Published online Nov 28, 2007. doi: 10.3748/wjg.v13.i44.5902
Revised: January 9, 2007
Accepted: September 24, 2007
Published online: November 28, 2007
Defining translational research is still a complex task. In oncology, translational research implies using our basic knowledge learnt from in vitro and in vivo experiments to directly improve diagnostic tools and therapeutic approaches in cancer patients. Moreover, the better understanding of human cancer and its use to design more reliable tumor models and more accurate experimental systems also has to be considered a good example of translational research. The identification and characterization of new molecular markers and the discovery of novel targeted therapies are two main goals in colorectal cancer translational research. However, the straightforward translation of basic research findings, specifically into colorectal cancer treatment and vice versa is still underway. In the present paper, a summarized view of some of the new available approaches on colorectal cancer translational research is provided. Pros and cons are discussed for every approach exposed.
- Citation: Gil-Bazo I. Novel translational strategies in colorectal cancer research. World J Gastroenterol 2007; 13(44): 5902-5910
- URL: https://www.wjgnet.com/1007-9327/full/v13/i44/5902.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i44.5902
In the current century, despite the recent achievements in the treatment of advanced colorectal carcinoma (CRC), this tumor remains a major public health concern. In fact, it comprises the third most common cancer type to occur in men and women and was the second leading cause of death among cancer patients in the United States during 2006[1].
Different surgical approaches can guarantee low recurrence rates and high survival expectancy in stagesIto III colon neoplasm patients[2]. Furthermore, adjuvant chemotherapy administration has been shown to effectively improve those rates[3]. However, the subset of stage II colon cancer patients to whom adjuvant therapy should be offered is still to be addressed[4]. In fact, different molecular pathology studies and genomic/proteomic investigations are working on that task[5].
In contrast, metastatic colorectal cancer is still far away from being a curable condition and the main goals in the treatment of stage IV colorectal cancer are to decrease tumor-related symptoms or, alternatively, to prolong symptom-free survival with tolerable toxicity[6,7]. However, the emergence of the highly selective therapeutic antibodies bevacizumab and cetuximab has definitely improved the survival of patients with metastatic CRC[8,9]. This fact has intensively boosted the search for other targeted therapies directed to other fundamental checkpoints in colorectal tumorigenesis[10,11].
Thus, due to colorectal cancer clinical and economic relevance, its basic and clinical research has become one of the most funded among all tumor types in most developed countries. However, the straightforward translation of basic research findings into colorectal cancer therapies is still underway.
In the present paper, a summarized view of some of the new available approaches on colorectal cancer translational research is provided.
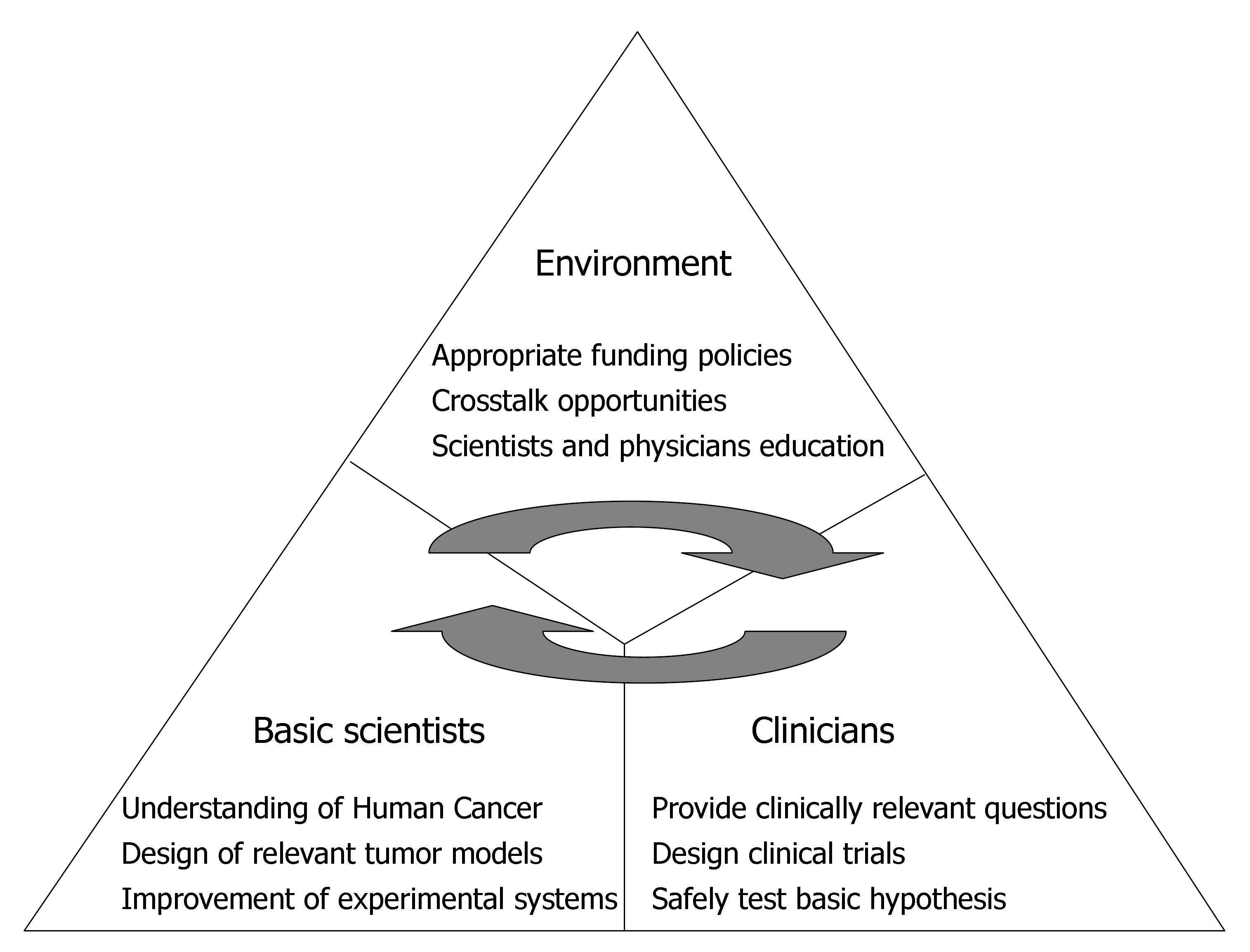
Translation of the exciting novel findings made in basic laboratories into testable hypotheses for evaluation in clinical trials is the ultimate aim of translational research in oncology[12-14]. Between a laboratory breakthrough and a real achievement in the clinic, there must be translational research. Thus, the job of the translational researcher is to take the knowledge gained in the laboratory and lay the groundwork needed to develop a new diagnostic tool for a human tumor or a novel drug to be tested in a clinical trial in human beings (Figure 1).
In other words, in order to improve human health, scientific discoveries must be translated into practical applications. Such discoveries typically start at “the bench” with basic research, in which scientists study disease at a molecular or cellular level[15-19], and then move on to the clinical level, or the patient’s “bedside”[20-22]. Scientists are increasingly aware that this bench-to-bedside approach to translational research should really be a two-way highway (Figure 1). Basic scientists provide clinicians with new tools to be used in patients and for assessment of their impact whereas physician-scientists formulate the clinically relevant questions to be tested by basic researchers in a better controlled and more simplified system. Actually, discoveries travel from the clinic to the laboratory in the form of clinical observations, human tissue, diagnostic images, and blood samples, which researchers use to further unlock the molecular and cellular features of cancer (Figure 1).
Often, translational research involves animal studies designed to mimic human conditions[23-26]. Such studies are generally performed with the same care and scrutiny as the best-planned human clinical trials, and comprise a complex set of supporting laboratory techniques that aim to determine how and why the new diagnostic tool or therapy works or fails in these models. Translational research studies may involve many years of investigation on tools and techniques, to try to estimate how safe and how effective the new treatment or diagnostic procedure will be in human trials.
One of the main scopes of translational research in cancer implies the identification and characterization of molecular markers[12]. These can be employed as diagnostic and prognostic tools but also for drug responsiveness assessment or even for targeted therapy design. Molecular markers of tumor responsiveness to drugs would help to select the patient populations that would most likely respond to the drug and identify therapeutic indications. Molecular markers of drug activity in normal tissue would allow pharmacodynamic monitoring of patients that could aid optimization of drug dosing and scheduling to maximize patient response[27]. Furthermore, biological markers involved in tumor initiation and progression can be specifically targeted by new drugs such as therapeutic antibodies[8,9] or anti-tumor vaccines[18].
In fact, another main goal in cancer research is targeted therapy[22]. Translational research is particularly feasible now because of the new understanding of what causes cancer in different individuals, which relates to different combinations of genetic events. This understanding has come primarily from the work of basic research scientists. Until fairly recently, the only effective armamentaria in cancer therapy were surgery, radiation therapy, and chemotherapy. These treatments generally affect neoplastic cells but also non-cancer tissues, leading to the often serious toxicity that characterizes most of traditional cancer treatments[28]. While these standard therapies will continue to play an important role in the treatment of patients with cancer, they can be vastly aided in this process by targeted drugs, which literally target the aberrant molecular pathways that are actually involved in tumor initiation and progression. Therefore, specifically delivering the targeted drug to the malignant cell and its closest environment can significantly relieve cancer treatment related collateral effects[27].
However, since extensive libraries of cytotoxic compounds are being developed for antitumor effect testing, it is becoming more and more common to find new therapies that are successfully developed, tested and commercialized against certain tumors but the ultimate molecular mechanisms involved in tumor response are not clearly known[29]. In those cases, the translational process is rather directed from clinical findings to basic cellular and molecular experiments (from “the bedside” to “the bench”), trying to unravel the complex pathway in which the new compound is playing a definitive role and the specific target or group of them that results inhibited. Therefore, the bidirectional nature of translational research needs to be emphasized[30].
There is still a widening gap between basic research and clinical practice, particularly for colorectal cancer. This might be due to the genetic and molecular complexity of this tumor, the lack of the ideal in vivo model for colorectal cancer, and the difficulties found in reproducing animal results into clinical trials in patients.
The principal directions toward which translational research has spread and grown in colorectal cancer in recent years are genomics and proteomics, oncogenic pathways assessment and new targeted therapies discovery (Table 1).
| Genomics | Proteomics |
| DNA microarrays | 2D-PAGE |
| DIGE | |
| LC-MS/MS | |
| ICAT | |
| iTRAQ | |
| Protein microarrays | |
| MALDI-TOF | |
| SELDI-TOF | |
| Tissue microarrays | |
| Oncogenic pathways | Preclinical models |
| AS-ODN | Min mice |
| miRNAs | Msh2, Msh4, Msh6 deficient mice |
| siRNAs | Apc163 8N mice |
| Smad4/Apc mice |
In the last years, there has been an increasingly high effort in the use of genome information in biomedical sciences. This genome information has greatly expanded the insight into the genetic basis of cancer, comprising one of the main fields of interest in translational cancer research. Traditional methods of identifying novel targets involved in cancer progression were based on studies of individual genes. The following understanding, however, has also shown that gene analysis alone is not sufficient to explain why cancer appears and progresses[31].
Now, the use of DNA microarrays facilitates the analysis of the expression of thousands of genes at the same time and rapidly[32,33]. Microarray analysis has been used for gene expression analysis of different neoplasms[34,35], including CRC[36-39]. However, the application of DNA microarray technology for analysis of CRC is of limited value since it fails to offer direct protein expression measurements[36,40]. In addition, it is already known that important pathways in colon tumorigenesis are regulated at the posttranscriptional level where RNA expression data cannot offer any further information. In fact, due to the alternative splicing of both mRNA and proteins, combined with protein posttranslational modifications, one gene can encode a considerable protein population. Actually, the proteome comprises all proteins that result from the whole genome. In contrast to the genome, the proteome is rather a dynamic parameter constituted by proteins and reflects both the intrinsic genetic program of the cell as well as the impact of its surrounding environment.
However, only a few studies have looked for a further insight into the function and/or importance of individual genes and their application to the proteome research of a tumor. Some of these genes have been proposed as candidate cancer biomarkers[41-43]. More recently a number of proteomic studies have also addressed the identification of potential targets in CRC[44-46].
In the proteomics field, several different technical strategies have been developed and applied to CRC translational research over the last years. Each one has its own advantages and drawbacks that should be considered before deciding the experimental design[47].
The technique leading the field for a long time was the two-dimensional polyacrilamide gel electrophoresis (2D-PAGE)[48]. The 2D-PAGE is based on the separation on a gel of the protein content of a sample in two dimensions according to mass and charge. The gels are stained and spots in samples are compared among different gels. However, a number of serious disadvantages such as its lack of real high-throughput capability (one sample per gel) is responsible for having been replaced by more advanced and capable techniques. Similar to 2D-PAGE, the two-dimensional difference gel electrophoresis (DIGE)[44,46] strengthened the 2D platform by allowing the detection and quantization of differences between three samples resolved on the same gel, or across multiple gels, when linked by an internal standard. Again, it also is a low-throughput technology that does not permit the comparison of many samples in a feasible manner.
Other low-throughput proteomic techniques have recently evolved for cancer protein profiling such as liquid chromatography coupled to tandem mass spectrometry detection (LC-MS/MS)[49], isotope-coded affinity tag (ICAT)[50] and a variation of the latter, isotope tags for relative and absolute quantification (iTRAQ)[51], (both consist of a differential tagging of proteins from samples that are compared using isotope-coded affinity tag in an isotope-dilution mass spectrometry experiment).
A study conducted by Wu et al[52] has recently compared some of these diverse proteomic strategies (2D-DIGE, ICAT and iTRAQ) on HCT-116 colon epithelial cells concluding that regarding the number of peptides detected for each protein by each method, the global-tagging iTRAQ technique was more sensitive than the cysteine-specific ICAT method, which in turn was as sensitive as, if not more sensitive than, the 2D-DIGE technique.
Nevertheless, as aforementioned, one of the most important goals in protein profiling in oncology is the discovery of new biomarkers[53]. The use of molecular markers in translational research has expanded considerably during the last 3 decades, and this increased analysis of specific molecular changes has been associated with a concomitant decline in the use of more general and less specific histochemical stains and biochemical assays. Some of the applications for molecular markers include diagnosis, early detection, and prognosis. Also, specific molecular markers are used to study the biology of the disease, to identify targets for novel therapies (e.g., use of Herceptin), and to aid the selection of specific therapies, as previously mentioned.
Therefore, cancer proteomic studies might identify disease-related biomarkers for early cancer diagnosis and new surrogate biomarkers for therapy efficacy and toxicity, but also for guidance of optimal anticancer drug combinations, enabling tailor-made therapy[54]. Furthermore, they could lead to new pharmacological targets. However, a crucial requisite for this purpose is to be able to perform a systematic analysis of a large number of proteins in an easy, reproducible, time-efficient and cost-effective way. High throughput technologies are therefore warranted.
Protein microarrays for instance[55], (targeted proteins bind to spotted probes on a “forward” microarray and specific probes bind to targeted proteins in spotted samples on a “reverse” microarray; bound proteins are detected by direct fluorescent labeling or by labeled secondary antibodies), provide a high throughput approach in terms of number of probes per “forward” array and number of samples per “reverse” array with the advantage of previously knowing the biomarker identity. On the other hand, the synthesis of many different probes is necessary, the identity of biomarkers has to be known and cross-reactivity of probes along with possible impaired binding of proteins with post-translational modifications (PTM) exists.
In 2002, the Nobel committee acknowledged the advances in mass spectrometry of biopolymers with the recognition of the discovery of electrospray ionization (ESI) mass spectrometry[56,57] and for the discovery of soft laser desorption (SLD) ionization, which led to the development of matrix-assisted laser desorption ionization (MALDI)[58]. These discoveries for peptides, proteins and other macromolecules have been revolutionary, providing easy measurements of molecular weight with unprecedented accuracy. Because the dominant ions generated under SLD and MALDI conditions are singly charged, the technique is most often used in combination with a time-of-flight (TOF) analyzer to extend the m/z range to 100000 Da and beyond[58]. MALDI-TOF technology is a highly capable tool allowing the measurement of up to 1536 samples per plate, also possessing access to PTM. On the negative side, this technique is unsuitable for high molecular weight proteins (> 100 kDa) and sample fractioning is needed when measuring complex samples.
Surface-enhanced laser desorption ionization time-of-flight (SELDI-TOF) technology is a variant of MALDI-TOF in which a selected part of a protein mixture is bound to a specific chromatographic surface and the rest is washed away[47]. Although SELDI-TOF technology only permits 96 samples to be tested by bioprocessor, fractioning of the sample is not necessary and direct application of the whole sample is possible. However, compared to MALDI-TOF, SELDI-TOF provides lower resolution and mass accuracy but requires smaller amounts of starting material. SELDI-TOF is also unsuitable for proteins heavier than 100 kDa.
SELDI-TOF is equally useful for the analysis of cell lysates from cell lines and tissue[59], however, in clinical practice its ultimate value derives from its application to easily accessible body fluids as serum or urine. In fact, in the last years several serum biomarker proteins have been identified through this technical approach[60-62].
In addition, low and high throughput techniques have been shown to be complementary and its combination can lead to a more efficient outcome[63].
In summary, compared to the genome, the proteome provides a more reliable picture of a biological status and is, thus, expected to be more useful than gene analysis for evaluating, for example, disease presence, progression and response to treatment.
A totally different approach for protein profiling has recently emerged in translational cancer research. To evaluate the clinical significance of newly detected potential cancer genes, it is usually required to examine a high number of well-characterized primary tumors. Using traditional methods of molecular pathology, this was a time consuming job that exploited precious tissue resources. However, a high throughput tissue analysis approach, [tissue microarray (TMA) technology], has been developed[64-66]. Using this TMA technology, samples from up to 1000 different tumors are arrayed in one recipient paraffin block, sections of which can be used for all kinds of in situ analyses[22,67].
Sections from TMA blocks can then be utilized for the simultaneous analysis of DNA, RNA or protein tumor levels. TMA protein analysis has also been performed in CRC samples for prognostic evaluation[68-72]. However, even though it has been suggested that minute arrayed tissue specimens are representative of their donor tissues, highly heterogeneous cancer types and low levels of protein expression could account for underestimating determined protein expression levels in certain tumors[68,73].
There are multiple different types of TMAs that can be utilized in cancer research including multi tumor arrays (containing different tumor types), tumor progression arrays (tumors of different stages) and prognostic arrays (tumors with clinical endpoints). The combination of multiple different TMAs allows a very quick but comprehensive characterization of biomarkers of interest.
Despite what proteomics have added to translational research in cancer, there are some novel approaches that combine the information provided by genomic and proteomic assays run in parallel in order to complement the translational impact of both procedures[74]. This has also been applied to CRC profiling. Kwong et al[75], for instance, studied gene and protein expression performed in parallel across progressive stages of human CRC. For this purpose, they applied cDNA microarray and 2D-PAGE technologies in parallel to analyzed samples collected from 60 CRC cases at various stages of disease progression. Of 47 genes analyzed, 12 (26%) showed significant correlation between mRNA level and protein levels, suggesting that protein abundance is regulated at the transcriptional level. The remaining 31 genes showed either a non-significant correlation between mRNA and protein expression levels or, in 28% of the genes, a negative correlation. Therefore, the authors conclude that posttranscriptional mechanisms play an important role in the regulation of gene products activities in CRC, underline the importance of analyzing gene expression at multiple levels and claim that genomic and proteomic approaches actually complement each other.
In another recent study to identify new biomarkers, Madoz-Gurpide et al[76] investigated the feasibility of expressing soluble proteins corresponding to up-regulated genes in surgically resected CRC samples. They used cDNA microarrays (CNIO Oncochip)[77] to identify differentially expressed genes in malignant compared to normal samples isolated from 22 different CRC patients. After investigating different sources of cDNA clones for protein expression, from 29 selected genes, 21 different proteins were finally expressed soluble with, at least, one distinct fusion protein. Additionally, seven of these potential markers were tested for antibody production and/or validation, confirming six of them to be overexpressed in CRC tissues by immunoblotting and TMA analysis[76]. Authors suggest that this kind of approach may provide relevant biological information of the neoplastic processes and lead to a better characterization of potentially interesting markers in a quite straightforward way for early diagnosis or individualized prognosis assessment.
The previously reviewed development of genomics and proteomics in cancer research has yielded an uncountable number of new potential oncogenic mediators and checkpoints, in CRC, worth further investigating. These novel gene-depending elements, potential new targets for future drugs, are commonly involved in a variety of molecular pathways and their intimate upstream/downstream regulators as well as their crosstalk networks and functional relevance still need to be addressed.
Most widely used experimental methods for molecular pathway research in oncology are performed on fairly well-controlled in vitro systems. Recent cell biology achievements and discoveries however, have led to more reliable and physiologically relevant settings where observations on cell behavior and cell fate under particular conditions can be imported into in vivo experiments employing animal cancer models and even translating findings into new human therapeutic trials.
In the last few years, several approaches to find molecules able to inhibit the expression of genes (so-called gene-silencing molecules) involved in colorectal cancer progression and therapeutic resistance have been pursued. Sequence-specific gene suppression strategies using antisense oligonucleotides (AS-ODN), ribozymes and deoxyribozymes were initially described and developed[78-82]. AS-ODN derivatives, depending on their type, recruit RNase H to cleave the target mRNA or inhibit translation by steric hindrance. Ribozymes though, directly bind to RNA via Watson-Crick base pairing and cleave the phosphodiester backbone of the RNA target by transesterification. Similarly, deoxyribozymes also bind to their RNA substrates via Watson-Crick base pairing and specifically cleave the target RNA.
Currently, in addition to their value in target valida-tion studies, different AS-ODN strategies are under evaluation in phase II and III clinical trials, particularly in hematological malignancies, malignant melanoma and prostate cancer[83,84]. However, consolidating AS-ODN as a broadly applicable functional genomic and therapeutic tool has proven difficult. For instance, difficulties in delivery of the AS-ODN into target tissues, instability of AS-ODN in vivo, poor oral availability, uncertainties about the precise mode of action, and toxic effects in animal and human studies have been argued[80,83]. Moreover, a number of class effects are observed with AS-ODN that are unrelated to the specific targeted mRNA sequence. Acute effects include activation of the alternative complement pathway and inhibition of the intrinsic coagulation pathway. In fact, given repeated doses of AS-ODN to animals, accumulation of AS-ODN and/or metabolites occurs in the form of basophilic granules in various tissues, including the kidney, lymph nodes and liver. Although several approaches are known to overcome some of these difficulties[85], very few contributions have firmly supported the use of AS-ODN technology in CRC research[86-88].
But in the field of gene-silencing molecules, the most recent and fascinating tools discovered for studying gene regulation and gene expression control are microRNAs (miRNAs) and small interfering RNA (siRNAs). miRNAs and siRNAs are typically 21 to 25 nucleotide RNA molecules that induce gene silencing by RNA interference (RNAi)[89-91]. Since the description of RNA interference (RNAi) in 1998[92], this gene-silencing technology has been developed into a widely used methodology in basic as well translational research. RNAi was originally discovered as a naturally occurring pathway in plants and invertebrates[92]. Once long double-stranded RNA molecules are inserted into these organisms, they are processed by the endonuclease Dicer into siRNAs. These siRNAs are subsequently incorporated into the multicomponent RNA-induced silencing complex (RISC), which unwinds the duplex and uses the anti-sense strand as a guide to look for homologous mRNAs and degrade them, as previously reviewed by others[93,94]. More strikingly, synthetic short siRNAs (20-25 bp) can be either delivered exogenously or expressed endogenously from RNA polymerase II or III promoters (in the form of siRNAs or short hairpin (sh)RNAs that are processed by Dicer into functional siRNAs) and used as a new powerful technology for achieving specific down-regulation of target mRNAs in mechanistic research or even therapeutic development in CRC[11,95-98].
Once potential targets are discovered and their expression is successfully inhibited in vitro, the safety, efficacy and feasibility of their inhibitors need to be evaluated in animal models in which human disease can be faithfully reproduced. In fact, in the last years, the need of relevant in vivo models in colorectal cancer research has prompted many investigators to work on developing reliable, reproducible and human colorectal cancer-mimicking animal models[25,99,100].
However, in colorectal cancer, much has been learned from human inherited syndromes, such as familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC)[101-103]. That knowledge in fact, has been translated into the design and development of CRC animal models.
Although several rat models have been created for the study of colorectal cancer[104-106], in this review, we will focus our attention on mouse models which have profusely evolved in the last few years because of their abundant genetic/genomic information, and easy mutagenesis using transgenic and gene knockout technology. Genetically engineered mice have become essential tools in both mechanistic studies and drug development in CRC, as previously reviewed by others[107]. In fact, mice provide unique opportunities to define and identify genes that are involved in colorectal cancer progression.
The first mouse model obtained to carry a mutation in the adenomatous polyposis coli (APC) tumor suppressor gene was named multiple intestinal neoplasia (Min)[108]. The Min mutation results in a truncated protein and induces the development of multiple intestinal adenomas (even more than one hundred) and a reduced lifespan of on average 150 d in heterozygous mice. Posterior models carrying mutations in different APC alleles have also been developed and each one possesses its own clinical manifestations. However, the majority of them shows small intestine adenomas and colonic tumors and distant metastases are rarely observed. Interestingly, it has been shown that different mutations in the APC gene, in Apc1638N mice for instance, confer distinct tumor susceptibility phenotypes and that fact resembles the heterogeneity observed in human FAP families[109]. Other models of hereditary non-polyposis colorectal cancer (HNPCC) have been developed through the mutation of several mismatch repair genes. One representative example are Msh2 deficient mice that are fertile and develop normally, however, these animals develop T-cell lymphomas early in their life and die because of the disease. Msh2 deficient mice that survive more than 6 months develop gastrointestinal adenomas, carcinomas and skin tumors and can also be used for tumorigenesis studies[110].
Finally, other more recent models have also been developed to better study colorectal cancer. Smad4 heterozygous mice bearing Apc mutations present an enhanced progression and a more malignant phenotype[111]. Other combinations responsible for increased gastrointestinal tumorigenesis are APC and oncogenic KRAS that seem to be synergistic in enhancing Wnt signaling[112].
Translational research is a key developing field in biome-dicine. The direct application of basic research findings to the patient’s diagnosis and treatment is even more important in cancer. In addition, clinical observations can dramatically contribute to basic research improvement and relevant enhancement. Colorectal cancer, due to its epidemiological importance and economic impact, is one of the main entities in which translational research is a reality today.
However, there still is a long way to go until basic researchers and clinical investigators share information and work together in colorectal cancer research on a daily basis.
Several new technologies and tools have demonstrated a great value in cancer and are in fact responsible for the last crucial pieces of research work allowing a new conception of cancer diagnosis and treatment. Among them, the development of new biomarkers for colorectal cancer combining proteomics and genomics is especially relevant.
Also, anti-sense strategies have recently opened the path for new target-specific therapy development. These new therapeutic discoveries need to be tested in preclinical animal models.
Since extensive validation of the above mentioned research fields is necessary, adequate funding is required. This may imply some adjustments in the current funding policy because it involves non-innovative studies. Furthermore, the pool of researchers/clinicians capable of performing translational research must be increased. Additionally, there should be an enhanced participation of patients in clinical trials and an optimization of the efficiency of these trials using validated surrogate markers. Only when these conditions are fulfilled the 'post-genomic' era of biomedical research will have unprecedented opportunities to innovate and improve therapy for cancer.
In the present paper, a summarized view of some of the new available approaches on colorectal cancer translational research is provided. Translational research in colorectal cancer comprises the identification and characterization of new molecular markers and the discovery of novel targeted therapies. The better understanding of human cancer and the design of more reliable tumor models and more accurate experimental systems is also part of translational research in cancer.
The principal directions toward which translational research has spread and grown in colorectal cancer in recent years are genomics and proteomics, oncogenic pathways assessment and new targeted therapies discovery.
To our knowledge, there is no other published paper specifically focused on translational research in colorectal cancer. Therefore, we consider this review as a unique and inspiring one.
The main objective of this manuscript is to help scientists and physicians working on colorectal cancer determine which findings have been already achieved and which others are still underway and provide a better knowledge of new tools and techniques available for this purpose. This focus might inspire other authors in their own research projects and emphasize the need of a new approach to colorectal cancer research.
Translational research: Investigation directed to the link of basic and clinical research in order to better define aims and better control tools and experimental systems. Genomics: Part of the bioscience that studies the genome and its implications in disease appearance, progression and response to treatment. Proteomics: Part of the bioscience responsible for peptide and protein investigation and their role in the diagnosis, treatment and research of disease. Targeted therapies: Group of drugs specifically designed to a certain target of the tumor cell such as growth factor receptors, membrane proteins and others.
This manuscript is a very good and complete review of the topic exposed.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4370] [Cited by in RCA: 4261] [Article Influence: 224.3] [Reference Citation Analysis (0)] |
| 2. | A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2518] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 3. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2732] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 4. | André T, Sargent D, Tabernero J, O'Connell M, Buyse M, Sobrero A, Misset JL, Boni C, de Gramont A. Current issues in adjuvant treatment of stage II colon cancer. Ann Surg Oncol. 2006;13:887-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Barozzi C, Ravaioli M, D'Errico A, Grazi GL, Poggioli G, Cavrini G, Mazziotti A, Grigioni WF. Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer. 2002;94:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Aggarwal G, Glare P, Clarke S, Chapuis P. Palliative and shared care concepts in patients with advanced colorectal cancer. ANZ J Surg. 2006;76:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Griffin-Sobel JP. Symptom management of advanced colorectal cancer. Surg Oncol Clin N Am. 2006;15:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 9. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7731] [Article Influence: 368.1] [Reference Citation Analysis (1)] |
| 10. | Diaz-Gonzalez JA, Russell J, Rouzaut A, Gil-Bazo I, Montuenga L. Targeting hypoxia and angiogenesis through HIF-1alpha inhibition. Cancer Biol Ther. 2005;4:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833-842; discussion 843-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Grizzle WE, Manne U, Jhala NC, Weiss HL. Molecular characterization of colorectal neoplasia in translational research. Arch Pathol Lab Med. 2001;125:91-98. [PubMed] |
| 13. | Steinert R, Buschmann T, van der Linden M, Fels LM, Lippert H, Reymond MA. The role of proteomics in the diagnosis and outcome prediction in colorectal cancer. Technol Cancer Res Treat. 2002;1:297-304. [PubMed] |
| 14. | Takayama O, Yamamoto H, Ikeda K, Ishida H, Kato T, Okuyama M, Kanou T, Fukunaga M, Tominaga S, Morita S. Application of RT-PCR to clinical diagnosis of micrometastasis of colorectal cancer: A translational research study. Int J Oncol. 2004;25:597-604. [PubMed] |
| 15. | Fichtner I, Slisow W, Gill J, Becker M, Elbe B, Hillebrand T, Bibby M. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer. 2004;40:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Gerner EW, Ignatenko NA, Besselsen DG. Preclinical models for chemoprevention of colon cancer. Recent Results Cancer Res. 2003;163:58-71; discussion 264-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Gupta RA, DuBois RN. Translational studies on Cox-2 inhibitors in the prevention and treatment of colon cancer. Ann N Y Acad Sci. 2000;910:196-204; discussion 204-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hörig H, Wainstein A, Long L, Kahn D, Soni S, Marcus A, Edelmann W, Kucherlapati R, Kaufman HL. A new mouse model for evaluating the immunotherapy of human colorectal cancer. Cancer Res. 2001;61:8520-8526. [PubMed] |
| 19. | Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res. 2005;11:1618-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003;30:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Goldberg RM. Advances in the treatment of metastatic colorectal cancer. Oncologist. 2005;10 Suppl 3:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Tabernero J, Macarulla T, Ramos FJ, Baselga J. Novel targeted therapies in the treatment of gastric and esophageal cancer. Ann Oncol. 2005;16:1740-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cai SR, Garbow JR, Culverhouse R, Church RD, Zhang W, Shannon WD, McLeod HL. A mouse model for developing treatment for secondary liver tumors. Int J Oncol. 2005;27:113-120. [PubMed] |
| 24. | Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16-26. [PubMed] |
| 25. | Rashidi B, Sun FX, Jiang P, An Z, Gamagami R, Moossa AR, Hoffman RM. A nude mouse model of massive liver and lymph node metastasis of human colon cancer. Anticancer Res. 2000;20:715-722. [PubMed] |
| 26. | Sturm JW, Magdeburg R, Berger K, Petruch B, Samel S, Bönninghoff R, Keese M, Hafner M, Post S. Influence of TNFA on the formation of liver metastases in a syngenic mouse model. Int J Cancer. 2003;107:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Eastman A, Perez RP. New targets and challenges in the molecular therapeutics of cancer. Br J Clin Pharmacol. 2006;62:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Rojas AM, Lyn BE, Wilson EM, Williams FJ, Shah N, Dickson J, Saunders MI. Toxicity and outcome of a phase II trial of taxane-based neoadjuvant chemotherapy and 3-dimensional, conformal, accelerated radiotherapy in locally advanced nonsmall cell lung cancer. Cancer. 2006;107:1321-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Jimeno A, Kulesza P, Kincaid E, Bouaroud N, Chan A, Forastiere A, Brahmer J, Clark DP, Hidalgo M. C-fos assessment as a marker of anti-epidermal growth factor receptor effect. Cancer Res. 2006;66:2385-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Finkelstein SE, Heimann DM, Klebanoff CA, Antony PA, Gattinoni L, Hinrichs CS, Hwang LN, Palmer DC, Spiess PJ, Surman DR. Bedside to bench and back again: how animal models are guiding the development of new immunotherapies for cancer. J Leukoc Biol. 2004;76:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Craven RA, Banks RE. Laser capture microdissection and proteomics: possibilities and limitation. Proteomics. 2001;1:1200-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2351] [Cited by in RCA: 2099] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 33. | Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6477] [Cited by in RCA: 5103] [Article Influence: 170.1] [Reference Citation Analysis (0)] |
| 34. | DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, Chen Y, Su YA, Trent JM. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996;14:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1150] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 35. | Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7015] [Cited by in RCA: 6310] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 36. | Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124-3130. [PubMed] |
| 37. | Williams NS, Gaynor RB, Scoggin S, Verma U, Gokaslan T, Simmang C, Fleming J, Tavana D, Frenkel E, Becerra C. Identification and validation of genes involved in the pathogenesis of colorectal cancer using cDNA microarrays and RNA interference. Clin Cancer Res. 2003;9:931-946. [PubMed] |
| 38. | Zou TT, Selaru FM, Xu Y, Shustova V, Yin J, Mori Y, Shibata D, Sato F, Wang S, Olaru A. Application of cDNA microarrays to generate a molecular taxonomy capable of distinguishing between colon cancer and normal colon. Oncogene. 2002;21:4855-4862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Birkenkamp-Demtroder K, Christensen LL, Olesen SH, Frederiksen CM, Laiho P, Aaltonen LA, Laurberg S, Sørensen FB, Hagemann R, ØRntoft TF. Gene expression in colorectal cancer. Cancer Res. 2002;62:4352-4363. [PubMed] |
| 40. | Hegde P, Qi R, Abernathy K, Gay C, Dharap S, Gaspard R, Hughes JE, Snesrud E, Lee N, Quackenbush J. A concise guide to cDNA microarray analysis. Biotechniques. 2000;29:548-550, 552-554, 556 passim. [PubMed] |
| 41. | Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellström KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695-3700. [PubMed] |
| 42. | Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974-5978. [PubMed] |
| 43. | Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci USA. 2001;98:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 44. | Alfonso P, Núñez A, Madoz-Gurpide J, Lombardia L, Sánchez L, Casal JI. Proteomic expression analysis of colorectal cancer by two-dimensional differential gel electrophoresis. Proteomics. 2005;5:2602-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Olesen SH, Christensen LL, Sørensen FB, Cabezón T, Laurberg S, Orntoft TF, Birkenkamp-Demtröder K. Human FK506 binding protein 65 is associated with colorectal cancer. Mol Cell Proteomics. 2005;4:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Friedman DB, Hill S, Keller JW, Merchant NB, Levy SE, Coffey RJ, Caprioli RM. Proteome analysis of human colon cancer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 2004;4:793-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 275] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | Engwegen JY, Gast MC, Schellens JH, Beijnen JH. Clinical proteomics: searching for better tumour markers with SELDI-TOF mass spectrometry. Trends Pharmacol Sci. 2006;27:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Lilley KS, Razzaq A, Dupree P. Two-dimensional gel electrophoresis: recent advances in sample preparation, detection and quantitation. Curr Opin Chem Biol. 2002;6:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 49. | Johansson C, Samskog J, Sundström L, Wadensten H, Björkesten L, Flensburg J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics. 2006;6:4475-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Yan W, Lee H, Deutsch EW, Lazaro CA, Tang W, Chen E, Fausto N, Katze MG, Aebersold R. A dataset of human liver proteins identified by protein profiling via isotope-coded affinity tag (ICAT) and tandem mass spectrometry. Mol Cell Proteomics. 2004;3:1039-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Zieske LR. A perspective on the use of iTRAQ reagent technology for protein complex and profiling studies. J Exp Bot. 2006;57:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 52. | Wu WW, Wang G, Baek SJ, Shen RF. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J Proteome Res. 2006;5:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 53. | Shau H, Chandler GS, Whitelegge JP, Gornbein JA, Faull KF, Chang HR. Proteomic profiling of cancer biomarkers. Brief Funct Genomic Proteomic. 2003;2:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Alessandro R, Belluco C, Kohn EC. Proteomic approaches in colon cancer: promising tools for new cancer markers and drug target discovery. Clin Colorectal Cancer. 2005;4:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | MacBeath G. Protein microarrays and proteomics. Nat Genet. 2002;32 Suppl:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 554] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 56. | Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5583] [Cited by in RCA: 4409] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 57. | Whitehouse CM, Dreyer RN, Yamashita M, Fenn JB. Electrospray interface for liquid chromatographs and mass spectrometers. Anal Chem. 1985;57:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 851] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 58. | Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988;60:2299-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4452] [Cited by in RCA: 3504] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 59. | Melle C, Ernst G, Schimmel B, Bleul A, Mothes H, Kaufmann R, Settmacher U, Von Eggeling F. Different expression of calgizzarin (S100A11) in normal colonic epithelium, adenoma and colorectal carcinoma. Int J Oncol. 2006;28:195-200. [PubMed] |
| 60. | Engwegen JY, Helgason HH, Cats A, Harris N, Bonfrer JM, Schellens JH, Beijnen JH. Identification of serum proteins discriminating colorectal cancer patients and healthy controls using surface-enhanced laser desorption ionisation-time of flight mass spectrometry. World J Gastroenterol. 2006;12:1536-1544. [PubMed] |
| 61. | Melle C, Ernst G, Schimmel B, Bleul A, Thieme H, Kaufmann R, Mothes H, Settmacher U, Claussen U, Halbhuber KJ. Discovery and identification of alpha-defensins as low abundant, tumor-derived serum markers in colorectal cancer. Gastroenterology. 2005;129:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Albrethsen J, Bøgebo R, Gammeltoft S, Olsen J, Winther B, Raskov H. Upregulated expression of human neutrophil peptides 1, 2 and 3 (HNP 1-3) in colon cancer serum and tumours: a biomarker study. BMC Cancer. 2005;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Wang YJ, Zhang GY, Xiao ZQ, Wang HM, Chen ZC. Preliminary proteomic analysis of indomethacin's effect on tumor transplanted with colorectal cancer cell in nude mice. J Biochem Mol Biol. 2006;39:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Mobasheri A, Airley R, Foster CS, Schulze-Tanzil G, Shakibaei M. Post-genomic applications of tissue microarrays: basic research, prognostic oncology, clinical genomics and drug discovery. Histol Histopathol. 2004;19:325-335. [PubMed] |
| 65. | Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Vrolijk H, Sloos W, Mesker W, Franken P, Fodde R, Morreau H, Tanke H. Automated acquisition of stained tissue microarrays for high-throughput evaluation of molecular targets. J Mol Diagn. 2003;5:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 394] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Bibeau F, Boissière-Michot F, Sabourin JC, Gourgou-Bourgade S, Radal M, Penault-Llorca F, Rochaix P, Arnould L, Bralet MP, Azria D. Assessment of epidermal growth factor receptor (EGFR) expression in primary colorectal carcinomas and their related metastases on tissue sections and tissue microarray. Virchows Arch. 2006;449:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass JR. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2007;60:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Knösel T, Emde A, Schlüns K, Chen Y, Jürchott K, Krause M, Dietel M, Petersen I. Immunoprofiles of 11 biomarkers using tissue microarrays identify prognostic subgroups in colorectal cancer. Neoplasia. 2005;7:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Xie D, Sham JS, Zeng WF, Che LH, Zhang M, Wu HX, Lin HL, Wen JM, Lau SH, Hu L. Oncogenic role of clusterin overexpression in multistage colorectal tumorigenesis and progression. World J Gastroenterol. 2005;11:3285-3289. [PubMed] |
| 72. | Shinto E, Tsuda H, Ueno H, Hashiguchi Y, Hase K, Tamai S, Mochizuki H, Inazawa J, Matsubara O. Prognostic implication of laminin-5 gamma 2 chain expression in the invasive front of colorectal cancers, disclosed by area-specific four-point tissue microarrays. Lab Invest. 2005;85:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Tzankov A, Went P, Zimpfer A, Dirnhofer S. Tissue microarray technology: principles, pitfalls and perspectives--lessons learned from hematological malignancies. Exp Gerontol. 2005;40:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Huber M, Bahr I, Krätzschmar JR, Becker A, Müller EC, Donner P, Pohlenz HD, Schneider MR, Sommer A. Comparison of proteomic and genomic analyses of the human breast cancer cell line T47D and the antiestrogen-resistant derivative T47D-r. Mol Cell Proteomics. 2004;3:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Kwong KY, Bloom GC, Yang I, Boulware D, Coppola D, Haseman J, Chen E, McGrath A, Makusky AJ, Taylor J. Synchronous global assessment of gene and protein expression in colorectal cancer progression. Genomics. 2005;86:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Madoz-Gúrpide J, López-Serra P, Martínez-Torrecuadrada JL, Sánchez L, Lombardía L, Casal JI. Proteomics-based validation of genomic data: applications in colorectal cancer diagnosis. Mol Cell Proteomics. 2006;5:1471-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Tracey L, Villuendas R, Ortiz P, Dopazo A, Spiteri I, Lombardia L, Rodríguez-Peralto JL, Fernández-Herrera J, Hernández A, Fraga J. Identification of genes involved in resistance to interferon-alpha in cutaneous T-cell lymphoma. Am J Pathol. 2002;161:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Calogero A, Hospers GA, Mulder NH. Synthetic oligonucleotides: useful molecules? A review. Pharm World Sci. 1997;19:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Castanotto D, Li JR, Michienzi A, Langlois MA, Lee NS, Puymirat J, Rossi JJ. Intracellular ribozyme applications. Biochem Soc Trans. 2002;30:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Farman CA, Kornbrust DJ. Oligodeoxynucleotide studies in primates: antisense and immune stimulatory indications. Toxicol Pathol. 2003;31 Suppl:119-122. [PubMed] |
| 81. | Förster Y, Meye A, Krause S, Schwenzer B. Antisense-mediated VEGF suppression in bladder and breast cancer cells. Cancer Lett. 2004;212:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Kraemer K, Fuessel S, Schmidt U, Kotzsch M, Schwenzer B, Wirth MP, Meye A. Antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clin Cancer Res. 2003;9:3794-3800. [PubMed] |
| 83. | Miyake H, Hara I, Fujisawa M, Gleave ME. The potential of clusterin inhibiting antisense oligodeoxynucleotide therapy for prostate cancer. Expert Opin Investig Drugs. 2006;15:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Tamm I. Antisense therapy in malignant diseases: status quo and quo vadis? Clin Sci (Lond). 2006;110:427-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Chen Y, Ji YJ, Roxby R, Conrad C. In vivo expression of single-stranded DNA in mammalian cells with DNA enzyme sequences targeted to C-raf. Antisense Nucleic Acid Drug Dev. 2000;10:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Jiang YA, Luo HS, Fan LF, Jiang CQ, Chen WJ. Effect of antisense oligodeoxynucleotide of telomerase RNA on telomerase activity and cell apoptosis in human colon cancer. World J Gastroenterol. 2004;10:443-445. [PubMed] |
| 87. | Fan Y, Zheng S, Xu ZF, Ding JY. Apoptosis induction with polo-like kinase-1 antisense phosphorothioate oligodeoxynucleotide of colon cancer cell line SW480. World J Gastroenterol. 2005;11:4596-4599. [PubMed] |
| 88. | Wong SC, Yu H, Moochhala SM, So JB. Antisense telomerase induced cell growth inhibition, cell cycle arrest and telomerase activity down-regulation in gastric and colon cancer cells. Anticancer Res. 2003;23:465-469. [PubMed] |
| 89. | Pushparaj PN, Melendez AJ. Short interfering RNA (siRNA) as a novel therapeutic. Clin Exp Pharmacol Physiol. 2006;33:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Dallas A, Vlassov AV. RNAi: a novel antisense technology and its therapeutic potential. Med Sci Monit. 2006;12:RA67-RA74. [PubMed] |
| 91. | Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13:644-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 375] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 92. | Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10522] [Cited by in RCA: 10144] [Article Influence: 375.7] [Reference Citation Analysis (1)] |
| 93. | Huppi K, Martin SE, Caplen NJ. Defining and assaying RNAi in mammalian cells. Mol Cell. 2005;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 788] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 95. | Charames GS, Bapat B. Cyclooxygenase-2 knockdown by RNA interference in colon cancer. Int J Oncol. 2006;28:543-549. [PubMed] |
| 96. | de Souza Nascimento P, Alves G, Fiedler W. Telomerase inhibition by an siRNA directed against hTERT leads to telomere attrition in HT29 cells. Oncol Rep. 2006;16:423-428. [PubMed] |
| 97. | Jubb AM, Chalasani S, Frantz GD, Smits R, Grabsch HI, Kavi V, Maughan NJ, Hillan KJ, Quirke P, Koeppen H. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene. 2006;25:3445-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Lee JH, Cho ES, Kim MY, Seo YW, Kho DH, Chung IJ, Kook H, Kim NS, Ahn KY, Kim KK. Suppression of progression and metastasis of established colon tumors in mice by intravenous delivery of short interfering RNA targeting KITENIN, a metastasis-enhancing protein. Cancer Res. 2005;65:8993-9003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Alencar H, King R, Funovics M, Stout C, Weissleder R, Mahmood U. A novel mouse model for segmental orthotopic colon cancer. Int J Cancer. 2005;117:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Bresalier RS, Hujanen ES, Raper SE, Roll FJ, Itzkowitz SH, Martin GR, Kim YS. An animal model for colon cancer metastasis: establishment and characterization of murine cell lines with enhanced liver-metastasizing ability. Cancer Res. 1987;47:1398-1406. [PubMed] |
| 101. | de Wind N, Dekker M, van Rossum A, van der Valk M, te Riele H. Mouse models for hereditary nonpolyposis colorectal cancer. Cancer Res. 1998;58:248-255. [PubMed] |
| 102. | Heyer J, Yang K, Lipkin M, Edelmann W, Kucherlapati R. Mouse models for colorectal cancer. Oncogene. 1999;18:5325-5333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Colnot S, Niwa-Kawakita M, Hamard G, Godard C, Le Plenier S, Houbron C, Romagnolo B, Berrebi D, Giovannini M, Perret C. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest. 2004;84:1619-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 104. | Anant S, Mukhopadhyay D, Hirano K, Brasitus TA, Davidson NO. Apobec-1 transcription in rat colon cancer: decreased apobec-1 protein production through alterations in polysome distribution and mRNA translation associated with upstream AUGs. Biochim Biophys Acta. 2002;1575:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Nakagama H, Nakanishi M, Ochiai M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005;96:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Pories SE, Ramchurren N, Summerhayes I, Steele G. Animal models for colon carcinogenesis. Arch Surg. 1993;128:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 107. | Taketo MM. Mouse models of gastrointestinal tumors. Cancer Sci. 2006;97:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1131] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 109. | Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA. 1994;91:8969-8973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 379] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 110. | de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 599] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 111. | Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 433] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 112. | Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, Rosty C, Abal M, El Marjou F, Smits R. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |