INTRODUCTION
Chronic hepatitis B virus (HBV) infection continues to be a major human health problem, and there are about 350 million chronic HBV carriers worldwide[1]. Chronic HBV infection is associated with serious complications as a result of long-term sequelae such as liver cirrhosis or hepatocellular carcinoma[2]. The host immune response to HBcAg and HBeAg appears critical in both viral clearance and clinical resolution. The ultimate objective for rational vaccine design is the induction of pathogen immunity. In laboratory animals, DNA vaccine has proven to be a simple and effective method to generate protective immunity against a variety of pathogens, including HBV[3,4]. DNA vaccination that can induce both cellular and humoral immune responses has become an attractive immunization strategy against chronic HBV infection. Although it is known that DNA applied either i.m. or intradermally is primarily taken up by muscle cells or keratinocytes, it has become clear in recent years that professional APCs are essential for priming naive T cells following DNA injection. Accumulating evidence indicates that dendritic cells (DCs), the most potent APCs, play a critical role in the induction of immune responses by DNA vaccines[5-7]. Thus, enhancement of antigen presentation by DCs is an attractive strategy to increase the potency of DNA vaccines. However, a major problem of DNA vaccines is its limited potency, because only a very limited fraction of injected DNA molecules are taken up by DCs.
Recently, heat shock protein (HSP) was observed to elicit protective immunity to cancers and infectious agents. The abilities of HSP include: (a) to chaperone peptides, including antigenic; (b) to interact with antigen presenting cells through a receptor; (c) to stimulate antigen presenting cells to secrete inflammatory cytokines; and (d) to mediate maturation of DCs, making them a one-stop shop for the immune system[8]. These properties also permit to use of HSP for developing a new vaccine. HSP has been reported to activate innate immune responses, to mediate the maturation of DCs, to upregulate proinflammatory cytokines[9-12], and to induce specific CTL responses[13,14].
In this study, we describe a novel DNA vaccination strategy to enhance uptake and presentation of antigens by DCs. Specifically, we developed a DNA vaccination based upon expression of the HBV e antigen fused to HSP, which are versatile immune regulators that chaperone antigenic peptides for MHC classIand II presentation by DCs. After vaccination, DNA is taken up by various cells that produce and secrete the antigen-HSP fusion proteins. The secreted fusion proteins, in addition to inducing B-cells, are efficiently captured and processed by DCs via receptor-mediated endocytosis, and then presented via MHC classIand class II molecules. This study demonstrates a broad enhancement of antigen-specific CD4+ helper, CD8+ cytotoxic T-cell, and B-cell responses by this DNA vaccination strategy, which may be superior to currently described HBV DNA vaccination approaches for the treatment of chronic HBV infection.
MATERIALS AND METHODS
Mice and cell lines
The mice used were female C57BL/6 and Balb/c mice, aged 4-5 wk. All mice were maintained in the animal facility at Baylor College of Medicine with approval of the Institutional Animal Care and Use Committee. The tumor cell lines EL-4, Trampc-2, and CT26 were purchased from the ATCC. EL-4 and Tampc-2 cells were cultured in DMEM medium and CT26 cells in RPMI 1640 medium both containing 10% heat-inactivated FBS (GIBCO) at 37°C in an humidified 5% CO2 atmosphere.
DNA constructs
The pCMV-HBeAg-HSP construct was generated by inserting HBeA (be derived from the precore open reading frame by cleavage of its C-terminus, nucleotide: 1901-2452 of HBV genome) & HSP-70 (StressGen Biotechnologies, Victoria, British Columbia, Canada) plasmid into a pCMV vector (Invitrogen, Carlsbad, CA,USA) with the cloning site HindIII & XbaI. Two control vectors, pCMV-HBeAg & pCMV-HSP, were also generated.
DNA preparation and immunization
Plasmid DNA was amplified in Escherichia coli DH5α and purified using an endotoxin-free purification kit (Qiagen) according to a standard protocol. Concentration was determined using the UV/Visible Spectrophotometer (Pharmacia Biotech) at 260 and 280 nm, and the material was adjusted to a final concentration of 1 mg/mL with endotoxin-free PBS (Sigma) and stored at -20°C. Mice were divided into 4 groups, which were immunized with different DNA vaccines including pCMV-HBeAg-HSP, pCMV-HBeAg, and pCMV-HSP. Controls were injected with PBS (C57BL/6 mice) or pCMV (Balb/c mice). Immunization method: mice were injected s.c. (C57BL/6) and i.m. (Balb/c) in quadriceps with 100 μg of DNA in 100 μL, two inoculations were carried out with an interval of 2 wk. Two weeks after the second immunization blood and spleens were collected, and BALB/c mice were challenged with CT26-HBeAg tumor cells.
