, Lebreton JP, Ténière P, Scotté M. Differential regenerative response and expression of growth factors following hepatectomy of variable extent in rats. Liver. 1999;19:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Tomiya T, Ogata I, Yamaoka M, Yanase M, Inoue Y, Fujiwara K. The mitogenic activity of hepatocyte growth factor on rat hepatocytes is dependent upon endogenous transforming growth factor-alpha. Am J Pathol. 2000;157:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Okamoto K, Suzuki S, Kurachi K, Sunayama K, Yokoi Y, Konno H, Baba S, Nakamura S. Beneficial effect of deletion variant of hepatocyte growth factor for impaired hepatic regeneration in the ischemically damaged liver. World J Surg. 2002;26:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Ikegami T, Nishizaki T, Uchiyama H, Kakizoe S, Yanaga K, Sugimachi K. Deletion variant of hepatocyte growth factor prolongs allograft survival after liver transplantation in rats. Surgery. 1999;125:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Tashiro H, Fudaba Y, Itoh H, Mizunuma K, Ohdan H, Itamoto T, Asahara T. Hepatocyte growth factor prevents chronic allograft dysfunction in liver-transplanted rats. Transplantation. 2003;76:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Jones DE, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268:G872-G878. [PubMed] |
| 71. | Noguchi S, Ohba Y, Oka T. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in mice. J Endocrinol. 1991;128:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Hashimoto M, Kothary PC, Eckhauser FE, Raper SE. Treatment of cirrhotic rats with epidermal growth factor and insulin accelerates liver DNA synthesis after partial hepatectomy. J Gastroenterol Hepatol. 1998;13:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA. 1989;86:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 349] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 74. | Webber EM, Wu JC, Wang L, Merlino G, Fausto N. Overexpression of transforming growth factor-alpha causes liver enlargement and increased hepatocyte proliferation in transgenic mice. Am J Pathol. 1994;145:398-408. [PubMed] |
| 75. | Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog. 1996;15:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | Kiso S, Kawata S, Tamura S, Inui Y, Yoshida Y, Sawai Y, Umeki S, Ito N, Yamada A, Miyagawa J. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology. 2003;124:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Berasain C, García-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 79. | Bucher ML, Swaffield MN. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci USA. 1975;72:1157-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 191] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Hwang TL, Chen MF, Chen TJ. Augmentation of liver regeneration with glucagon after partial hepatectomy in rats. J Formos Med Assoc. 1993;92:725-728. [PubMed] |
| 81. | Scheving LA, Stevenson MC, Taylormoore JM, Traxler P, Russell WE. Integral role of the EGF receptor in HGF-mediated hepatocyte proliferation. Biochem Biophys Res Commun. 2002;290:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Terada Y, Inoshita S, Nakashima O, Kuwahara M, Sasaki S, Marumo F. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int. 1999;56:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Fanger GR. Regulation of the MAPK family members: role of subcellular localization and architectural organization. Histol Histopathol. 1999;14:887-894. [PubMed] |
| 85. | Coutant A, Rescan C, Gilot D, Loyer P, Guguen-Guillouzo C, Baffet G. PI3K-FRAP/mTOR pathway is critical for hepatocyte proliferation whereas MEK/ERK supports both proliferation and survival. Hepatology. 2002;36:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Thoresen GH, Guren TK, Christoffersen T. Role of ERK, p38 and PI3-kinase in EGF receptor-mediated mitogenic signalling in cultured rat hepatocytes: requirement for sustained ERK activation. Cell Physiol Biochem. 2003;13:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608-10613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 88. | Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530-34536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Sérandour AL, Loyer P, Garnier D, Courselaud B, Théret N, Glaise D, Guguen-Guillouzo C, Corlu A. TNFalpha-mediated extracellular matrix remodeling is required for multiple division cycles in rat hepatocytes. Hepatology. 2005;41:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, Dent P. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556-3565. [PubMed] |
| 91. | Ozawa K, Fujimoto T, Nakatani T, Asano M, Aoyama H, Tobe T. Changes in hepatic energy charge, blood ketone body ratio, and indocyanine green clearance in relation to DNA synthesis after hepatectomy. Life Sci. 1982;31:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Ngala Kenda JF, de Hemptinne B, Lambotte L. Role of metabolic overload in the initiation of DNA synthesis following partial hepatectomy in the rat. Eur Surg Res. 1984;16:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Maruyama H, Harada A, Kurokawa T, Kobayashi H, Nonami T, Nakao A, Takagi H. Duration of liver ischemia and hepatic regeneration after hepatectomy in rats. J Surg Res. 1995;58:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | McGowan J, Atryzek V, Fausto N. Effects of protein-deprivation on the regeneration of rat liver after partial hepatectomy. Biochem J. 1979;180:25-35. [PubMed] |
