Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5642
Revised: June 15, 2007
Accepted: August 23, 2007
Published online: November 14, 2007
AIM: To evaluate safety and effect on hepatitis B virus (HBV) suppression of a long-term treatment with lamivudine (LAM) at standard (100 mg/d) or double (200 mg/d) dose in chronic hepatitis B.
METHODS: This was a case study with matched controls (1:3) in patients with chronic hepatitis B with anti-HBe antibodies.
RESULTS: Twelve patients received LAM 200 mg/d and 35 LAM 100 mg/d, for a median of 28 mo. A primary response (PR; i.e., negative HBV-DNA with Amplicor assay) was achieved in 100% of LAM-200 patients and 83% of LAM-100 patients. A virological breakthrough occurred in 16.7 and 24.7%, respectively, of the PR-patients, with the appearance of typical LAM resistance mutations in all but one patient. Viremia blips (i.e., transient HBV-DNA below 80 IU/mL in patients who tested negative at Amplicor assay) were detected using a real time polymerase chain reaction (PCR) and occurred in seven out of nine patients with subsequent BT and in four out of 32 patients with end-of-study response (77.7% vs 12.5%; P = 0.001) at chi-square test). At the end of the study, 51.4% of LAM-100 patients and 83.3% of LAM-200 patients had remained stably HBV-DNA negative. Double-dose LAM was well tolerated.
CONCLUSION: Long-term treatment of anti-HBe positive chronic hepatitis B with double dose lamivudine causes a more profound and stable viral suppression as compared to conventional treatment.
- Citation: Stornaiuolo G, Stanzione M, Brancaccio G, Cuomo G, Precone V, Di Biase S, Felaco FM, Piccinino F, Gaeta GB. Viral blips during long-term treatment with standard or double dose lamivudine in HBe antigen negative chronic hepatitis B. World J Gastroenterol 2007; 13(42): 5642-5647
- URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5642
Lamivudine (LAM) treatment has changed the therapeutic approach to chronic hepatitis B (CHB), since it has a potent antiviral effect and an excellent tolerability profile[1-4]. In chronic hepatitis B with anti-HBe antibodies, lamivudine must be administered indefinitely, since drug discontinuation causes an immediate rebound in the vast majority of patients[5,6]. Unfortunately, the drug has a low genetic barrier which causes the emergence of resistant mutants and disease resurgence at a rate of approximately 15% a year[7-10]. In addition, a percentage of patients ranging from 11% to 32% are primary non-responders to treatment[11-14], which limits further the efficacy of lamivudine in this setting.
At present, about 80% of patients with CHB in the Mediterranean area lack HBeAg in serum and present anti-HBe antibodies with the presence of HBV-DNA in serum[15]. This percentage is expanding in other parts of the world[16], due to the control measures against HBV and the subsequent decline in the proportion of hepatitis B virus (HBV) infections in young patients.
Lamivudine is registered for the treatment of chronic hepatitis B at a standard daily dose of 100 mg. This dosage derives from studies which compared the efficacy of 25, 100 and 300 mg/d given for 12-24 wk and found that viral suppression was similar at 100 and 300 mg[17,18]. As a consequence, 100 mg was adopted as the standard of therapy and the potential of higher doses in improving long-term outcomes has never been investigated.
In this pilot study we investigated the feasibility and the effects on viral suppression of a long-term treatment with a daily dose of 200 mg lamivudine in patients with HBeAg-negative chronic hepatitis B compared with matched patients treated with the standard dose.
The study enrolled consecutive patients with HBeAg-negative, anti-HBe positive CHB between June 1999 and December 2002. The patients had to be negative for anti-HDV, anti-HCV and anti-HIV antibodies and present HBV-DNA in serum at levels > 2 × 104 IU/mL. Basal evaluation comprised routine liver function tests, abdominal ultrasound (US) and liver biopsy. Histological specimens were evaluated using the Ishak's scores for necroinflammation and fibrosis[19]; minimal requirements for a specimen to be evaluated were a length of at least 2 cm and the presence of ≥ 10 portal tracts.
Twelve consecutive patients received lamivudine at the daily dose of 200 mg (LAM 200; 100 mg b.i.d.). As a reference group, we selected three age matched patients for each case, with anti-HBe positive CHB treated in the same period with the standard dose of 100 mg according to an open label, long-term study. In both cases and controls, the therapy was continued indefinitely; the therapy was stopped in patients who did not clear HBV-DNA after 12 mo of treatment. The study was closed on June 30, 2005.