Elispot for T-cell response assay
Elispot assays were used as a measure for T-cell response. Ninety-six well filtration plates (Milipore, Bedford, MA, USA) were coated with AN18 (anti-mouse IFN-γ, Mebtech) at the concentration of 10 μg/mL and kept at 4°C overnight. Splenocytes were cultured in 96-well plates (1 × 106 cells/mL and 2 × 106 cells/mL) with RPMI 1640/10% FBS containing HEPES, 2MC, and NEAAS. Splenocytes from mice of different groups were stimulated with HBeAg classIpeptide (HBcAg93-100 peptide), HBeAg class II peptide (HBcAg 120-131), or HBV protein (HBsAg, 227 amino acids, 24kD) (BD Pharmingen, SD, CA, USA) for comparing the effect of different DNA vaccinations on the T-cell response. The splenocytes derived from mice vaccinated with HBeAg-HSP were also stimulated with Trampc-2 classIpeptide (P117-139, WT1), CT26 classIpeptide (peptide AH1), Tyrosinase protein, Tyrosinase classIpeptide (Ty-4), Tyrosinase class II peptide (Ty-5), and PMSA4 class II peptide as controls. Proteins were added at a final concentration of 60 μg/mL, peptides at a final concentration of 30 μg/mL. All assays were performed in triplicates. After stimulation for 20 h at 37°C, the plate was washed with PBS and the second antibody (anti-mouse IFN-γ, Mebtech Mab R4-6A2 biotin) was added for a further incubation at 37°C for 2 h. Avidin-HRP was added for 1 h at room temperature after washing, then 100 μL AEC was added to each well for coloring for 4 min after washing. The reaction was stopped by drying the membrane. The results were sent to Zellnet Consulting, Inc. (NY, USA) for test.
CTL cytotoxicity assays
CTL cytotoxic activity was determined using a 51Cr-release assay. In brief, splenocytes obtained from mice 2 wk after the second immunization were cultured in 24 well plates with RPMI 1640/10% FBS containing HEPES, 2MC, NEAAS, and IL-2 (50 U/mL). Splenocytes were stimulated with HBeAg classIpeptide (HbcAg93-100 peptide) for 7 d, with changing half of the medium every 2 d. Target cell lines were cultured with IFN-γ (100 U/mL) for 24 h. EL-4 cells were pulsed with HBeAg classIpeptide and HBV protein (HBsAg, 227 amino acids, 24 kDa) as target cells. EL-4 cells pulsed with HBV non-related classIpeptide such as CT26 and Trampc-2 classIpeptides, non-pulsed EL-4 cells, and CT26 cells pulsed with HBeAg classIpeptide served as controls. All target and control cells were labeled with 51Cr for 90 min. Cells were added to the wells at effector-to-target ratios ranging from 100:1 to 6.25:1 in triplicates. Plates were incubated at 37°C for 5 h, before supernatants were collected and activity was assessed in a Gamma counter (Beckman, Fullerton, CA, USA).
Anti-HBeAg antibodies assays
An ELISA assay (BD Biosciences) was used to quantify the antibody response after immunization. Sera were obtained 2 wk after the second immunization. Microtiter plates (Maxisorp) were coated with HBeAg overnight at 4°C. The coated plate was washed with PBS to stop reaction for 1 h at 37°C. Sera were added at different dilutions and the plate was incubated at 37°C for 2 h. The second antibody was added at 37°C for 2 h after washing with PBS. Finally, substrate was added and the plate was stored at room temperature for 30 min before stopping reactions with 4N sulfuric acid. Reading of the plate was done in an ELISA reader at 450 nm.
CT26-HBeAg tumor cell challenge test
CT26 cells were transfected with HBeAg using GenePoter reagent (Gene Therapy Systems) according to the manufacture’s instructions. Forty-eight hours after transfection, cells were harvested and plated into selective medium in 10 cm dishes, 5 × 104 CT26 cells were plated into 250 μg/mL Geneticin. Two weeks after the second vaccination, 5 × 105 CT26 cells with HBeAg were injected s.c. into mice. Tumor incidence and tumor growth were monitored and tumor size was measured (v = 1/2ab2: v: volume; a: largest diameter; b: smallest diameter).
Statistical analysis
All statistical analyses were performed using student t-test. Values of P < 0.05 was considered significant.
RESULTS
Enhancement of T cell response and CTL activity by HBeAg-HSP DNA vaccine
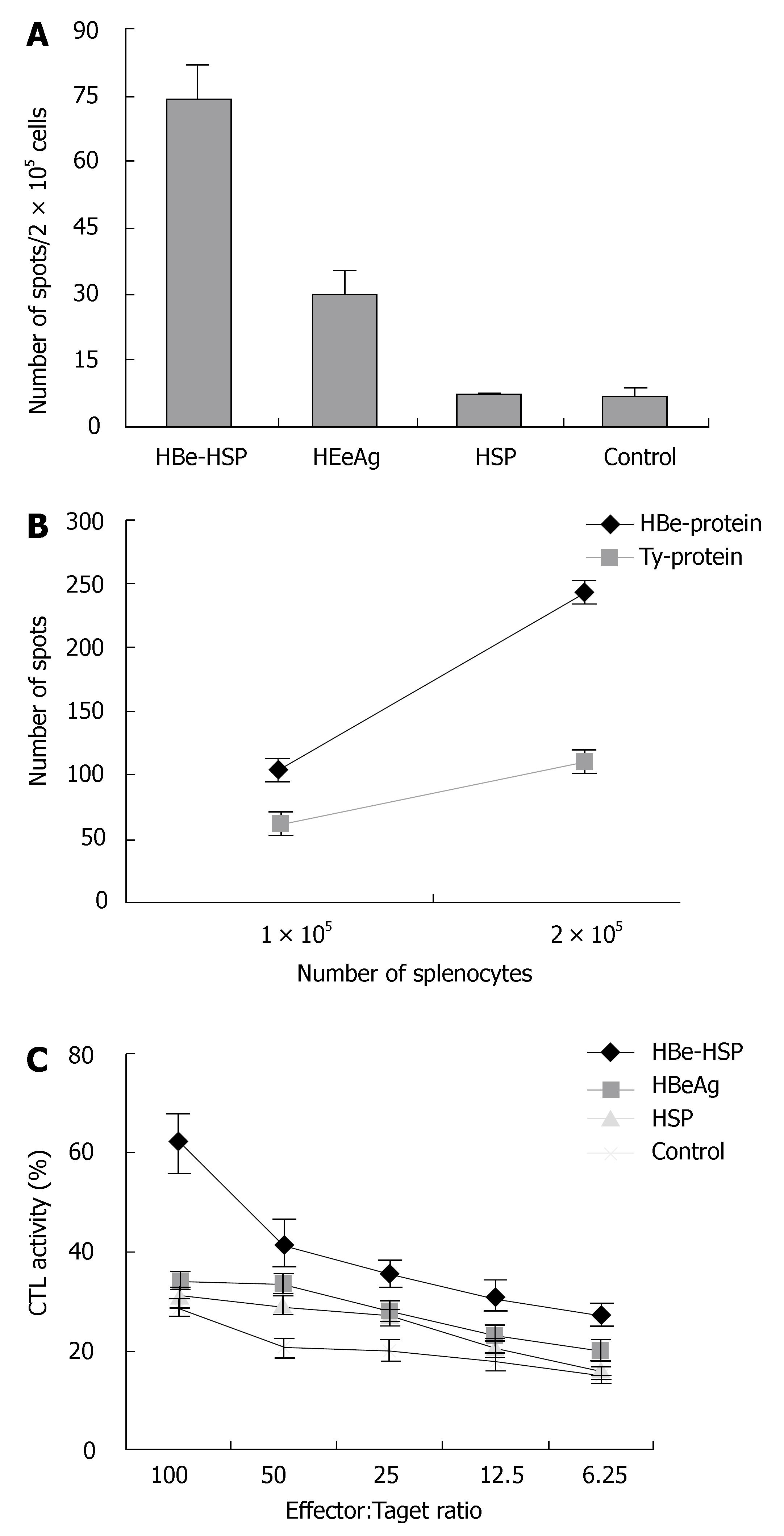
To evaluate whether HBeAg-HSP DNA vaccine can enhance immune response in vivo, splenocytes were obtained for T cell response and CTL activity. These immune responses were first stimulated with HBV protein and were compared between C57BL/6 and Balb/c mice immunized with different DNA vaccines. In CTL assay, EL-4 cells were pulsed with HBV protein as target cells. ELISPOT showed a stronger T-cell response from the mice immunized with HBeAg-HSP than that from HBeAg immunized mice after stimulation with HBV protein (Figure 1A, spots 74 ± 5 vs 31 ± 6, P < 0.01). A specific T-cell response was obtained in HBV protein stimulation in comparison with Tyrosinase protein stimulation (Figure 1B). Superior CTL assay to HBV protein pulsed target cell was also observed in mice immunized with HBeAg-HSP in comparison to those immunized with HBeAg (Figure 1C, 46% ± 10% vs 35% ± 8% in E: T > 50:1, P < 0.05).
Figure 1 A: Comparison of T cell proliferation between different groups after stimulation with HBV protein (C57BL/6 mice); B: Comparison of T cell proliferation between stimulation with HBV protein and with non-related protein (Balb/c mice); C: Comparison of CTL activity to HBV protein pulsed target cells between different groups (C57BL/6 mice).
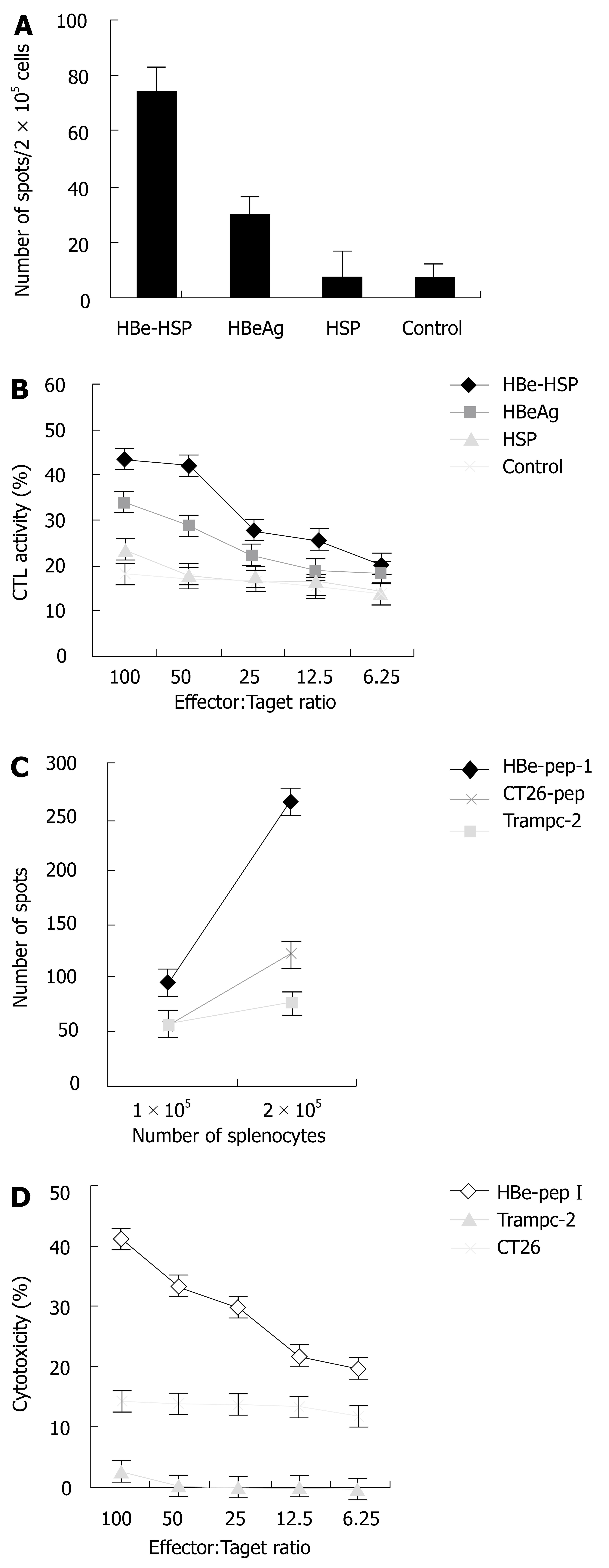
We also evaluated the specific stimulating effect of HBV MHC classIpeptide on T cell response and CTL activity. After splenocytes were stimulated with HBV MHC classIpeptide, ELISPOT showed a stronger T-cell response from mice immunized with HBeAg-HSP than from those which had been immunized with HBeAg (Figure 2A, 76 ± 6 vs 29 ± 5, P < 0.01). CTL activity to HBV classIpeptide pulsed target cells is also stronger in mice immunized with HBeAg-HSP than in mice immunized with HBeAg (Figure 2B, 44 ± 5 vs 30 ± 6 in E:T > 50:1, P < 0.05). A specific effect on T cell response is also obtained after stimulation with HBV classIpeptide in comparison with CT26 classIpeptide and TrampC-2 classIpeptide stimulation (Figure 2C). A stronger CTL activity to HBV classIpeptide pulsed target cells was shown in comparison with target cells pulsed with CT 26 classIpeptide and TrampC-2 classIpeptide (Figure 2D). T cell response to HBV classIpeptide and CTL activity to HBV classIpeptide pulsed target cells proved cytotoxic T cell activity.
Figure 2 A: Comparison of T cell proliferation between different groups after stimulation with HBV classIpeptide (C57BL/6 mice); B: Comparison of CTL activity to HBV classIpeptide pulsed target cells between different groups (C57BL/6 mice); C: Comparison of T cell proliferation between stimulation with HBV and with non-related classI peptide (Balb/c mice); D: Comparison of CTL activity to HBV classIpeptide pulsed target cell between HBV and non-related classIpeptide (Balb/c mice).
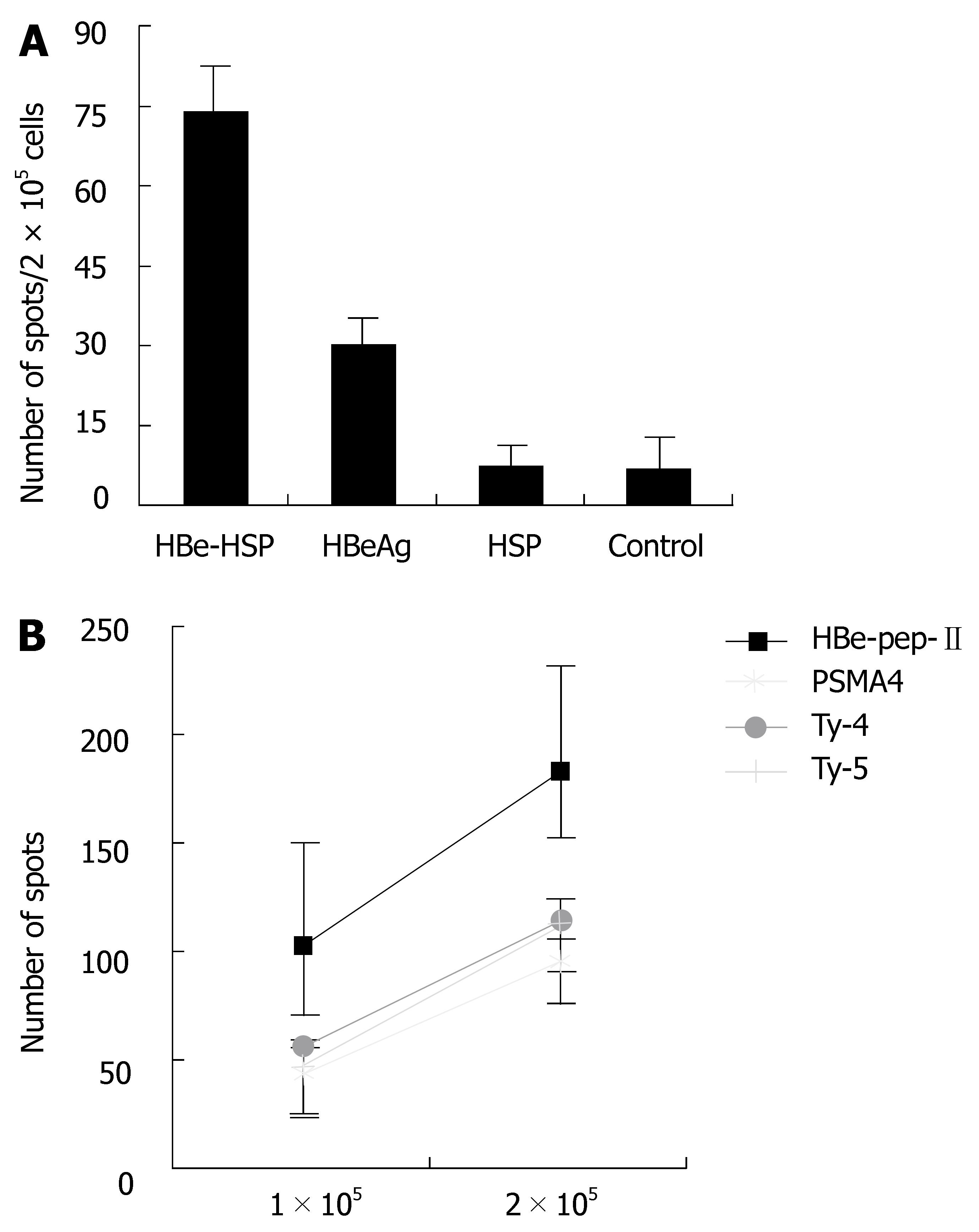
To evaluate helper T cell activity, the effects of HBV MHC class II peptides on T cell response and CTL activity were studied. Splenocytes were stimulated with HBV class II peptides for T cell response. A stronger T cell response was obtained from HBeAg-HSP DNA vaccine immunized mice in comparison with that in mice immunized with HBeAg (A, spots 74 ± 9 vs 31 ± 6, P < 0.01) and HSP DNA vaccine (Figure 3). A specific stronger T cell response by HBV class II peptide stimulation was shown in comparison with that by PSMA4 class II peptide, Tyrosin-4 and Tyrosin-5 class II peptide stimulation (Figure 3B).
Figure 3 A: Comparison of T cell proliferation between different groups after stimulation with HBV class II peptide (C57BL/6 mice); B: Comparison of T cell proliferation after stimulation with different class II peptide (Balb/c mice).
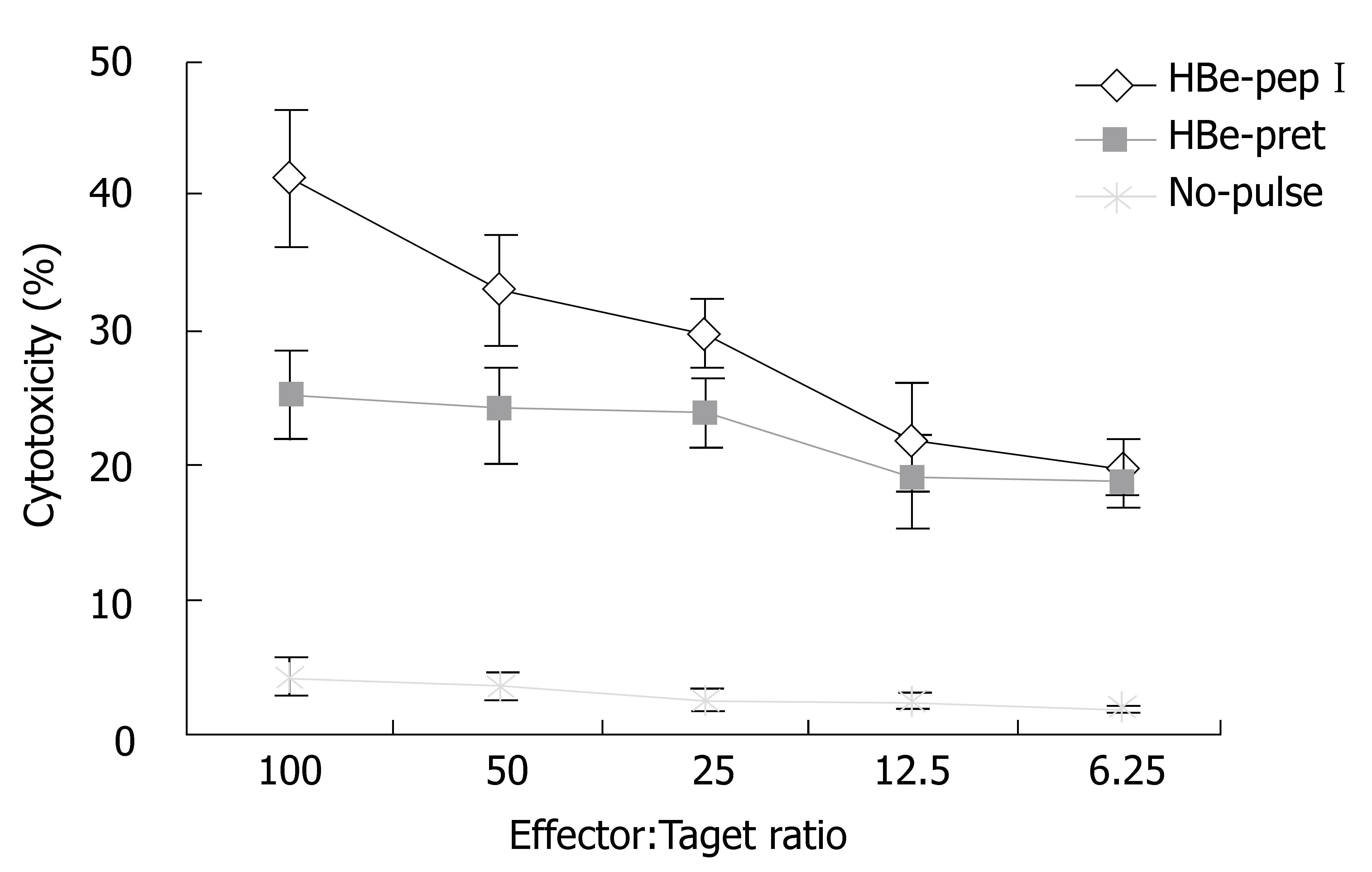
We also studied whether HBV related proteins and classIpeptides can increase target cell antigenicity. Results suggested that splenocytes from mice immunized with HBeAg-HSP DNA vaccine have a stronger CTL activity to target cells pulsed with HBV protein and HBV classIpeptide in comparison to non-target cells and CT 26 cells (Figure 4).
Figure 4 Comparison of CTL activity to target cells pulsed with different agents (Balb/c mice).
Serum antibody response to HBeAg antigen after DNA vaccination
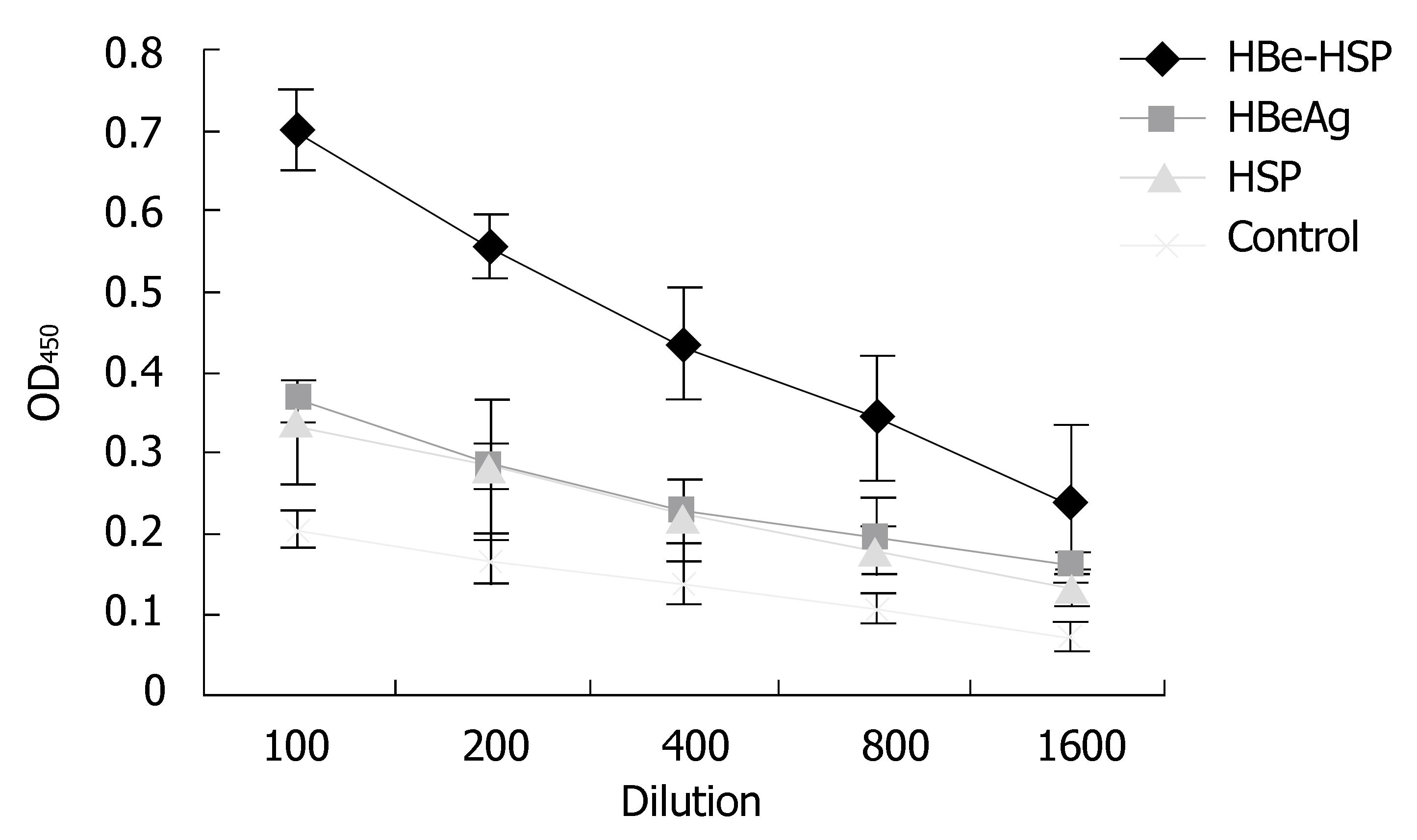
To determine whether HBeAg-HSP DNA vaccination can also induce an antibody response to HBeAg antigen, we measured serum anti-HBeAg antibody responses by ELISA assay. As shown in Figure 5, antibody levels detected in mice immunized with the HBeAg-HSP DNA vaccine were markedly higher than those immunized with the HBeAg or the HSP DNA vaccines (P < 0.05). The results indicate the superiority of HBeAg-HSP in inducing humoral immunity.
Figure 5 Comparison of antibody titers to HBV between different groups after immunization (Balb/c mice).
Systemic immunity enhancement in vivo by HBeAg-HSP DNA vaccine
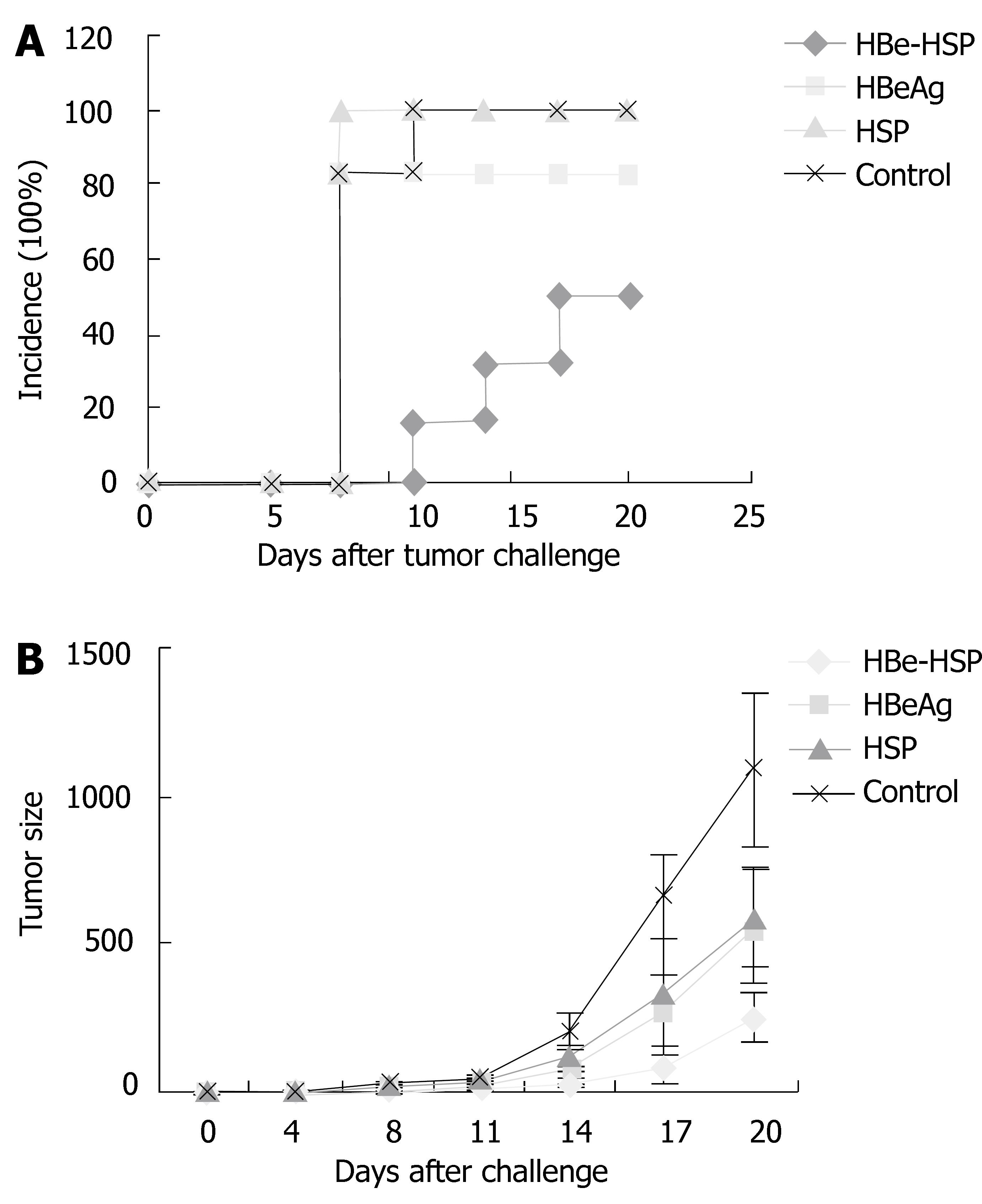
To evaluate systemic immunity enhancement in vivo by the HBeAg-HSP DNA vaccine, Balb/c mice (10 mice/group) were challenged by CT26 cells transfected with HBeAg to observe the antitumor effect after different DNA vaccine immunizations. CT26-HBeAg cells were injected s.c. at 5 × 105/mouse and tumor incidence and tumor growth were monitored. Results showed that there is a low tumor incidence in mice immunized with HBeAg DNA vaccine and a lower tumor incidence in HBeAg-HSP DNA vaccinated mice (Figure 6A), the incidence of tumor are 6/10 in HBeAg-HSP group, 8/10 in HBeAg group and 10/10 in the other two groups. Tumor growth was slowest in mice immunized with the HBeAg-HSP DNA vaccine (Figure 6B). The results suggested that HBeAg-HSP DNA vaccination can induce a stronger immune response to the related antigen.
Figure 6 A: Tumor incidence after CT26-HBeAg challenge of Balb/c mice; B: Tumor growth after CT26-HBeAg challenge of Balb/c mice.
DISCUSSION
HBV infection is a major human health problem and it is associated with a risk of developing liver cirrhosis or hepatocellular carcinoma[1,2]. Thus, effective preventive and therapeutic strategy to chronic HBV infection has been a major exploration[15,16]. Only a small proportion of patients with chronic HBV infection benefit from a treatment with interferon-α (IFN-α)[2]. Antigen-based vaccines have some disadvantages, such as the possibility of reversion to a virulent form, especially in immunocompromised individuals; whole-killed or subunit vaccines do not induce intracellular synthesis of antigen because there is poor or absent presentation of antigen on classIMHC and thus poor induction of a CTL response[17]. DNA-based vaccination is an efficient new technique to stimulate specific immune responses and specific for HBV antigen to induce a strong humoral and cell-mediated immunity against HBV infection[18,19]. HBV(HBsAg, HBc/eAg) DNA vaccine has been popularly studied for prophylaxis or therapy against HBV infection[15,16,20-24].
DNA vaccine has made an attractive alternative to conventional methods of vaccination. HBV DNA vaccination can induce CD8+ T cells as well as a dominant Th1 phenotype among the splenic lymphocytes, so eliciting strong CTL and protective levels of antibody[25-28]. Antigen-presenting cells (APCs) play a key role in induction of immune responses by DNA vaccines. DNA vaccines express native protein antigens in situ which can be recognized by B cells and presented by MHC classIand II molecules to prime helper T cells and CTLs. Dendritic cells (DCs) are usually thought of as a specific APCs for T cell and B cell activation and regulation of antibody synthesis, presentation of antigen by DCs is a potent stimulus to immune response, particularly to cell-mediated immunity and the development of CTLs. Thus, DCs are critical for initiating and modulating B and T cell responses elicited by DNA vaccination[29-31]. However, only a very limited fraction of injected DNA molecules is taken up by DCs, the intracellular antigens expressed by DCs are difficult to be processed and presented to MHC class II[32].
In this study, we designed a novel DNA vaccination strategy to enhance uptake and presentation of antigen by DCs, specifically, we developed a DNA vaccine based upon the expression of the HBV e antigen fused to HSP, which are versatile immune regulators that chaperone peptides for MHC classIand II presentation by DCs. The abilities of HSP include: to chaperone peptides, including antigen peptides; to interact with antigen presenting cells through a receptor; to stimulate antigen presenting cells, such as DCs to secrete inflammatory cytokines; and to mediate DC maturation[14]. The HSP70 peptide complex has been shown to elicit CD4+ helper T cells and CD8+ cytotoxic T cells and has been used for inducing antitumor immunity and for therapy of infectious diseases[33-37]. The novel vaccination strategy-HBeAg-HSP we developed has been shown to induce a stronger CTL activity, T cell proliferative response, and antibody response than that of HBeAg DNA vaccine. Moreover, it also showed a stronger anti-tumor immunity to tumor with HBV antigen challenge than that of the HBeAg DNA vaccine. To date, many kinds of cancer vaccines have been tested worldwide and have shown their own advantages. HSP-based cancer vaccine is one of the outstanding representatives[38]. HSP complexes isolated from tumor have been shown to induce specific anti-tumor immunity, HSP alone can also induce non-specific immunity[39]. Recent works by Enomoto and Chan indicated HSP70 based vaccine possess superior properties such as stimulation of DC maturation and T cell proliferation[40,41]. HSP vaccine has been extensively tested in animals and more recently in clinical trials[42,43]. HSP vaccine can induce immune responses against mutated tumor-specific antigens, as well as normal self-antigens. Immune responses to self-antigens by HSP may thus produce damage to normal tissues, however, there are no reports about toxic side effects in mouse models or clinical trials with HSP[44,45].
It is important that exploit of effective DNA vaccination to induce HBV specific immune response to clear HBV infection. The results of this study demonstrate the broad enhancement of antigen-specific CD4+ helper, CD8+ cytotoxic T-cell, B-cell response, and specific anti-tumor immunity by this DNA vaccination strategy, which may be superior to currently described HBV DNA vaccination for the treatment of chronic HBV infection.
COMMENTS
Background
Chronic HBV infection is associated with serious complications as a result of long-term sequelae such as liver cirrhosis or hepatocellular carcinoma. The host immune response to HBcAg and HBeAg appears critical in both viral clearance and clinical resolution. DNA vaccination that can induce both cellular and humoral immune responses has become an attractive immunization strategy against chronic HBV infection. However, a major problem of DNA vaccine is its limited potency, because only a very limited fraction of injected DNA molecules are taken up by DCs. In this study, we describe a novel DNA vaccination strategy to enhance uptake and presentation of antigens by DCs.
Research frontiers
A DNA vaccination based upon expression of the HBV e antigen fused to a heat shock protein (HSP) was developed, this study demonstrate that this DNA vaccination strategy may be superior to currently described HBV DNA vaccination for the treatment of chronic HBV infection.
Innovations and breakthroughs
DNA vaccine has made an attractive alternative to conventional methods of vaccination. In this study, we designed a novel DNA vaccination strategy to enhance uptake and presentation of antigen by DCs, it may be superior to currently described HBV DNA vaccination for the treatment of chronic HBV infection.
Applications
HSP vaccine has been extensively tested in animals and more recently in clinical trials. The results in this study suggested that HBeAg-HSP DNA vaccine may be superior to currently described HBV DNA vaccination for the treatment of chronic HBV infection.
Terminology
DNA vaccine has made an attractive alternative to conventional methods of vaccination. HBV DNA vaccination can induce CD8+ T cells as well as a dominant Th1 phenotype among the splenic lymphocytes, so elicit strong CTL and protective levels of antibody. The novel vaccination strategy-HBeAg-HSP we developed has been shown to induce a stronger CTL activity, T cell proliferative response, and antibody response than the HBeAg DNA vaccine, and it also showed a stronger anti-tumor immunity to tumor with HBV antigen challenge than that of HBeAg DNA vaccine.
Peer review
The novel vaccination strategy-HBeAg-HSP was studied and it is one of the outstanding representatives for the treatment of chronic HBV infection. Moreover, it will be interesting in the treatment of cancer in future. It is deserved to be published.