| 95. | Nelsen CJ, Rickheim DG, Tucker MM, McKenzie TJ, Hansen LK, Pestell RG, Albrecht JH. Amino acids regulate hepatocyte proliferation through modulation of cyclin D1 expression. J Biol Chem. 2003;278:25853-25858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Ueda J, Chijiiwa K, Nakano K, Zhao G, Tanaka M. Lack of intestinal bile results in delayed liver regeneration of normal rat liver after hepatectomy accompanied by impaired cyclin E-associated kinase activity. Surgery. 2002;131:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Sainz GR, Monte MJ, Barbero ER, Herrera MC, Marin JJ. Bile secretion by the rat liver during synchronized regeneration. Int J Exp Pathol. 1997;78:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Xu HS, Rosenlof LK, Jones RS. Bile secretion and liver regeneration in partially hepatectomized rats. Ann Surg. 1993;218:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Meier PJ. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am J Physiol. 1995;269:G801-G812. [PubMed] |
| 100. | Gerloff T, Geier A, Stieger B, Hagenbuch B, Meier PJ, Matern S, Gartung C. Differential expression of basolateral and canalicular organic anion transporters during regeneration of rat liver. Gastroenterology. 1999;117:1408-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 102. | Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 103. | Kim DH, Sabatini DM. Raptor and mTOR: subunits of a nutrient-sensitive complex. Curr Top Microbiol Immunol. 2004;279:259-270. [PubMed] |
| 104. | Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 105. | Jiang YP, Ballou LM, Lin RZ. Rapamycin-insensitive regulation of 4e-BP1 in regenerating rat liver. J Biol Chem. 2001;276:10943-10951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Goggin MM, Nelsen CJ, Kimball SR, Jefferson LS, Morley SJ, Albrecht JH. Rapamycin-sensitive induction of eukaryotic initiation factor 4F in regenerating mouse liver. Hepatology. 2004;40:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Volarevic S, Stewart MJ, Ledermann B, Zilberman F, Terracciano L, Montini E, Grompe M, Kozma SC, Thomas G. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 108. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5539] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 109. | Jenkins DP, Baxter GF, Yellon DM. The pathophysiology of ischaemic preconditioning. Pharmacol Res. 1995;31:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 112. | Howell JG, Zibari GB, Brown MF, Burney DL, Sawaya DE, Olinde JG, Granger DN, McDonald JC. Both ischemic and pharmacological preconditioning decrease hepatic leukocyte/endothelial cell interactions. Transplantation. 2000;69:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Arai M, Thurman RG, Lemasters JJ. Involvement of Kupffer cells and sinusoidal endothelial cells in ischemic preconditioning to rat livers stored for transplantation. Transplant Proc. 1999;31:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Arai M, Thurman RG, Lemasters JJ. Contribution of adenosine A(2) receptors and cyclic adenosine monophosphate to protective ischemic preconditioning of sinusoidal endothelial cells against Storage/Reperfusion injury in rat livers. Hepatology. 2000;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Carini R, De Cesaris MG, Splendore R, Bagnati M, Albano E. Ischemic preconditioning reduces Na(+) accumulation and cell killing in isolated rat hepatocytes exposed to hypoxia. Hepatology. 2000;31:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Ricciardi R, Meyers WC, Schaffer BK, Kim RD, Shah SA, Wheeler SM, Donohue SE, Sheth KR, Callery MP, Chari RS. Protein kinase C inhibition abrogates hepatic ischemic preconditioning responses. J Surg Res. 2001;97:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 118. | Peralta C, Closa D, Hotter G, Gelpí E, Prats N, Roselló-Catafau J. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 120. | Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Koti RS, Seifalian AM, McBride AG, Yang W, Davidson BR. The relationship of hepatic tissue oxygenation with nitric oxide metabolism in ischemic preconditioning of the liver. FASEB J. 2002;16:1654-1656. [PubMed] |
| 122. | Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 123. | Ishikawa Y, Yamamoto Y, Kume M, Yamagami K, Yamamoto H, Kimoto S, Sakai Y, Yamamoto M, Yamaoka Y. Heat shock preconditioning on mitochondria during warm ischemia in rat livers. J Surg Res. 1999;87:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Ricciardi R, Schaffer BK, Kim RD, Shah SA, Donohue SE, Wheeler SM, Quarfordt SH, Callery MP, Meyers WC, Chari RS. Protective effects of ischemic preconditioning on the cold-preserved liver are tyrosine kinase dependent. Transplantation. 2001;72:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Sindram D, Rüdiger HA, Upadhya AG, Strasberg SM, Clavien PA. Ischemic preconditioning protects against cold ischemic injury through an oxidative stress dependent mechanism. J Hepatol. 2002;36:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Ricciardi R, Shah SA, Wheeler SM, Quarfordt SH, Callery MP, Meyers WC, Chari RS. Regulation of NFkappaB in hepatic ischemic preconditioning. J Am Coll Surg. 2002;195:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 128. | Funaki H, Shimizu K, Harada S, Tsuyama H, Fushida S, Tani T, Miwa K. Essential role for nuclear factor kappaB in ischemic preconditioning for ischemia-reperfusion injury of the mouse liver. Transplantation. 2002;74:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 130. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 131. | Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L. Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia and reperfusion. Transplant Proc. 2006;38:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Szijártó A, Hahn O, Lotz G, Schaff Z, Madarász E, Kupcsulik PK. Effect of ischemic preconditioning on rat liver microcirculation monitored with laser Doppler flowmetry. J Surg Res. 2006;131:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Tsuyama H, Shimizu K, Yoshimoto K, Nezuka H, Ito H, Yamamoto S, Hasebe K, Onishi I, Muraoka K, Ninomiya I. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury in mice. Transplant Proc. 2000;32:2310-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Fernández L, Heredia N, Peralta C, Xaus C, Roselló-Catafau J, Rimola A, Marco A, Serafín A, Deulofeu R, Gelpí E. Role of ischemic preconditioning and the portosystemic shunt in the prevention of liver and lung damage after rat liver transplantation. Transplantation. 2003;76:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Bedirli A, Kerem M, Pasaoglu H, Erdem O, Ofluoglu E, Sakrak O. Effects of ischemic preconditioning on regenerative capacity of hepatocyte in the ischemically damaged rat livers. J Surg Res. 2005;125:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Teoh N, Field J, Farrell G. Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice. J Hepatol. 2006;45:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 641] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 139. | Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 140. | Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 141. | Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Clavien PA, Camargo CA, Gorczynski R, Washington MK, Levy GA, Langer B, Greig PD. Acute reactant cytokines and neutrophil adhesion after warm ischemia in cirrhotic and noncirrhotic human livers. Hepatology. 1996;23:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 413] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 144. | Ohmura T, Ledda-Columbano GM, Piga R, Columbano A, Glemba J, Katyal SL, Locker J, Shinozuka H. Hepatocyte proliferation induced by a single dose of a peroxisome proliferator. Am J Pathol. 1996;148:815-824. [PubMed] |
| 145. | Ledda-Columbano GM, Curto M, Piga R, Zedda AI, Menegazzi M, Sartori C, Shinozuka H, Bluethmann H, Poli V, Ciliberto G. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 146. | Enami Y, Kato H, Murakami M, Fujioka T, Aoki T, Niiya T, Murai N, Ohtsuka K, Kusano M. Anti-transforming growth factor-beta1 antibody transiently enhances DNA synthesis during liver regeneration after partial hepatectomy in rats. J Hepatobiliary Pancreat Surg. 2001;8:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Raz R, Durbin JE, Levy DE. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J Biol Chem. 1994;269:24391-24395. [PubMed] |
| 148. | Boulton R, Woodman A, Calnan D, Selden C, Tam F, Hodgson H. Nonparenchymal cells from regenerating rat liver generate interleukin-1alpha and -1beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49-58. [PubMed] |
| 149. | Nakamura T, Arakaki R, Ichihara A. Interleukin-1 beta is a potent growth inhibitor of adult rat hepatocytes in primary culture. Exp Cell Res. 1988;179:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 150. | Franco-Gou R, Roselló-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, Bartrons R, Peralta C. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 226] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 152. | Scotté M, Masson S, Lyoumi S, Hiron M, Ténière P, Lebreton JP, Daveau M. Cytokine gene expression in liver following minor or major hepatectomy in rat. Cytokine. 1997;9:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 153. | Russell WE, Coffey RJ, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA. 1988;85:5126-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 154. | Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 155. | Herrera B, Fernández M, Alvarez AM, Roncero C, Benito M, Gil J, Fabregat I. Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor beta in rat fetal hepatocytes. Hepatology. 2001;34:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 156. | Herrera B, Murillo MM, Alvarez-Barrientos A, Beltrán J, Fernández M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 157. | Liu P, Xu B, Spokas E, Lai PS, Wong PY. Role of endogenous nitric oxide in TNF-alpha and IL-1beta generation in hepatic ischemia-repefusion. Shock. 2000;13:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 158. | Liu P, Xu B, Hock CE. Inhibition of nitric oxide synthesis by L-name exacerbates acute lung injury induced by hepatic ischemia-reperfusion. Shock. 2001;16:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 159. | Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). J Immunol. 1998;161:4122-4128. [PubMed] |
| 160. | Franco-Gou R, Roselló-Catafau J, Peralta C. Protection against lung damage in reduced-size liver transplantation. Crit Care Med. 2006;34:1506-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 161. | Ye J, Laychock SG. A protective role for heme oxygenase expression in pancreatic islets exposed to interleukin-1beta. Endocrinology. 1998;139:4155-4163. [PubMed] |
| 162. | LoCicero J, Xu X, Zhang L. Heat shock protein suppresses the senescent lung cytokine response to acute endotoxemia. Ann Thorac Surg. 1999;68:1150-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 163. | Gao Y, Shan YQ, Pan MX, Wang Y, Tang LJ, Li H, Zhang Z. Protein kinase C-dependent activation of P44/42 mitogen-activated protein kinase and heat shock protein 70 in signal transduction during hepatocyte ischemic preconditioning. World J Gastroenterol. 2004;10:1019-1027. [PubMed] |
| 164. | Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 165. | Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 450] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 166. | Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 386] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 167. | Hsu BR, Juang JH, Chen ST, Hsu S, Fu SH. Cobalt-Protoporphyrin treatment renders islets tolerant to interleukin-1 beta suppression. Transplant Proc. 2004;36:1181-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416-5423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 169. | Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 220] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 170. | Harada H, Wakabayashi G, Takayanagi A, Shimazu M, Matsumoto K, Obara H, Shimizu N, Kitajima M. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation. 2002;74:1434-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Barrier A, Olaya N, Chiappini F, Roser F, Scatton O, Artus C, Franc B, Dudoit S, Flahault A, Debuire B. Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J. 2005;19:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 172. | Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 898] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 173. | Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 174. | Li XC, Ma YF, Wang XH. Role of NF-kappaB as effector of IPC in donor livers before liver transplantation in rats. Transplant Proc. 2006;38:1584-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 175. | Franco-Gou R, Peralta C, Massip-Salcedo M, Xaus C, Serafín A, Roselló-Catafau J. Protection of reduced-size liver for transplantation. Am J Transplant. 2004;4:1408-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 176. | Oe S, Hiros T, Fujii H, Yasuchika K, Nishio T, Iimuro Y, Morimoto T, Nagao M, Yamaoka Y. Continuous intravenous infusion of deleted form of hepatocyte growth factor attenuates hepatic ischemia-reperfusion injury in rats. J Hepatol. 2001;34:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 177. | Ozaki M, Haga S, Zhang HQ, Irani K, Suzuki S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: role of PI3-K and Akt kinase upon rac1. Cell Death Differ. 2003;10:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 178. | Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1448] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 179. | Ladilov YV, Balser C, Piper HM. Protection of rat cardiomyocytes against simulated ischemia and reoxygenation by treatment with protein kinase C activator. Circ Res. 1998;82:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 180. | Rehring TF, Shapiro JI, Cain BS, Meldrum DR, Cleveland JC, Harken AH, Banerjee A. Mechanisms of pH preservation during global ischemia in preconditioned rat heart: roles for PKC and NHE. Am J Physiol. 1998;275:H805-H813. [PubMed] |
| 181. | Crenesse D, Gugenheim J, Hornoy J, Tornieri K, Laurens M, Cambien B, Lenegrate G, Cursio R, De Souza G, Auberger P. Protein kinase activation by warm and cold hypoxia- reoxygenation in primary-cultured rat hepatocytes-JNK(1)/SAPK(1) involvement in apoptosis. Hepatology. 2000;32:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 182. | Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1243] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 183. | Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1-13. [PubMed] |
| 184. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3190] [Cited by in RCA: 3241] [Article Influence: 124.7] [Reference Citation Analysis (0)] |
| 185. | Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 478] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 186. | Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2015] [Cited by in RCA: 2064] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 187. | Tsakiridis T, McDowell HE, Walker T, Downes CP, Hundal HS, Vranic M, Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315-4322. [PubMed] |
| 188. | Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372-31378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 973] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 189. | Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 551] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 190. | Izuishi K, Fujiwara M, Hossain MA, Usuki H, Maeta H. Significance of phosphoinositide 3-kinase pathway on ischemic preconditioning followed by ischemia reperfusion in mice liver. Transplant Proc. 2003;35:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 191. | Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1527] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 192. | Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501-534. [PubMed] |
| 193. | Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1243] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 194. | Pagano M, Theodoras AM, Tam SW, Draetta GF. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 1994;8:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 195. | Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397-404. [PubMed] |
| 196. | Cai FG, Xiao JS, Ye QF. Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats. World J Gastroenterol. 2006;12:2936-2940. [PubMed] |
| 197. | Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785-5799. [PubMed] |
| 198. | Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429-30435. [PubMed] |
| 199. | Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163-G171. [PubMed] |
| 200. | Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 201. | Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 202. | Compagnon P, Wang HB, Southard JH, Mangino MJ. Ischemic preconditioning in a rodent hepatocyte model of liver hypothermic preservation injury. Cryobiology. 2002;44:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 203. | Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 204. | Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843-850; discussion 851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 205. | Li SQ, Liang LJ, Huang JF, Li Z. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2004;10:2580-2584. [PubMed] |
| 206. | Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, Pollwein B, Niklas M, Rau HG, Jauch KW. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 207. | Choukèr A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, Peter K, Thiel M. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg. 2005;140:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 208. | Choukér A, Martignoni A, Schauer RJ, Dugas M, Schachtner T, Kaufmann I, Setzer F, Rau HG, Löhe F, Jauch KW. Alpha-gluthathione S-transferase as an early marker of hepatic ischemia/reperfusion injury after liver resection. World J Surg. 2005;29:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 209. | Nuzzo G, Giuliante F, Vellone M, De Cosmo G, Ardito F, Murazio M, D'Acapito F, Giovannini I. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl. 2004;10:S53-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 210. | Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, de la Torre AN, Merchant A, Arora R, Samanta AK. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 211. | Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 212. | Azoulay D, Del Gaudio M, Andreani P, Ichai P, Sebag M, Adam R, Scatton O, Min BY, Delvard V, Lemoine A. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the ying and the yang. Ann Surg. 2005;242:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 213. | Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, Varotti G, D'Errico A, Ballardini G, Pinna AD. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006;12:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 214. | Kwon AH, Matsui Y, Ha-Kawa SK, Kamiyama Y. Functional hepatic volume measured by technetium-99m-galactosyl-human serum albumin liver scintigraphy: comparison between hepatocyte volume and liver volume by computed tomography. Am J Gastroenterol. 2001;96:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 215. | Sugai Y, Komatani A, Hosoya T, Yamaguchi K. Response to percutaneous transhepatic portal embolization: new proposed parameters by 99mTc-GSA SPECT and their usefulness in prognostic estimation after hepatectomy. J Nucl Med. 2000;41:421-425. [PubMed] |
| 216. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 951] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 217. | Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D, Jarnagin W, Fong Y, Blumgart LH. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 218. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1117] [Article Influence: 48.6] [Reference Citation Analysis (0)] |