The patients were followed-up monthly, including medical examination and alanine aminotransaminase (ALT) determination; at each visit a serum sample was stored at -40°C for HBV-DNA testing. An abdominal US was performed every six months. Lamivudine tablets were dispensed directly monthly.
Routine quantitative testing for HBV-DNA in serum was performed using Amplicor HBV Monitor (Roche, Branchburg, NJ, USA; detection limit 80 IU/mL).
In addition, analysis of HBV-DNA in serum was performed by the use of a Real Time PCR (RT-PCR) and Sybr Green as a fluorescent intercalating agent of the double strand DNA. The reference standards used for the external calibration were the WHO hepatitis B virus DNA International Standards No. 97/746 and the working reagent HBV No. 98/780, both provided by the National Institute of Biological Standards and Controls. HBV-DNA was extracted from serum by a slightly modified guanidinium thiocyanate method. Briefly, 100 μL of serum or plasma were added to 400 μL of the extraction solution and 0.5 mL of 2-propanol were added to the tube. The mixture was centrifuged for 10 min, then supernatant was removed and pellets washed twice with 70% ethanol. Each sample was dissolved in 50 μL sterile water and 10 μL were used for PCR (sensitivity = 10 IU/mL).
Processed specimens were added to 40 μL of an amplification buffered (pH = 8.0) mixture containing 0.5 μmol/L each of the reverse and forward primers directed toward the highly conserved HBV pre-core/core region and Sybr Green (BMA Molecular Probe), 2.5 IU of Taq Gold (Applied Biosystem) and 0.5 IU of Uracil DNA Glycosylase (Amersham Life Science - USB). The PCR process was carried out with the following amplification cycles: 50°C for 2 min; 94°C for 10 min; two cycles, each of 1 min duration, at 94°C, 60°C and 72°C; 38 cycles at 94°C, 60°C and 72°C, of 20 s each. Real time detection was performed using a ABI PRISM 7000 Sequence Detection System (Applied Biosystem).
Polymerase mutants conferring LAM resistance were detected using a commercial assay (InnoLipa HBV-DR, Innogenetics, Gent, Belgium) in all patients who presented serum HBV-DNA reappearance while on therapy and in those who remained viremic from the beginning of the therapy. HBV genotypes were identified using the INNOLIPA HBV-Genotyping test (Innogenetics, Gent, Belgium).
Virological response (VR) to treatment was a fall in HBV-DNA below 80 IU/mL with or without a biochemical response (BR). Patients who remained constantly serum HBV-DNA positive over a 12 mo therapy period were defined as primary non-responders (PNR). A virological breakthrough (VBT) was defined as an increase ≥ 1 log in serum HBV-DNA in a previous responder patient while continuing LAM therapy; a biochemical breakthrough (BBT) was an ALT elevation above 2 × in a virological BT. An ALT flare was an ALT elevation > 500 U/L. A viral blip was a transient HBV-DNA below 80 IU/mL detected by RT-PCR in patients who tested negative at Amplicor assay. Complete viral suppression was a stably undetectable HBV-DNA in serum using RT-PCR.
A clinical examination was performed monthly. At the same time, differential blood count and serum biochemistry were performed, including serum amylase, lipase and creatinine phosphokinase (CPK) determination. Any adverse event was registered.
The data were analyzed according to an intention to treat procedure. Patients who were lost at follow-up were considered as non-responders. Chi-square and Fisher's exact test were used to analyze categorical variables and Student's t test for continuous data. The data were analyzed using SPSS software, version 12. The study was approved by the local Ethics Committee.
Twelve patients received LAM 200 mg/d and 35 patients LAM 100 mg/d (one patient receiving 200 mg LAM was matched with two controls only). The basal characteristics of the patients are reported in Table 1. There was no difference between the groups relating to demographic data and ALT values, serum HBV-DNA values and liver histology. Overall, a liver biopsy taken within 18 mo before therapy was available in 34 patients; 50% of whom had cirrhosis. In both groups median treatment duration was 28 mo. Based on Roche Monitor assay, a primary VR was observed in all the patients treated with 200 mg lamivudine and in 83% of those treated with the standard regimen (Table 2). In both groups, median HBV-DNA disappearance time was 3 mo and in no patients did HBV-DNA become undetectable at Monitor test later than the sixth months of therapy. Primary non-response was observed in six patients, all belonging to the 100 mg group. In PNRs LAM therapy was discontinued after 12 mo; during treatment, HBV-DNA levels remained above 2 × 104 IU/mL.
| Lam-200 | Lam-100 | P | |
| Number of patients | 12 | 35 | NS |
| Age (median; range) | 44.5 (32-60) | 44 (23-72) | NS |
| BMI median (range) | 24.3 (23.2-28.7) | 25.5 (20.9-34.3) | NS |
| Gender (M/F) | 11/1 | 26/9 | NS |
| Histology | NS | ||
| Minimal-Mild | 1 (14%) | 6 (22%) | |
| Moderate-Severe | 3 (43%) | 7 (26%) | |
| Cirrhosis | 3 (43%) | 14 (52%) | |
| ALT (x n.v.) | NS | ||
| < 3 | 4 (33.3%) | 16 (50%) | |
| 3-5 | 3 (25%) | 5 (15.6%) | |
| > 5 | 5 (41.7%) | 11 (34.4%) | |
| HBV-DNA IU/mL median (range) | 1.06 × 106 (3.2 × 104-3.8 × 106) | 6 × 105 (2.4 × 104-3.5 × 106) | NS |
| Months of therapy median (range) | 28 (8-50) | 28 (9-48) | NS |
During treatment, a VBT was detected in nine patients, of whom two belonged to the 200 mg group (2/12; 16.6%) and seven to the 100 mg group (7/29; 24.1%). A BBT followed in all patients with VBT; in these patients, the ALT values were below 2 × the basal value in eight out of nine patients and in the range of a hepatitis flare in one patient (belonging to 100 mg group). At the end of the study, 63% of the patients in LAM-100 group and 83% in LAM-200 group were still VR (Table 2). None of the basal variables considered in Table 1 was associated with the outcome of the treatments.
In 11 patients who were constantly negative at the HBV-Monitor test, the RT-PCR revealed transient viremia blips below 80 IU/mL. This feature was recorded in seven out of the nine patients who subsequently presented a BT and in four out of the 32 patients with end of study response, the latter belonging to the 100 mg group (Table 3; P = 0.001).The negative predictive value and positive predictive value for BT were 0.93 and 0.63, respectively. Viremia blips occurred in 10 out of 29 (34.4%) patients who were initially responders to LAM 100 mg and in one out of 12 (8.4%) of those responding to LAM 200 mg. On the whole, the patients who showed absent or partial viral suppression (i.e., a PNR or a VBT or viral blips) were 17 out of 35 treated with LAM 100 mg and two out of 12 treated with LAM 200 mg (P = 0.051).
Lamivudine resistance mutations were researched in 13 cases, five of whom were PNRs and eight BTs (Table 4). None of the primary non responders had detectable mutations at baseline; however, all of them developed rtM204V/I mutant (none with concomitant rtL180M) while continuing LAM therapy, two with an ALT increase after the appearance of the mutation and one with a hepatitis-like flare. Among BT patients, seven presented rtM204V/I (four with concomitant rtL180M) and one patient presented an ALT flare with no detectable mutations. Mutations preceded the clinical BT by one to eight months. HBV genotypes were detected in 24 patients, all but one were genotype D.
| Patients | Genotype | Baseline | Mutation (mo) |
| Breakthrough | |||
| 11 | D | Wt | rtM204V (11) |
| 12 | D | Wt | None (19) |
| 23 | D | Wt | rtM204V (24) |
| 14 | A | Wt | rtM204V |
| rtL180M (28) | |||
| 15 | D | Wt | rtM204V |
| rtL180M (32) | |||
| 16 | D | Wt | rtM204V |
| rtL180M (19) | |||
| 17 | D | Wt | rtM204V |
| rtL180M (21) | |||
| 18 | D | Wt | rtM204I (12) |
| Primary non-response | |||
| 11 | D | Wt | rtM204I (9) |
| 12 | D | Wt | rtM204V (11) |
| 13 | D | Wt | rtM204I (11) |
| 14 | D | Wt | rtM204I (9) |
| 15 | D | Wt | rtM204I (12) |
Lamivudine was well tolerated; one patient belonging to the 100 mg group discontinued the treatment due to an increase in serum amylase to eight times the UNL. No serious adverse events were recorded in either treatment group.
This study was designed to check whether a long-term, double dose lamivudine treatment is feasible in patients with chronic hepatitis B and has the potential for a more profound and stable suppression of viral replication.
Basically, lamivudine pharmacokinetic is subjected to significant individual variability[20-22]. Plasma concentrations of lamivudine do not reflect its antiviral activity, since it depends on the 5-triphosphate anabolite of the drug, which is formed through an intracellular, saturable enzymatic process. Higher dosage of lamivudine produced higher intracellular lamivudine triphosphate concentrations. The intracellular half-life of active compound varies from 17 to 19 h in hepatic cell lines and from 10.5 to 15.5 h in peripheral blood mononuclear cells (PBMCs)[23,24] which suggested a dose interval of 12 h in HIV patients. In our study giving daily LAM 200 mg in two refracted doses may have enhanced intracellular active drug availability and even reached adequate anti-viral concentrations in extrahepatic sites of HBV replication.
In chronic hepatitis B, lamivudine doses ranging from 25 to 300 mg/d were used obtaining a steady viral inhibition at 100 mg[17,18] as measured by a hybridization assay. Looking at long term efficacy, Yuen et al[25] found no difference in the cumulative incidence of resistance mutations between patients treated with lamivudine 100 mg/d and those who received 25 mg/d for one to three years, though viral suppression was less effective at lamivudine 25 mg than at lamivudine 100 mg. This suggests that viral suppression may be sub-optimal even at the dose of 100 mg and is in keeping with our results at 100 and 200 mg.
In our study, the use of a sensitive PCR method highlighted that viral suppression was less efficient at 100 mg/d and this was the background for mutant selection and viral breakthroughs. Indeed, some patients classified as VR by Monitor assay had viremia blips below 80 IU/mL. This phenomenon was significantly associated with subsequent stable resurgence of viral replication and was mostly observed in patients receiving 100 mg lamivudine. At the end of the study a stable viral suppression was achieved in 83% of the patients receiving 200 mg LAM and in 51% of those treated at 100 mg/d.
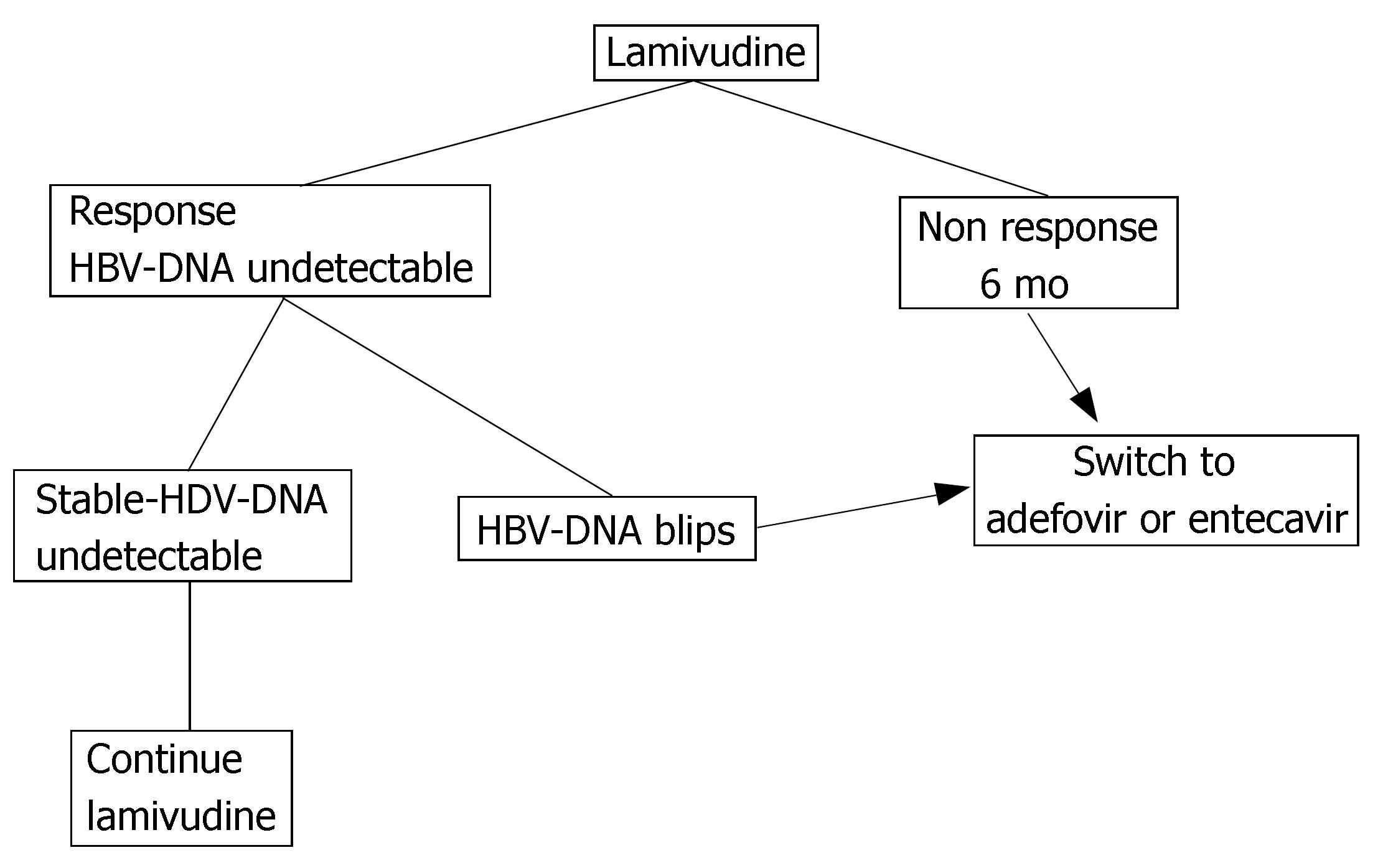
There are some practical consequences from these observations, which are summarized in Figure 1. First of all, patients under treatment with lamivudine require strict follow-up with a high-sensitivity PCR assay, in order to detect, as soon as possible, even low level viremia. Secondly, an early change in therapeutic strategy is advisable for patients with viremia blips, by switching to adefovir or to entecavir[26-28], in order to prevent virological and biochemical BTs. Finally, patients with undetectable HBV-DNA and no viremia blips are candidates for continuing long-term lamivudine monotherapy, since the absence of viremia blips is predictive of long-term response.
Genotype D of HBV was largely prevalent in our series and this raises the question as to whether the results are applicable to different HBV genotypes. There is evidence that response rates to Peg-interferon depend on HBV genotypes while less clear-cut results were obtained with anti-HBV analogs[1,29-31].
None of the primary non-response was associated with a pre-existing mutation, supporting the concept that the pharmacokinetic of the drug may play an important role in primary non-response and that higher drug doses may force this pharmacokinetic defect. Alternatively, PNR patients might harbour viral variants not detected by our method. Lamivudine resistance mutations were detected in all but one patient with virological BT.
In all primary non-responders continuing the treatment caused the appearance of resistance mutations, with further ALT elevation. From a practical point of view, patients who do not respond to a standard lamivudine dose should stop the treatment within the first six months (Figure 1), since a response after this period is unlikely. There is no data on early shifting to a higher lamivudine dose after an initial non-response to a standard dose.
In conclusion, our results indicate that the patients under lamivudine should be monitored using a high sensitivity PCR assay. An extended treatment with a double dose of lamivudine is feasible in chronic hepatitis B, and has the potential for a more pronounced viral suppression than a standard dose. Since lamivudine is a well-used, non-toxic and non-expensive anti-viral agent, these data should stimulate more powered studies aimed at optimizing treatment strategies.
Sustained viral suppression is the main goal of antiviral therapy in chronic hepatitis B. Lamivudine was the first antiviral drug approved for HBV treatment, at the standard daily dose of 100 mg. Although it was safe and potent, it caused the emergence of drug-resistant viral strains, at a rate of about 15% a year. At present, other antiviral drugs against HBV are available (adefovir, entecavir, telbivudine) that show a higher genetic barrier than lamivudine. Recent guidelines do not recommend the use of lamivudine as first line therapy in chronic hepatitis B.
Thousands of patients with chronic hepatitis B are under lamivudine treatment. Furthermore, lamivudine is the cheapest anti-HBV drug. After five years of continued treatment approximately 25% of the patients have an undetectable HBV-DNA. Can we improve the efficacy of lamivudine and predict the long-term response to treatment? There is a lack of studies with a higher than standard dosage of lamivudine in immunocompetent patients. This article examines the possibility of prolonged treatment with double daily dose of lamivudine (200 mg) and the predictive value of close monitoring of serum HBV-DNA using a highly sensitive real-time PCR.
Patients treated with lamivudine 200 mg/d achieved a more rapid primary response and a more profound and long-lasting viral suppression. In fact, the use of a sensitive PCR for HBV-DNA detection showed that transient low viremia levels ("viral blips") were more frequently detected in patients receiving lamivudine 100 mg and predicted a virological breakthrough and the emergence of lamivudine resistance mutations.
Patients under lamivudine treatment require a strict follow-up using a highly sensitive PCR. Real time PCR has a lower detection limit of 10 IU/mL and seems the best method for this purpose. Detecting even low level viremia during treatment may predict the emergence of resistance mutations. In this case, an early change in therapeutic strategy is advisable as indicated in Figure 1 of the paper. The paper suggests that therapy with double dose lamivudine is feasible and may achieve better results than the standard dose.
Virological breakthrough is the increase of at least one log of the plasma viral concentration during anti-viral therapy. In most of the cases it is due to the emergence of viral resistant strains. Biochemical breakthrough is the increase of ALT value that follows the virological breakthrough. Genetic barrier may be defined as the probability of not reaching any resistant escape strains.
The aim and content of the study were considered innovative. However, some limits come from the low number of patients enrolled in the 200 mg lamivudine group. As the authors stated, these results should encourage further trials.
S- Editor Liu Y L- Editor Roberts SE E- Editor Lu W
| 1. | Tillmann HL. Antiviral therapy and resistance with hepatitis B virus infection. World J Gastroenterol. 2007;13:125-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Lai CL, Ching CK, Tung AK, Li E, Young J, Hill A, Wong BC, Dent J, Wu PC. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology. 1997;25:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1346] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 4. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 352] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Wong VW, Chan HL, Wong ML, Tam JS, Leung NW. Clinical course after stopping lamivudine in chronic hepatitis B patients with lamivudine-resistant mutants. Aliment Pharmacol Ther. 2004;19:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 270] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Lok AS, Hussain M, Cursano C, Margotti M, Gramenzi A, Grazi GL, Jovine E, Benardi M, Andreone P. Evolution of hepatitis B virus polymerase gene mutations in hepatitis B e antigen-negative patients receiving lamivudine therapy. Hepatology. 2000;32:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Papatheodoridis GV, Dimou E, Laras A, Papadimitropoulos V, Hadziyannis SJ. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology. 2002;36:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 500] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 11. | Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000;32:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 279] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Gaia S, Marzano A, Smedile A, Barbon V, Abate ML, Olivero A, Lagget M, Paganin S, Fadda M, Niro G. Four years of treatment with lamivudine: clinical and virological evaluations in HBe antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2004;20:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Rizzetto M, Tassopoulos NC, Goldin RD, Esteban R, Santantonio T, Heathcote EJ, Lagget M, Taak NK, Woessner MA, Gardner SD. Extended lamivudine treatment in patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2005;42:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, Rizzetto M, Craxì A. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Gaeta GB, Stornaiuolo G, Precone DF, Lobello S, Chiaramonte M, Stroffolini T, Colucci G, Rizzetto M. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J Hepatol. 2003;39:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 619] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Nevens F, Main J, Honkoop P, Tyrrell DL, Barber J, Sullivan MT, Fevery J, De Man RA, Thomas HC. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3776] [Article Influence: 125.9] [Reference Citation Analysis (1)] |
| 20. | Yuen GJ, Morris DM, Mydlow PK, Haidar S, Hall ST, Hussey EK. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 210] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Moore KH, Yuen GJ, Hussey EK, Pakes GE, Eron JJ, Bartlett JA. Population pharmacokinetics of lamivudine in adult human immunodeficiency virus-infected patients enrolled in two phase III clinical trials. Antimicrob Agents Chemother. 1999;43:3025-3029. [PubMed] |
| 23. | Gao WY, Agbaria R, Driscoll JS, Mitsuya H. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2',3'-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994;269:12633-12638. [PubMed] |
| 24. | Kewn S, Veal GJ, Hoggard PG, Barry MG, Back DJ. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem Pharmacol. 1997;54:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419-1427. [PubMed] |
| 27. | Lampertico P, Viganò M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Kramvis A, Kew MC. Relationship of genotypes of hepatitis B virus to mutations, disease progression and response to antiviral therapy. J Viral Hepat. 2005;12:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Westland C, Delaney W, Yang H, Chen SS, Marcellin P, Hadziyannis S, Gish R, Fry J, Brosgart C, Gibbs C. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil1. Gastroenterology. 2003;125:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |