Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5618
Revised: April 16, 2007
Accepted: August 12, 2007
Published online: November 14, 2007
AIM: To review and summarize drug metabolism and its related interactions in prescribing drugs within the similar therapeutic or structural class for gastrointestinal disease treatment so as to promote rational use of medicines in clinical practice.
METHODS: Relevant literature was identified by performing MEDLINE/Pubmed searches covering the period from 1988 to 2006.
RESULTS: Seven classes of drugs were chosen, including gastric proton pump inhibitors, histamine H2-receptor antagonists, benzamide-type gastroprokinetic agents, selective 5-HT3 receptor antagonists, fluoroquinolones, macrolide antibiotics and azole antifungals. They showed significant differences in metabolic profile (i.e., the fraction of drug metabolized by cytochrome P450 (CYP), CYP reaction phenotype, impact of CYP genotype on interindividual pharmacokinetics variability and CYP-mediated drug-drug interaction potential). Many events of severe adverse drug reactions and treatment failures were closely related to the ignorance of the above issues.
CONCLUSION: Clinicians should acquaint themselves with what kind of drug has less interpatient variability in clearance and whether to perform CYP genotyping prior to initiation of therapy. The relevant CYP knowledge helps clinicians to enhance the management of patients with gastrointestinal disease who may require treatment with polytherapeutic regimens.
- Citation: Zhou Q, Yan XF, Zhang ZM, Pan WS, Zeng S. Rational prescription of drugs within similar therapeutic or structural class for gastrointestinal disease treatment: Drug metabolism and its related interactions. World J Gastroenterol 2007; 13(42): 5618-5628
- URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5618
More and more drugs within the similar therapeutic or structural class are emerging and it is essential to compare the alternative drug choices according to their efficacy, safety, suitability and cost. However, irrational prescription is common in many countries. Drug metabolism and its related interactions are most prone to be ignored in clinical practice. Actually, metabolism by cytochrome P450 (CYP) represents an important clearance mechanism for the majority of drugs, thus affecting their oral bioavailability, duration and intensity of pharmacological action[1]. The metabolic profile of a drug depicts its amount metabolized by CYP, CYP reaction phenotype, impact of CYP genotype on interindividual pharmacokinetics (PK) variability and CYP-mediated drug-drug interaction potential. It is closely related to the three-dimensional chemical structure of drug and may exhibit significant differences among drugs within the similar therapeutic or structural class, although the efficacy of these similar drugs do not show sharp differences at the dose used clinically[2,3]. The voluntary market withdrawal of cerivastatin by Bayer and withdrawal of medications such as terfenadine, astemizole, cisapride, and mibefradil from the market by the Food and Drug Administration (FDA) further demonstrate the relevance of metabolic drug-drug interaction profile. Although the FDA has published guidance for in vitro and in vivo drug metabolism/drug interaction studies in the drug development process[4,5], systematic summary is not yet available on metabolic differences in market products within the similar therapeutic or structural class. This review focuses on seven classes of drugs for gastrointestinal diseases treatment and aims to help clinicians realize what kind of drug has less interpatient variability in clearance, whether to perform CYP genotyping prior to the initiation of therapy, and how to enhance the management of patients on polytherapy regimens.
Seven classes of drugs for gastrointestinal diseases treatment were chosen, including gastric proton pump inhibitors, histamine H2-receptor antagonists, benzamide-type gastroprokinetic agents, selective 5-HT3 receptor antagonists, fluoroquinolones, macrolide antibiotics and azole antifungals. Relevant literature, focusing on drug metabolism, metabolic interaction potentials and clinical events of adverse drug reactions and treatment failures caused by drug-drug interaction, was identified by performing MEDLINE/Pubmed searches covering the period from 1988 to 2006.
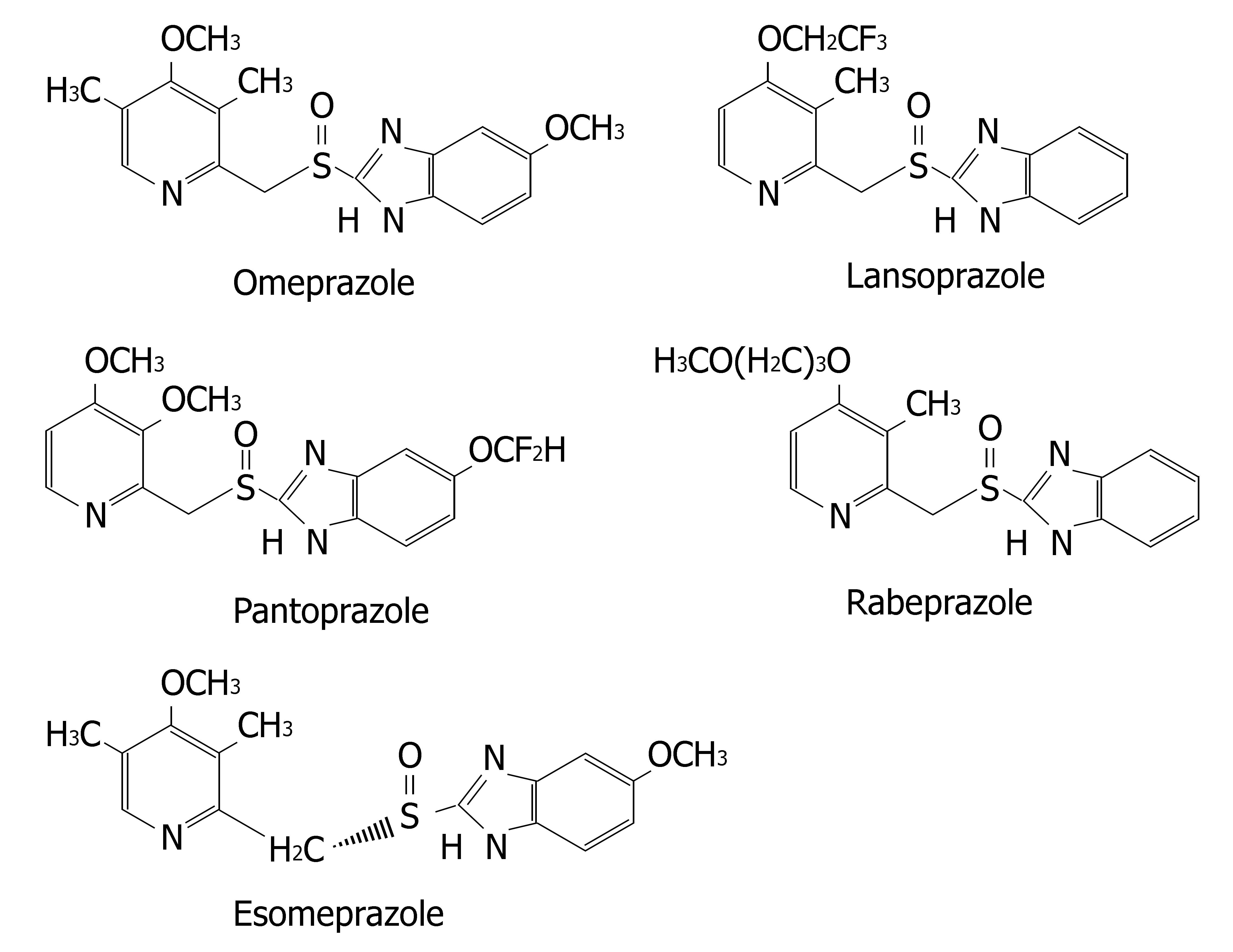
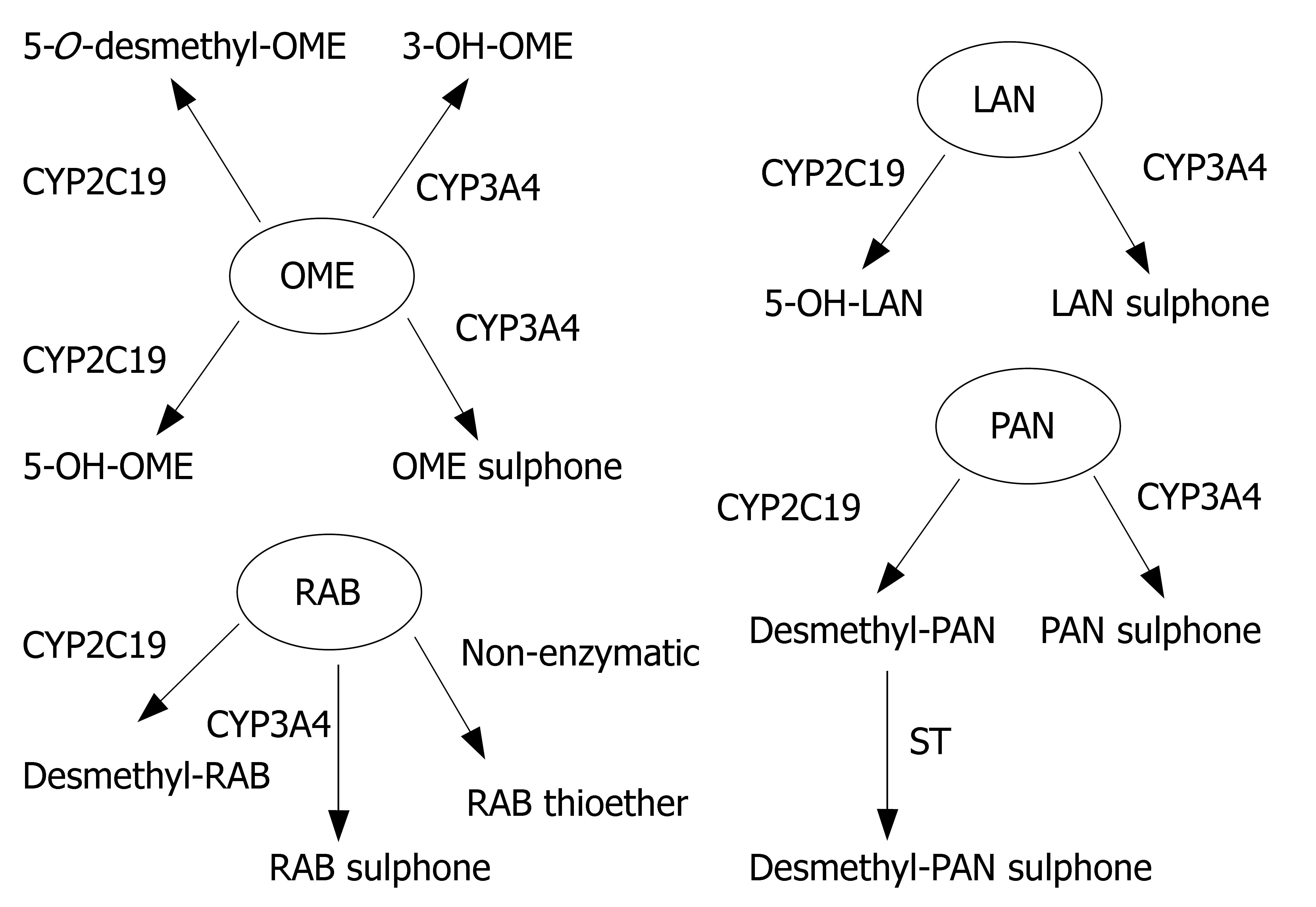
Proton pump inhibitors (or "PPI"s) are a group of drugs widely prescribed for the treatment of acid-related diseases such as peptic ulcer, gastroesophageal reflux disease (GERD), nonsteroidal anti-inflammatory drug induced gastropathy and Zollinger-Ellison syndrome. Currently used PPIs in clinical practice are as follows: omeprazole, lansoprazole, pantoprazole, rabeprazole and esomeprazole. All are benzimidazole derivatives (Figure 1). Schematic depiction of metabolic differences among four PPIs is described in Figure 2.
Lansoprazole, omeprazole and pantoprazole are all primarily metabolized by CYP2C19, an isoenzyme that exhibits genetic polymorphism with 15%-20% of Asian populations being poor⁄slow metabolizers, whereas the prevalence is much lower (3%-5%) among Caucasians and Blacks[6]. Their PK behaviors are all dependent on CYP2C19 genotype. AUCpo(PM)/AUCpo(EM), the ratio of parent drug area-under-the concentration vs time curve after oral dosing (AUCpo) derived from poor metabolizers (PM) and extensive metabolizers (EM), is 7.4, 3.7 and 6.0 for omeprazole, lansoprazole and pantoprazole, respectively[7]. CYP2C19 polymorphism is also a major predictor of treatment failures in patients receiving lansoprazole-, omeprazole- or pantoprazole based polytherapy for eradication of H pylori[8].
Omeprazole has also been known as a potent inhibitor of CYP2C19, and may cause pharmacokinetic interactions with other CYP2C19 substrates such as diazepam, phenytoin and moclobemide[9-11]. Both lansoprazole and omeprazole also induce CYP1A2 in vitro[12]. Omeprazole can reduce clozapine plasma concentrations by 40%[13]. However, concomitant intake of omeprazole or lansoprazole at high therapeutic doses does not affect the PK behavior of theophylline and caffeine[14,15]. The underlying explanation of the discrepancy may be that inducibility of CYP1A2 by omeprazole in vivo is related to the genetic polymorphism of CYP1A2, dose and course of treatment[16-18]. Potential interactions between omeprazole or lansoprazole and CYP1A2 substrates with narrow therapeutic windows should be kept in mind in long-term concurrent therapy. Among these three old PPIs, pantoprazole has by far the lowest potential for interactions[19].
Rabeprazole, although metabolized partially by CYP2C19, is primarily metabolized by nonenzymatic reduction and hence genotype and modifiers of CYP2C19 have less impacts on its PK (AUCpo(PM)/AUCpo(EM)≤ 1.8) and clinical efficacy[20].
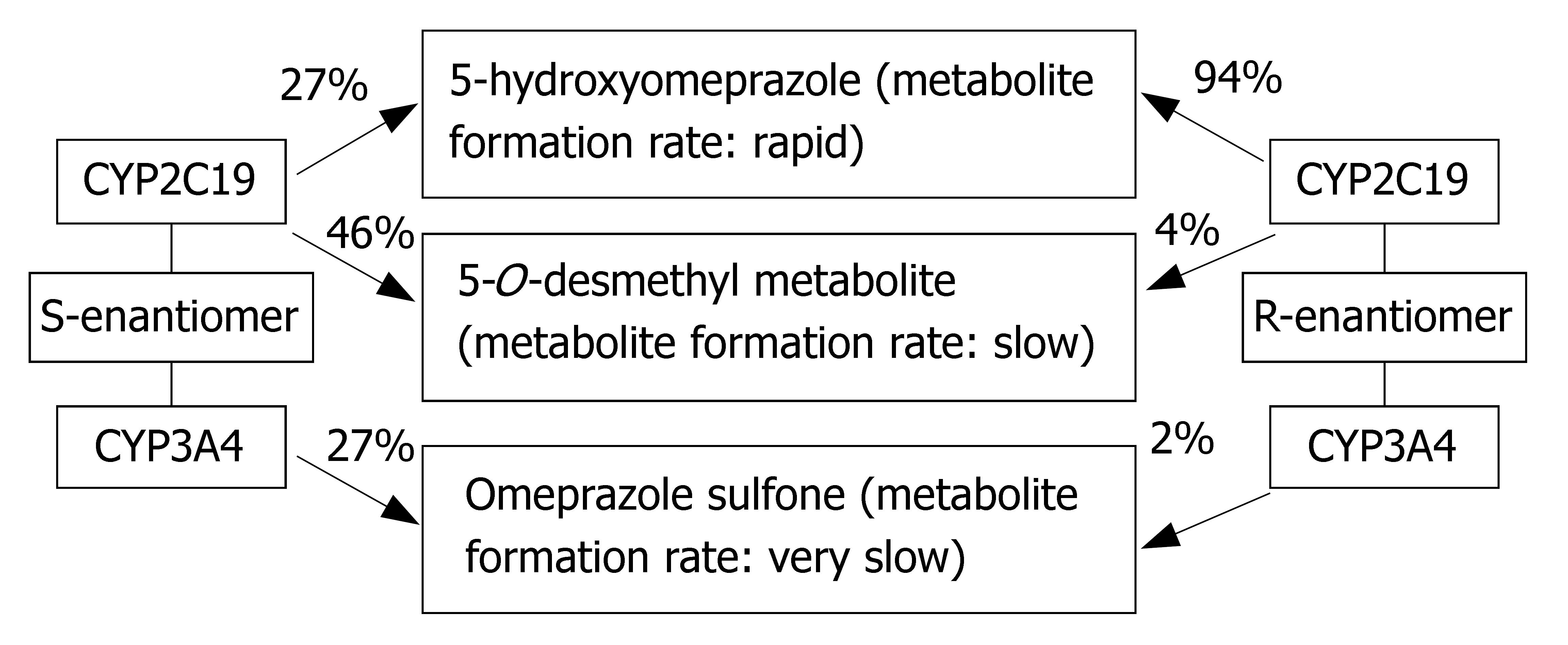
Esomeprazole is the S-enantiomer of omeprazole. Its metabolism involves CYP2C19, but to a lesser extent than omeprazole (Figure 3). Its PK is less dependent on CYP2C19 genotype (AUCpo(PM)/AUCpo(EM) approximate 3.0) and hence, it has less interpatient variability in clearance than omeprazole. Moreover, esomeprazole is cleared more slowly in vivo and has an improved oral bioavailability, leading to the greater inhibition of gastric acid secretion compared to omeprazole[21,22].
The enantiomers of pantoprazole are differentially affected by CYP2C19 genotype, such that the AUCpo(PM)/AUCpo(EM) ratio is 11 and 2.5 for the R-(+)- and S-(−)-enantiomers, respectively[23]. Comparative clinical trial of S-(−)-pantoprazole vs racemic pantoprazole in the treat-ment of GERD has been carried out by Pai et al[24]. S-(−)-pantoprazole (20 mg) was found to be more effective than racemic pantoprazole (40 mg) in improving symptoms. Consequently, the use of S-(−)-pantoprazole offers both pharmacokinetic and pharmacodynamic advantages.
Many recent cost-effectiveness analyses have provided an economic basis to employ CYP2C19 genotyping prior to initiating omeprazole-, lansoprazole- or pantoprazole-based polytherapy. However, pharmacogenetic tests may be unnecessary if rabeprazole or esomeprazole based therapy are considered.
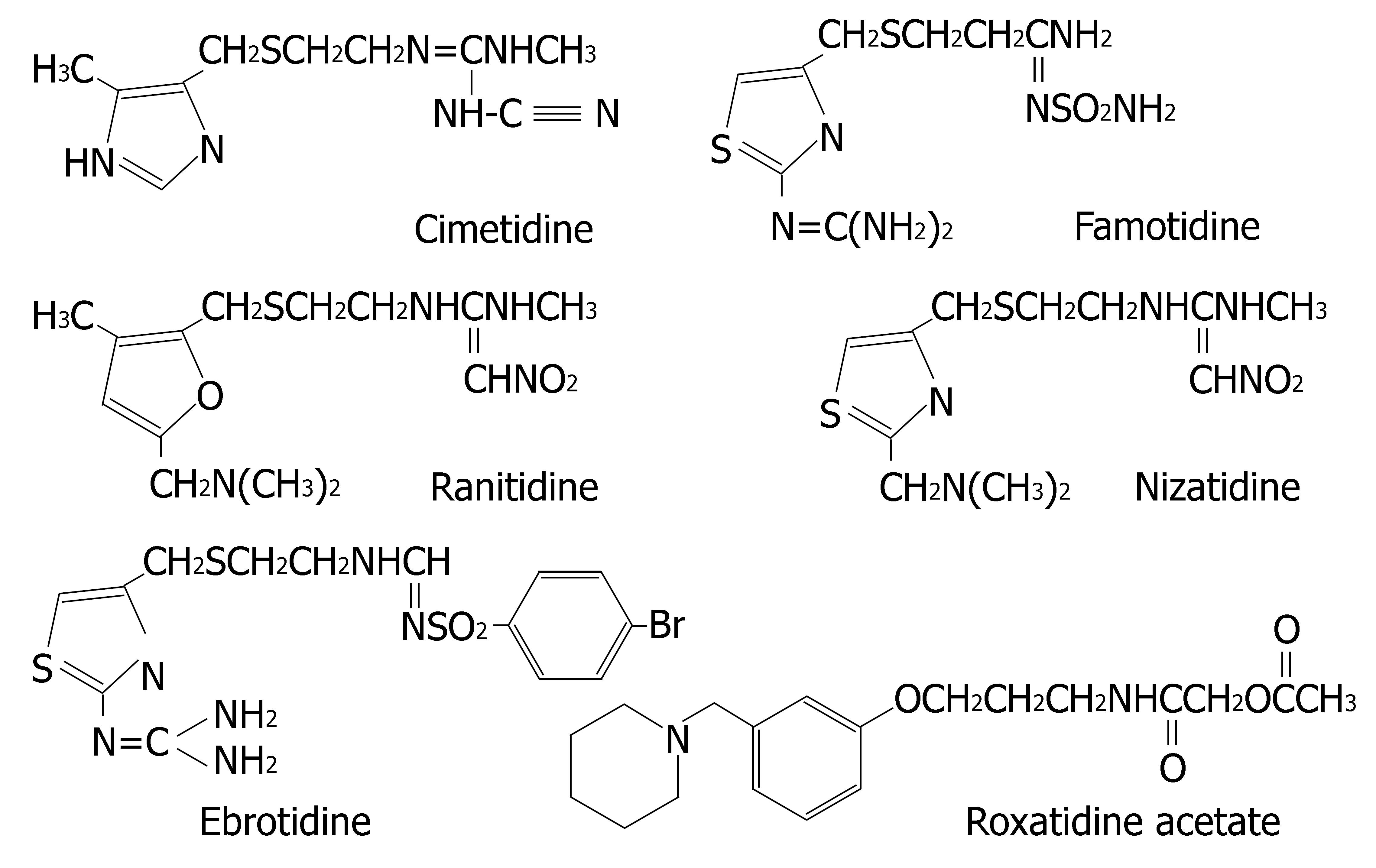
Histamine H2-receptor antagonists are clinically applied for the treatment of gastritis, gastric and duodenal ulcers[25]. Six H2-receptor antagonists are currently on the market: cimetidine, ranitidine, famotidine, nizatidine, ebrotidine and roxatidine acetate. Their chemical structures are depicted in Figure 4.
Martinez et al[26] compared the inhibitory effect of the H2-receptor antagonists on the enzymes activities in human liver microsomes. The results were as follows: CYP1A2: cimetidine > ranitidine = ebrotidine; CYP2D6: cimetidine > ranitidine = ebrotidine; CYP3A4: ebrotidine > cimetidine > ranitidine. However, it should be cautiously considered when these in vitro data were extrapolated to
in vivo situations. Firstly, cimetidine only selectively inhibits in vivo activities of CYP3A4 and CYP2D6[27]. For example, coadministered cimetidine increased the degree of beta-blockade of timolol (CYP2D6 substrate) ophthalmic solution and the maximum plasma concentrations of CYP3A4 substrates (e.g., midazolam and saquinavir) and disopyramide (CYP3A4 and CYP2D6 substrate)[26,28-30]. Coadministration of cimetidine 400 mg twice a day with saquinavir soft gel 1200 mg twice a day resulted in a significant increase in saquinavir AUC0-24 (120%) and Cmax (179%). From this view, coadministered cimetidine may be employed as a new pharmacoenhancer for boosting saquinavir for HIV infections. Beneficial effects of the inhibitory activity of cimetidine toward CYP are also used for the prevention of hepatotoxicity induced by overdoses with paracetamol, a substrate of several CYP isoenzymes which activate the drug by oxidation to the hepatotoxic metabolite N-acetyl-p-benzoquinone imine. Secondly, inhibition of CYP1A2 activity in humans by cimetidine has not been observed with clinical significance. In concurrent therapy of warfarin, disposition of the less potent R-warfarin (CYP1A2 substrate) was impaired. However, this interaction is likely to be of minimal clinical significance in most patients[31]. The interaction between cimetidine and theophylline was reported inconsistently. Degree of inhibition (absolute change in theophylline clearance) was closely related to route of administration, dosage, the basal theophylline clearance and smoking history[32-35]. Significant pharmacokinetic interaction between cimetidine and theophylline was not observed with low-dose cimetidine (200 mg twice daily), but with 800 mg cimetidine given once daily. Smokers or individuals with higher basal theophylline clearances had greater degree and percent of inhibition than non-smokers or individuals with lower basal theophylline clearances. It suggests that disposition of CYP1A2 substrates may still be impaired in smokers or other individuals with high CYP1A2 activities when coadministered with cimetidine. Thirdly, ebrotidine has no inhibitory effect on CYP3A4 in vivo, which is confirmed by lack of metabolic interaction with midazolam[26]. Overall, in contrast to cimetidine, the effects of the other H2-receptor antagonists on CYP in vivo seem to have little clinical significance.
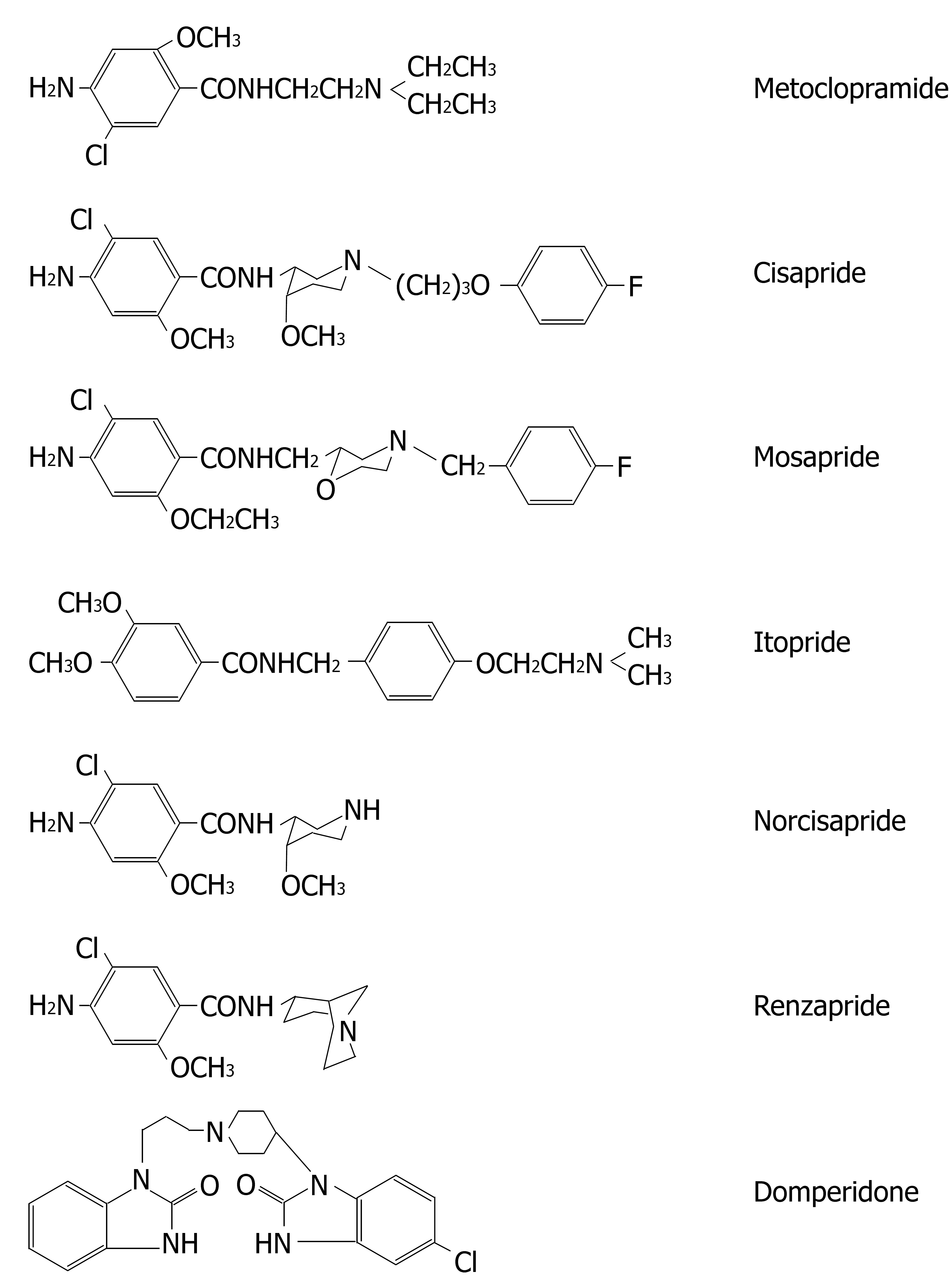
Benzamide-type gastroprokinetic agents (e.g., metoclo-pramide, cisapride, mosapride, itopride, renzapride and domperidone) are the mainstay of therapy in disorders of gastric motility such as non-ulcer dyspepsia (NUD), GERD, gastritis, diabetic gastroparesis and functional dyspepsia. Their chemical structures are depicted in Figure 5.
Among these gastroprokinetic agents, metoclopramide is predominantly metabolized by CYP2D6, thus its elimination being slow in PMs of CYP2D6 or in patients taking inhibitors of this isoform. Metoclopramide-induced acute dystonic reactions were more frequently observed in patients carrying homozygous CYP2D6 polymorphisms[36]. Meanwhile, it is also a potent inhibitor of CYP2D6 at therapeutically relevant concentrations and markedly inhibits in vitro codeine bioactivation[37,38]. Human pharmacokinetic interactions between metoclopramide and CYP2D6 substrates have not yet been documented.
Cisapride, mosapride and domperidone, are all predominantly metabolized by CYP3A4. Their dispositions could be strongly impaired by CYP3A4 inhibitors, causing greatly elevated plasma concentrations of parent drugs[39-41]. Among these prokinetic agents, only interactions of cisapride and CYP3A4 inhibitors induce severe clinical adverse events like QT interval prolongation and/or torsades de pointe, which is responsible for the withdrawal of cisapride by FDA. However, cisapride is still on the market in some countries following restriction imposed on its usage. The most important step that can be taken to minimize the risk of cisapride-associated arrhythmias is to avoid the concomitant administration of contraindicated drugs, particularly the macrolide antibiotics (e.g., ery-thromycin, clarithromycin) and the azole antifungals, (e.g., itraconazole and ketoconazole).
Itopride is primarily metabolized by flavin-containing monooxygenase and its PK is unlikely influenced by CYP3A4 inhibitors[39]. Norcisapride is a major active meta-bolite of cisapride via CYP3A4-mediated N-dealkylation. It possesses approximately 15% of the prokinetic activity of cisapride, but has no apparent effect on myocardial conduction[42]. Compared with cisapride, norcisapride elimination does not depend on CYP, and so norcisapride does not interact with azoles or macrolides. Janssen Pharmaceutica has licensed Sepracor's patent on (+) -norcisapride, and its clinical trials are undergoing. This new compound along with mosapride, domperidone and itopride are potentially safer alternatives to cisapride in the concurrent therapy of gastroprokinetic agents with potent CYP3A4 inhibitors.
Renzapride is not metabolized by CYP. It is excreted via the renal route primarily unchanged. Thus, its disposition is unsusceptible to CYP modulators and it does not interfere with CYP-mediated metabolism of other drugs[43]. It is currently in clinical development for constipation-predominant irritable bowel syndrome.
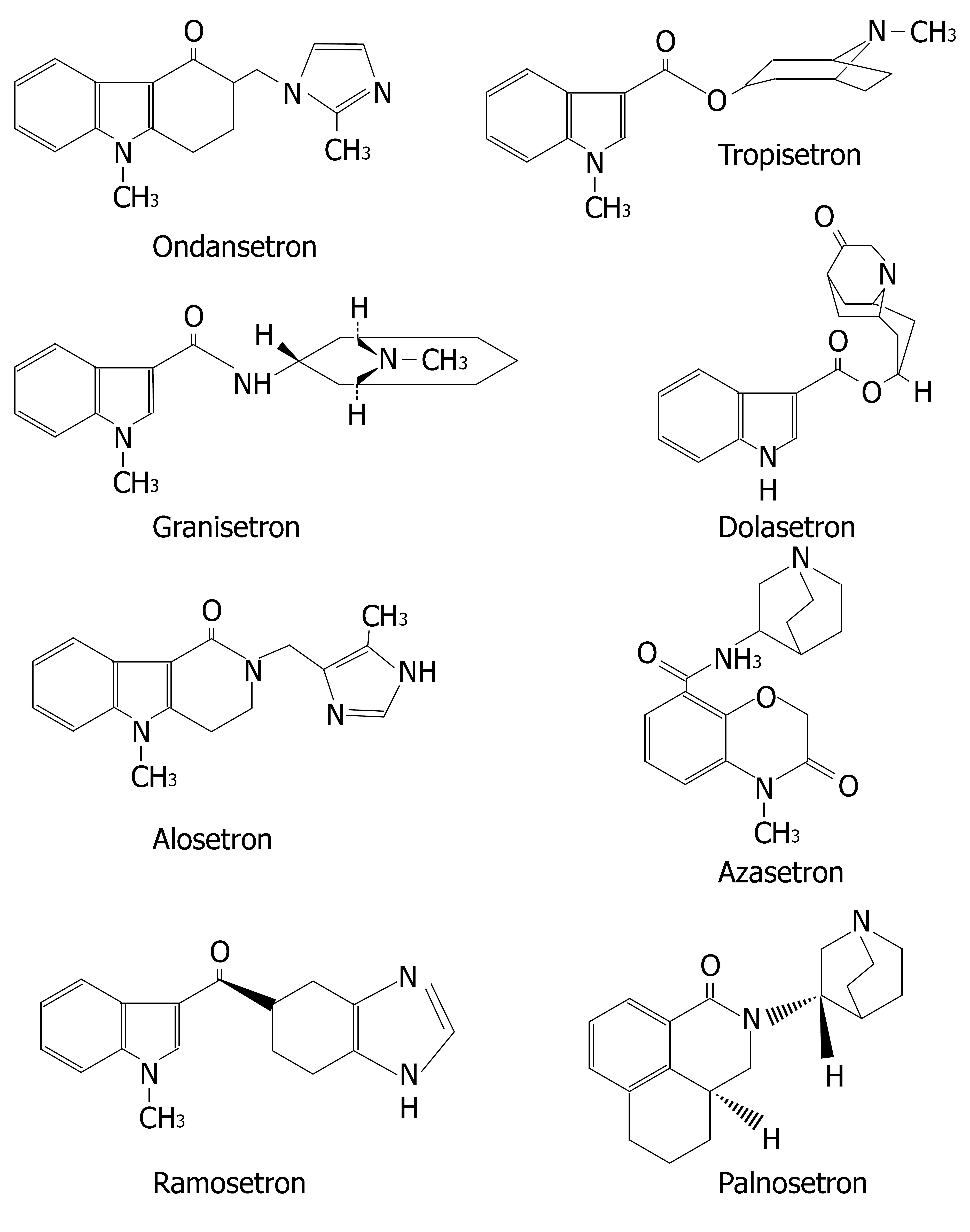
The selective 5-HT3-receptor antagonists or "setrons", including ondansetron, dolasetron, tropisetron, granisetron, alosetron, azasetron, palonosetron and ramosetron (Figure 6) represent a class of antiemetics that are currently used for chemotherapy- and radiotherapy-induced, or postoperative nausea and vomiting. However, these setrons have different metabolic profiles.
Ondansetron is cleared by multiple CYP forms in humans, with no single CYP form dominating the overall metabolism. Therefore, its PK lacks bimodality and seems unchanged when ondansetron is used concomitantly with specific CYP isoenzyme inhibitors[44].
Dolasetron is rapidly reduced by carbonyl reductase to its major active metabolite hydrodolasetron, which is eliminated by multiple routes, including renal excretion and metabolism mainly by glucuronidation and hydroxy-lation[45]. Hence, dolasetron appears to be insusceptible to clinically significant metabolic interactions posed by drugs commonly used in chemotherapy or surgery.
Tropisetron metabolism is almost exclusively CYP2D6-dependent and the metabolites are not pharmacologically active, thus the efficacy of antiemetic treatment with tropisetron largely depends on CYP2D6 genotype. The dose of tropisetron has to be patient-tailored according to CYP2D6 genotype[46,47].
Granisetron is unique because it is not metabolized via CYP2D6. Instead, it is metabolized via CYP3A4, which is not subject to significant genetic polymorphism and variation in patient response. Moreover, carriers of the duplication of the CYP2D6 allele predicting ultrarapid metabolizer status had less frequent vomiting episodes in subjects receiving granisetron than patients receiving tropisetron. Use of granisetron would obviate the need for CYP2D6 genotyping and may lead to improved prophylaxis of postoperative nausea and vomiting[48-50].
Alosetron is extensively metabolized in humans. In vivo data suggest that CYP1A2 plays a prominent role in alosetron metabolism[51,52]. In a pharmacokinetic study, 40 healthy female subjects received fluvoxamine (a known strong inhibitor of CYP1A2) in escalating doses from 50 to 200 mg per day for 16 d, with coadministration of alosetron 1 mg on the last day. Fluvoxamine increased mean alosetron AUC by approximately 6-fold and prolonged the half-life by approximately 3-fold. Thus, concomitant administration of alosetron and strong inhibitor of CYP1A2 is contraindicated. Otherwise, dose-related side effects of alosetron such as constipation may occur more frequently[53].
Azasetron is mainly excreted in urine as the unmeta-bolized form (approximately 60%-70%), which is different from the fact that other setrons undergo extensive metabolism[54]. In vitro data suggest that azasetron does not cause clinically significant CYP-mediated drug interactions.
Palonosetron is metabolized in the liver (approximately 50%). The two primary metabolites, N-oxide-palonosetron and 6-(S)-hydroxy-palonosetron, are essentially inactive. CYP2D6 is the major enzyme of palonosetron metabolism. Clinical pharmacokinetic parameters were not significantly different between PMs and EMs of CYP2D6[55-57]. As for ramosetron, in vitro data with human liver microsomes showed its minimal potential to cause clinically important CYP-mediated drug interactions[58].
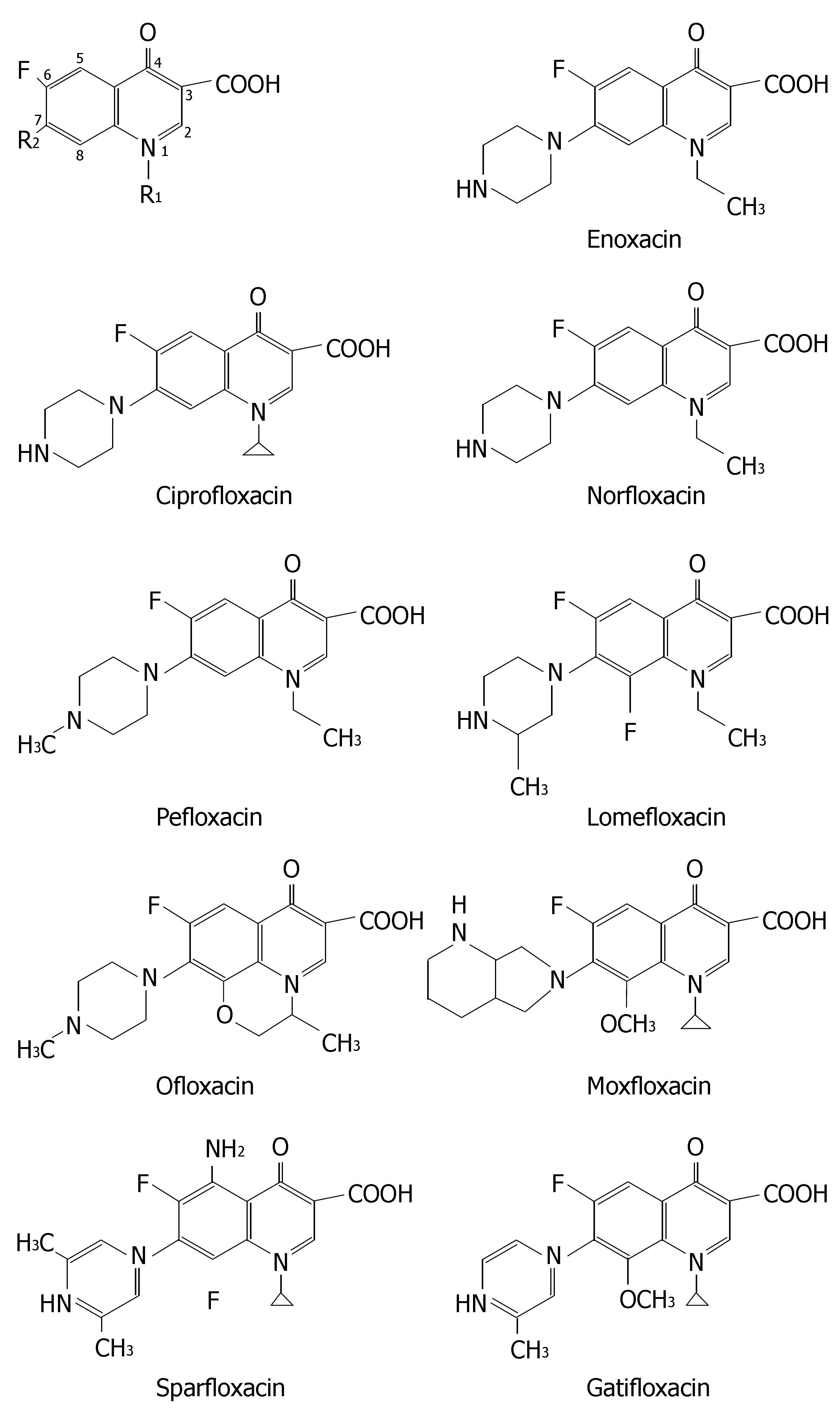
Fluoroquinolones are good choices in treatment of intesti-nal infections caused by sensitive bacterias. Meanwhile, fluoroquinolones-based polytherapy regimens are also used for H pylori infection in some occasions, especially after treatment failure in initial H pylori eradication[59-63].
The chemical structures of nine fluoroquinolones are listed in Figure 7. They have different CYP-mediated interaction potentials. Enoxacin, ciprofloxacin, norfloxacin and to a lesser extent pefloxacin all have inhibitory effects on metabolism of CYP1A2 substrates such as warfarin, tacrine, clozapine, tizanidine and theophylline. Ofloxacin, levofloxacin, sparfloxacin, lomexacin, gatifloxacin, sparfloxacin, lomefloxacin and moxifloxacin, are less prone to inhibit CYP1A2 and thus are alternative fluoroquinolones to patients receiving concurrent therapy of CYP1A2 substrate with narrow therapeutic window.
Moreover, ciprofloxacin and norfloxacin significantly depressed CYP3A4 in human microsomes[64]. Many case reports indicated their inhibitory effects on CYP3A4 in humans[65-69]. Clinicians should be wary of coadministration of norfloxacin or ciprofloxacin with CYP3A4 substrates with narrow therapeutic window. Table 1 lists the meta-bolic drug interactions related to fluoroquinolones with clinical relevance.
| Polytherapy regimen | Clinical consequence | Ref |
| Ciprofloxacin + tizanidine | Oral ciprofloxacin (500 mg twice daily for 3 d) increased AUC (0-infinity) of tizanidine by 10-fold and Cmax by 7-fold and dangerously potentiates its hypotensive and sedative effects, mainly by inhibiting CYP1A2. Care should be exercised when tizanidine is used concomitantly with ciprofloxacin. | 70 |
| Ciprofloxacin + clozapine | Ciprofloxacin (250 mg twice daily for 7 d) can moderately increase serum concentrations of clozapine and N-desmethylclozapine in patients with schizophrenia. A probable mechanism of interaction is an inhibition of CYP1A2 by ciprofloxacin. | 71 |
| Ciprofloxacin + theophylline | The interaction between oral ciprofloxacin (500 mg twice daily for 60 h) and theophylline can be clinically significant. Inter-individual variability in the magnitude of interaction can be attributed to inter-individual differences in the level of CYP1A2 expression. | 72 |
| Ciprofloxacin + olanzapine | Ciprofloxacin treatment (250 mg twice daily for 3 d) doubled olanzapine concentrations in one patient through the inhibition of CYP1A2. | 73 |
| Ciprofloxacin + sildenafil | Ciprofloxacin significantly increased sildenafil bioavailability (above 2-fold) in healthy volunteers, mainly by CYP3A4 inhibition. Dose adjustment of sildenafil is thus necessary. | 65 |
| Ciprofloxacin + methadone | Ciprofloxacin inhibited metabolism of methadone via CYP1A2 and CYP3A4, and caused profound sedation, confusion, and respiratory depression | 66 |
| Ciprofloxacin + cyclosporine | Ciprofloxacin and cyclosporine may be used together safely at the recommended dosage. However, case reports have suggested a possible pharmacokinetic interaction, e.g., ciprofloxacin substantially increased cyclosporine blood levels in a patient with pure red blood cell aplasia. However, levofloxacin therapy (500 mg/d IV) did not interfere with cyclosporine blood levels and thus it could be a therapeutic alternative. | 67, 68 |
| Enoxacin + fluvoxamine | Enoxacin (200 mg/d for 11 d) significantly increased the plasma concentrations at 2, 3 h and the Cmax of fluvoxamine in healthy volunteers. Sleepiness produced by fluvoxamine increased when coadministered with enoxacin. | 74 |
| Enoxacin + theophylline | A multidose regimen of enoxacin significantly slowed the clearance of theophylline and elevated theophylline concentrations in serum. The careful monitoring of serum theophylline level and modification of theophylline dosage in patients receiving enoxacin and theophylline were recommended. | 75 |
| Norfloxacin + cyclosporine | In pediatric patients undergoing renal transplantation norfloxacin impaired cyclosporine disposition by inhibition of CYP3A4, resulting in cyclosporine dose reduction from 7.4 mg/kg per day to 4.5 mg/kg per day. | 69 |
Macrolide antibiotics are usually included in polytherapy regimen for treatment of H pylori gastritis[76-80]. In addition, erythromycin, clarithromycin and azithromycin all exhibit prokinetic effects and may be used in the management of gastroparesis[81-84]. For example, erythromycin therapy is effective in the treatment of patients with gastroparesis, in whom metoclopramide or domperidone was ineffective.
Macrolides can be classified into 3 groups based on the propensity of these compounds to interfere with CYP3A4[85-87]. The first group (e.g., troleandomycin, erythromycin and clarithromycin) are potent mechanism-based CYP3A4 inhibitors. Because mechanism based inhibition is an irreversible inhibition where a covalent bond is formed between a metabolite and the active site of the enzyme, destroying the enzyme's activity, so the first group of macrolides could produce drug interactions with clinical relevance. The second group (e.g., flurithromycin, midecamycin, josamycin and roxithromycin) form complexes to a lesser extent and rarely produce drug interactions. The last group (e.g., azithromycin, dirithromycin and spiramycin) does not inhibit CYP3A4 and are unable to modify the PK behaviors of other compounds.
Metz et al[88] reported a potentially significant phar-macokinetic drug interaction between clarithromycin and carbamazepine in two patients with long-standing epilepsy who received omeprazole-clarithromycin therapy for H pylori gastritis. In both cases, clarithromycin therapy was temporally related to an increase in serum carbamazepine levels, which returned to the therapeutic range following cessation of clarithromycin therapy. If possible, erythromycin and clarithromycin should be avoided in patients taking CYP3A4 substrates such as atorvastatin, simvastatin, rifabutin, midazolam, cyclosporin, cisapride, pimozide, disopyramide, astemizole, nifedipine and carbamazepine. Azithromycin may be an alternative[89-92]. If clinical judgment suggests erythromycin and clari-thromycin should be used, it is necessary to adjust dosage of CYP3A4 substrates with narrow therapeutic window (e.g., decrease the dosage of carbamazepine by 30%-50%), monitor the serum drug levels closely, and warn the patient about the signs and symptoms of toxicity.
Moreover, both erythromycin and clarithromycin are also potent inhibitors of P-glycoprotein and can signi-ficantly interfere with the PK behaviors of P-glycoprotein substrate such as digoxin. For example, a case of a clarithromycin-associated digoxin toxicity in a patient with chronic atrial fibrillation and H pylori infection was reported by Gooderham et al[93].
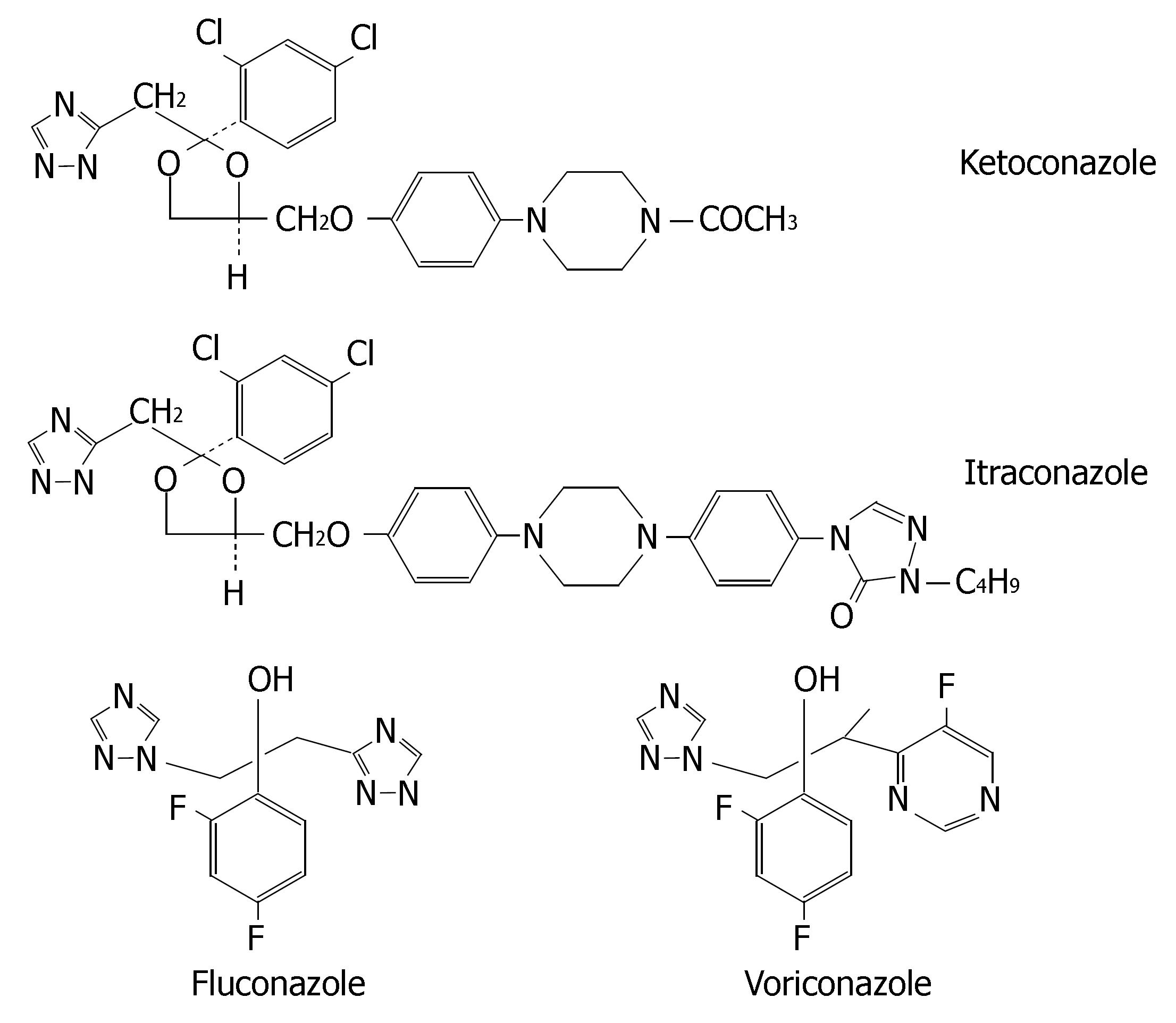
Azole antifungals (i.e., ketoconazole, itraconazole, fluconazole and voriconazole) may be used in treatment for fungus infections in digestive tracts. Their chemical structures are illustrated in Figure 8.
Ketoconazole is extensively metabolized into several inactive metabolites in the liver and the metabolites primarily excreted in bile[94]. Itraconazole is metabolized predominately by CYP3A4. Renal excretion of the parent drug is less than 0.03% of the dose[95]. Fluconazole is mainly excreted in urine as the unmetabolized form (approximately 80%). Accordingly, renal function is the major determinant of fluconazole PK[96]. The concurrent therapy of CYP3A4 inducers (e.g., rifampin and rifabutin) with itraconazole or ketoconazole results in poor antifungal response, thus their coadministrations are not recommended. However, fluconazole PK is less affected by CYP3A4 inducers[97], so fluconazole may be as an alternative for patients receiving comedicated CYP3A4 inducers.
Voriconazole is extensively metabolized by CYP2C19, CYP2C9 and CYP3A4. The major metabolite of voriconazole is the N-oxide, which has negligible antifungal activity. Inducers or inhibitors of these isoenzymes may increase or decrease voriconazole plasma concentra-tions. Coadministration of voriconazole with rifampicin, carbamazepine and phenobarbital is contraindicated. Allelic polymorphisms of CYP2C19 have been shown to be the most important determinants of the clearance of voriconazole, resulting in two phenotypes: PMs and EMs (both homozygous and heterozygous). Homozygous EMs have a two-fold lower exposure than heterozygous EMs and four-fold lower drug exposure than PMs[98-100]. Coadministration of a potent CYP3A4 inhibitor leads to a higher and prolonged exposure with voriconazole that might increase the risk of ADRs on a short-term basis, particularly in CYP2C19 PM patients[101]. Thus, it is necessary to implement CYP2C19 genotyping prior to initiation of voriconazole therapy or therapeutic drug monitoring in the course of treatment.
Ketoconazole and itraconazole are potent inhibitors of CYP3A4. Coadministration with CYP3A4 substrates can cause clinically significant drug interactions, some of which can be life-threatening. Cisapride, oral midazolam, pimozide, quinidine, triazolam, levacetylmethadol, statins metabolized by CYP3A4 (i.e., lovastatin, simvastatin and atorvastatin), ergot alkaloids metabolized by CYP3A4 (i.e., dihydroergotamine, ergometrine, ergotamine and methylergometrine) are contraindicated with ketoconazole and itraconazole.
The potency of fluconazole as a CYP3A4 inhibitor is much lower and thus its clinical interactions with CYP3A4 substrates are of less magnitude. Doses of less than 200 mg/d are not associated with significant CYP3A4-mediated interactions. So fluconazole (≤ 200 mg/d) is a relatively safe alternative azole antifungal when coadministered with statins metabolised by CYP3A4[102]. However, it is a potent inhibitor of CYP2C9. Coadministration of fluconazole with CYP2C9 substrates such as phenytoin, warfarin, fluvastatin and losartan leads to clinically significant drug interactions, whereas concurrent therapy of itraconazole or ketoconazole has minimal effect on PK of CYP2C9 substrates[103,104].
Voriconazole inhibits the activities of CYP2C19, CYP2C9 and CYP3A4. Thus, there is a potential for voriconazole to increase the plasma levels of substances metabolized by these CYPs. Coadministration of voriconazole with CYP3A4 substrates (e.g., terfenadine, astemizole, cisapride, quinidine and sirolimus) is contrain-dicated. When initiating voriconazole in patients already receiving cyclosporine or tacrolimus, it is recommended that the maintenance dosage of two immunosuppressive agents should be adjusted and that their level be carefully monitored. If patients receiving CYP2C9 substrates (e.g., warfarin, phenytoin or sulphonylureas) are treated simultaneously with voriconazole, pharmacotherapy monitoring and dosage adjustment for these drugs should be implemented accordingly.
The relationship between chemical structure and metabolic profile has been describled in the above summary on seven classes of drugs for gastrointestinal diseases treatment. The underlying molecular mechanism of interactions between drug and metabolizing enzymes is complex and it determines whether the drug is a substrate or inhibitor of the specific enzyme and how far it influences the enzyme activity.
Comparative molecular field analysis (CoMFA) modelling can reveal the key molecular characteristics of CYP inhibitors. For example, both electrostatic and steric interactions were found to account for the differences in the potencies of drugs to inhibit CYP2B6. The differences in inhibitory effects of H2-receptor antagonists on CYP enzymes may be attributed to the different ability of substituent to bind to the heme iron in CYP[105]. Cimetidine carries both the imidazole and the cyano groups which strongly bind to the heme iron and are responsible for its prominent interaction potential. In comparison to cimetidine, ranitidine has the following structural characteristics: (1) the imidazole ring is substituted with a furane ring, and (2) the side chain cyano group is substituted with a nitro group. Famotidine, nizatidine and ebrotidine all possess a thiazole nucleus instead of the imidazole ring, and the cyano-group in the side chain is substituted by aminosulfonyl- or nitro group. The affinity of binding with CYP isoenzymes is in the following order: imidazole ring (cimetidine), furane ring (ranitidine), thiazole ring (famotidine, nizatidine and ebrotidine). Roxatidine carries no imidazole group in its chemical structure, so it also has a weak inhibitory effect on CYPs.
The relationship between chemical structure of fluoroquinolone and its interaction magnitude has been determined[106,107]. Molecular modeling studies showed that it is possible to explain the potency of the quinolones to inhibit CYP1A2 on a molecular level. The keto group, the carboxylate group, and the core nitrogen at position 1 are likely to be the most important groups for binding to the active site of CYP1A2, because of the molecular electrostatic potential in these regions. Fluoroquinolones carrying an alkylated piperazinyl moiety at the position 7 (e.g., ofloxacin, levofloxacin, sparfloxacin, lomexacin and gatifloxacin) or a bulky substituent at the position 8 (e.g., sparfloxacin, lomefloxacin, gatifloxacin and moxifloxacin), are less prone to inhibit CYP1A2 than those without corresponding substituents.
The type of CYP metabolism and degree to which an azole antifungal is metabolized are governed by a number of factors including the physiochemical properties of the drug (lipophilicity) and its PK characteristics. Because ketoconazole and itraconazole are highly lipophilic, their clearance is heavily dependent upon CYP-mediated metabolism[108]. Fluconazole, on the other hand, is relatively less lipophilic and requires less CYP-mediated metabolism at low dosages (< 200 mg/day). Ketoconazole carries an imidazole ring, whereas itraconazole, fluconazole and voriconazole contain triazole rings. The four azole antifungals are strongly binding to hepatic microsome CYP enzymes in a Type II manner (i.e., involving the direct ligation of an azole nitrogen with the iron atom of the haem group in the CYP enzyme), which resulted in the broad-spectrum inhibition of multiple CYP isoforms, although the relative potencies towards the various isoforms vary from drug to drug[109].
Generally, if some type of CYP isoenzyme is the most important determinants of the clearance of a drug, metabolic drug interactions can be anticipated when the drug is coadministered with inducers or inhibitors of this isoenzyme. If this drug has a relative narrow therapeutic window, drug-drug interaction may be of clinical relevance. Morever, obvious inter-individual clinical outcome may be observed in patients if a CYP isoenzyme (the determinant of the clearance of a drug) exhibts polymorphism. Under all these situations in clinical practice, clinicians and pharmcists should show abilities in medication therapy management. Careful observations are needed in using new drugs in view of few clinical experiences.
In conclusions, the metabolic profile includes the fraction of drug metabolized by CYP, CYP reaction phenotype, impact of CYP genotype on interindividual PK variability and CYP-mediated drug-drug interaction potential. Significant differences may be observed with the metabolic profiles of medications for gastrointestinal disease treatment even if they belong to the same therapeutic or structural class. Many events of severe ADRs and treatment failures were closely related to the ignorance of this respect. Clinicians should acquaint themselves with what kind of drug has less interpatient variability in clearance and whether to perform CYP genotyping prior to initiation of therapy. The relevant CYP knowledge also helps clinicians enhance the management of patients on polytherapy regimens, i.e., better anticipate or avoid a drug interaction, choose an alternative agent with lower interaction potential, and perform pharmacotherapy monitoring (e.g., monitoring clinical symptoms and alterations in laboratory values) and dosage adjustment accordingly when concurrent therapy can not be avoided.
Metabolism by cytochrome P450 (CYP) represents an important clearance mechanism for the majority of drugs, thus affecting their oral bioavailability, duration and intensity of pharmacological action. The metabolic profile of a drug depicts its amount metabolized by CYP, the CYP reaction phenotype, impact of the CYP genotype on interindividual pharmacokinetics variability and CYP-mediated drug-drug interaction potential. It is closely related to the three-dimensional chemical structure of drug and may exhibit significant differences among drugs within the similar therapeutic or structural class, although the efficacy of these similar drugs do not show sharp differences at the dose used clinically. Many events of severe adverse drug reactions and treatment failures are attributed to the ignorance of above issues. In order to promote rational drug use in clinical practice, it is essential to let clinicians know what kind of drug has less interpatient variability in clearance, whether to perform CYP genotyping prior to therapy and how to enhance the management of patients on polytherapy regimens from the perspective of drug metabolism.
Food and Drug Administration (FDA) published guidance for in vitro and in vivo drug metabolism/drug interaction studies in the drug development process in 1999. Withdrawals of medications such as terfenadine, astemizole, cisapride, and mibefradil from the market by FDA demonstrate the relevance of metabolic drug-drug interaction profile. Some scientists tried to describe the three-dimensional quantitative structure activity relationships (QSARs) within substrates, inducers and inhibitors of CYP in recent years. There are also sporadic reports on metabolic differences in market products within the similar structural class.
This article is the first systematic summary on metabolic differences in market drug products within the similar therapeutic or structural class for gastrointestinal disease treatment.
The significance of this article is: (1) it helps doctors realize what kind of drug for gastrointestinal disease treatment has less interpatient variability in clearance and whether to perform CYP genotyping prior to therapy; (2) help doctors enhance management of patients on polytherapy regimens. Doctors will learn to better anticipate or avoid a drug interaction, choose an alternative agent with lower interaction potential, perform pharmacotherapy monitoring and adjust dosage accordingly when concurrent therapy cannot be avoided; and (3) help doctors attach equal importance to medicines for other disease treatment, and finally promote rational drug use in clinical practice.
Drug metabolism: the process by which the drug is chemically converted in the body to a metabolite, usually through specialized enzymatic systems. Its rate is an important determinant of the duration and intensity of the pharmacological action of drugs. Cytochrome P450: the most important element of oxidative metabolism of a large number of endogenous compounds (e.g., steroids) and xenobiotics (e.g., drugs). CYP is the standard abbreviation for mammalian cytochrome P450. CYP reaction phenotype: the relative contribution of the CYP isoforms to the metabolic pathways. CYP genotyping: the process of determining the CYP genotype of an individual by molecular biology techniques. It can be used to prospectively identify individuals at risk for adverse drug reactions or therapeutic failure due to altered drug metabolism. AUCpo(PM)/AUCpo(EM): the ratio of parent drug area-under-the concentration vs. time curve after oral dosing (AUCpo) derived from poor metabolizers (PM) and extensive metabolizers (EM).
The review by Zhou et al summarizes current literature on seven classes of drugs used in the treatment of gastrointestinal diseases with respect to the clearance of these substances. It is highly interesting and may help physicians to choose an equivalent drug or drug combination in clinical practice.
S- Editor Wang J L- Editor Ma JY E- Editor Liu Y
| 1. | Ansede JH, Thakker DR. High-throughput screening for stability and inhibitory activity of compounds toward cytochrome P450-mediated metabolism. J Pharm Sci. 2004;93:239-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Lewis DF, Modi S, Dickins M. Quantitative structure-activity relationships (QSARs) within substrates of human cytochromes P450 involved in drug metabolism. Drug Metabol Drug Interact. 2001;18:221-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Lewis DF, Modi S, Dickins M. Structure-activity relationship for human cytochrome P450 substrates and inhibitors. Drug Metab Rev. 2002;34:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | FDA Guidance for industry drug metabolism/drug interaction studies in the drug development process: studies in vitro. April 1997. Available from: http: //www.fda.gov/cder/guidance/clin3.pdf. |
| 5. | FDA Guidance for Industry In Vivo Drug Metabolism/Drug Interaction Studies -Study Design, Data Analysis, and Recommendations for Dosing and Labeling. November 1999. Available from: http: //www.fda.gov/cber/gdlns/metabol.htm. |
| 6. | McColl KE, Kennerley P. Proton pump inhibitors--differences emerge in hepatic metabolism. Dig Liver Dis. 2002;34:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Rodrigues AD, Rushmore TH. Cytochrome P450 pharmacogenetics in drug development: in vitro studies and clinical consequences. Curr Drug Metab. 2002;3:289-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Schwab M, Schaeffeler E, Klotz U, Treiber G. CYP2C19 polymorphism is a major predictor of treatment failure in white patients by use of lansoprazole-based quadruple therapy for eradication of Helicobacter pylori. Clin Pharmacol Ther. 2004;76:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Andersson T, Cederberg C, Edvardsson G, Heggelund A, Lundborg P. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin Pharmacol Ther. 1990;47:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Ko JW, Sukhova N, Thacker D, Chen P, Flockhart DA. Evaluation of omeprazole and lansoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab Dispos. 1997;25:853-862. [PubMed] |
| 11. | Yu KS, Yim DS, Cho JY, Park SS, Park JY, Lee KH, Jang IJ, Yi SY, Bae KS, Shin SG. Effect of omeprazole on the pharmacokinetics of moclobemide according to the genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;69:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Curi-Pedrosa R, Daujat M, Pichard L, Ourlin JC, Clair P, Gervot L, Lesca P, Domergue J, Joyeux H, Fourtanier G. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1994;269:384-392. [PubMed] |
| 13. | Frick A, Kopitz J, Bergemann N. Omeprazole reduces clozapine plasma concentrations. A case report. Pharmacopsychiatry. 2003;36:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Dilger K, Zheng Z, Klotz U. Lack of drug interaction between omeprazole, lansoprazole, pantoprazole and theophylline. Br J Clin Pharmacol. 1999;48:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Rizzo N, Padoin C, Palombo S, Scherrmann JM, Girre C. Omeprazole and lansoprazole are not inducers of cytochrome P4501A2 under conventional therapeutic conditions. Eur J Clin Pharmacol. 1996;49:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Han XM, Ouyang DS, Chen XP, Shu Y, Jiang CH, Tan ZR, Zhou HH. Inducibility of CYP1A2 by omeprazole in vivo related to the genetic polymorphism of CYP1A2. Br J Clin Pharmacol. 2002;54:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Sinués B, Fanlo A, Bernal ML, Val M, Mayayo E. Omeprazole treatment: genotoxicity biomarkers, and potential to induce CYP1A2 activity in humans. Hum Exp Toxicol. 2004;23:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Nousbaum JB, Berthou F, Carlhant D, Riche C, Robaszkiewicz M, Gouerou H. Four-week treatment with omeprazole increases the metabolism of caffeine. Am J Gastroenterol. 1994;89:371-375. [PubMed] |
| 19. | Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hepatol. 1996;8 Suppl 1:S21-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Hu YM, Mei Q, Xu XH, Hu XP, Hu NZ, Xu JM. Pharmacodynamic and kinetic effect of rabeprazole on serum gastrin level in relation to CYP2C19 polymorphism in Chinese Hans. World J Gastroenterol. 2006;12:4750-4753. [PubMed] |
| 21. | Chen CY, Lu CL, Luo JC, Chang FY, Lee SD, Lai YL. Esomeprazole tablet vs omeprazole capsule in treating erosive esophagitis. World J Gastroenterol. 2005;11:3112-3117. [PubMed] |
| 22. | Schwab M, Klotz U, Hofmann U, Schaeffeler E, Leodolter A, Malfertheiner P, Treiber G. Esomeprazole-induced healing of gastroesophageal reflux disease is unrelated to the genotype of CYP2C19: evidence from clinical and pharmacokinetic data. Clin Pharmacol Ther. 2005;78:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Tanaka M, Ohkubo T, Otani K, Suzuki A, Kaneko S, Sugawara K, Ryokawa Y, Ishizaki T. Stereoselective pharmacokinetics of pantoprazole, a proton pump inhibitor, in extensive and poor metabolizers of S-mephenytoin. Clin Pharmacol Ther. 2001;69:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Pai VG, Pai NV, Thacker HP, Shinde JK, Mandora VP, Erram SS. Comparative clinical trial of S-pantoprazole versus racemic pantoprazole in the treatment of gastro-esophageal reflux disease. World J Gastroenterol. 2006;12:6017-6020. [PubMed] |
| 25. | Zhou Q, Ruan ZR, Yuan H, Jiang B, Xu DH. Pharmacokinetics and bioequivalence of ranitidine and bismuth derived from two compound preparations. World J Gastroenterol. 2006;12:2742-2748. [PubMed] |
| 26. | Martínez C, Albet C, Agúndez JA, Herrero E, Carrillo JA, Márquez M, Benítez J, Ortiz JA. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther. 1999;65:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition & amp; induction in drug discovery. Curr Top Med Chem. 2001;1:403-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Ishii Y, Nakamura K, Tsutsumi K, Kotegawa T, Nakano S, Nakatsuka K. Drug interaction between cimetidine and timolol ophthalmic solution: effect on heart rate and intraocular pressure in healthy Japanese volunteers. J Clin Pharmacol. 2000;40:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Jou MJ, Huang SC, Kiang FM, Lai MY, Chao PD. Comparison of the effects of cimetidine and ranitidine on the pharmacokinetics of disopyramide in man. J Pharm Pharmacol. 1997;49:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Boffito M, Carriero P, Trentini L, Raiteri R, Bonora S, Sinicco A, Reynolds HE, Hoggard PG, Back DJ, Di Perri G. Pharmacokinetics of saquinavir co-administered with cimetidine. J Antimicrob Chemother. 2002;50:1081-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Niopas I, Toon S, Aarons L, Rowland M. The effect of cimetidine on the steady-state pharmacokinetics and pharmacodynamics of warfarin in humans. Eur J Clin Pharmacol. 1999;55:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | McEwen J, McMurdo ME, Moreland TA. The effects of once-daily dosing with ranitidine and cimetidine on theophylline pharmacokinetics. Eur J Drug Metab Pharmacokinet. 1998;13:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Nix DE, Di Cicco RA, Miller AK, Boyle DA, Boike SC, Zariffa N, Jorkasky DK, Schentag JJ. The effect of low-dose cimetidine (200 mg twice daily) on the pharmacokinetics of theophylline. J Clin Pharmacol. 1999;39:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Yoshimura N, Takeuchi H, Ogata H, Ishioka T, Aoi R. Effects of roxatidine acetate hydrochloride and cimetidine on the pharmacokinetics of theophylline in healthy subjects. Int J Clin Pharmacol Ther Toxicol. 1989;27:308-312. [PubMed] |
| 35. | Cremer KF, Secor J, Speeg KV. The effect of route of administration on the cimetidine-theophylline drug interaction. J Clin Pharmacol. 1989;29:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | van der Padt A, van Schaik RH, Sonneveld P. Acute dystonic reaction to metoclopramide in patients carrying homozygous cytochrome P450 2D6 genetic polymorphisms. Neth J Med. 2006;64:160-162. [PubMed] |
| 37. | Desta Z, Wu GM, Morocho AM, Flockhart DA. The gastroprokinetic and antiemetic drug metoclopramide is a substrate and inhibitor of cytochrome P450 2D6. Drug Metab Dispos. 2002;30:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Dayer P, Desmeules J, Striberni R. In vitro forecasting of drugs that may interfere with codeine bioactivation. Eur J Drug Metab Pharmacokinet. 1992;17:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Michalets EL, Williams CR. Drug interactions with cisapride: clinical implications. Clin Pharmacokinet. 2000;39:49-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Mushiroda T, Douya R, Takahara E, Nagata O. The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: comparison with cisapride and mosapride citrate. Drug Metab Dispos. 2000;28:1231-1237. [PubMed] |
| 41. | Ward BA, Morocho A, Kandil A, Galinsky RE, Flockhart DA, Desta Z. Characterization of human cytochrome P450 enzymes catalyzing domperidone N-dealkylation and hydroxylation in vitro. Br J Clin Pharmacol. 2004;58:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Shulman RJ, Boyle JT, Colletti RB, Friedman RA, Heyman MB, Kearns G, Kirschner BS, Levy J, Mitchell AA, Van Hare G. The use of cisapride in children. The North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 1999;28:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Tack J, Middleton SJ, Horne MC, Piessevaux H, Bloor JS, Meyers NL, Palmer RM. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1655-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Dixon CM, Colthup PV, Serabjit-Singh CJ, Kerr BM, Boehlert CC, Park GR, Tarbit MH. Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos. 1995;23:1225-1230. [PubMed] |
| 45. | Aventis Pharmaceuticals Inc. Prescribing information for ANZEMET ® Tablets (dolasetron mesylate). Available from: http: //redpoll.pharmacy.ualberta.ca/drugbank/drugBank/FDA_labels/020623.pdf. |
| 46. | Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay PB, Possinger K, Roots I, Brockmöller J. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol. 2002;20:2805-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Xu CT, Pan BR. Clinical application of 5a2HTa-3R antagonist tropisetron in chemotherapy patients. China Nati J New Gastroenterol. 1995;1:52-57. |
| 48. | Tan M. Granisetron: new insights into its use for the treatment of chemotherapy-induced nausea and vomiting. Expert Opin Pharmacother. 2003;4:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Blower PR. 5-HT3-receptor antagonists and the cytochrome P450 system: clinical implications. Cancer J. 2002;8:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Janicki PK. Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005;11:RA322-RA328. [PubMed] |
| 51. | Koch KM, Corrigan BW, Manzo J, James CD, Scott RJ, Stead AG, Kersey KE. Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Aliment Pharmacol Ther. 2004;20:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Koch KM, Palmer JL, Noordin N, Tomlinson JJ, Baidoo C. Sex and age differences in the pharmacokinetics of alosetron. Br J Clin Pharmacol. 2002;53:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Koch KM; GlaxoSmithKline. Prescribing information for Lotronex Tablets (alosetron hydrochloride). Available from: www.fda.gov/cder/foi/label/2002/21107s5lbl.pdf. |
| 54. | Tsukagoshi S. Pharmacokinetics of azasetron (Serotone), a selective 5-HT3 receptor antagonist. Gan To Kagaku Ryoho. 1999;26:1001-1008. [PubMed] |
| 55. | Helsinn Healthcare SA. Prescribing information for AloxiTM (palonosetron hydrochloride) injection. Available from: http: //www.fda.gov/cder/foi/label/2003/21372_aloxi_lbl.pdf. |
| 56. | Siddiqui MA, Scott LJ. Palonosetron. Drugs. 2004;64:1125-1132; discussion 1133-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Tonini G, Vincenzi B, Santini D. New drugs for chemotherapy-induced nausea and vomiting: focus on palonosetron. Expert Opin Drug Metab Toxicol. 2005;1:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Niwa T, Yamamoto S, Saito M, Kobayashi N, Ikeda K, Noda Y, Takagi A. Effects of serotonin-3 receptor antagonists on cytochrome P450 activities in human liver microsomes. Biol Pharm Bull. 2006;29:1931-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Sharara AI, Chaar HF, Aoun E, Abdul-Baki H, Araj GF, Kanj SS. Efficacy and safety of rabeprazole, amoxicillin, and gatifloxacin after treatment failure of initial Helicobacter pylori eradication. Helicobacter. 2006;11:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Nista EC, Candelli M, Zocco MA, Cazzato IA, Cremonini F, Ojetti V, Santoro M, Finizio R, Pignataro G, Cammarota G. Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2005;21:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Nista EC, Candelli M, Zocco MA, Cremonini F, Ojetti V, Finizio R, Spada C, Cammarota G, Gasbarrini G, Gasbarrini A. Levofloxacin-based triple therapy in first-line treatment for Helicobacter pylori eradication. Am J Gastroenterol. 2006;101:1985-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Guslandi M. Review article: alternative antibacterial agents for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2001;15:1543-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Dresner D, Coyle W, Nemec R, Peterson R, Duntemann T, Lawson JM. Efficacy of ciprofloxacin in the eradication of Helicobacter pylori. South Med J. 1996;89:775-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | McLellan RA, Drobitch RK, Monshouwer M, Renton KW. Fluoroquinolone antibiotics inhibit cytochrome P450-mediated microsomal drug metabolism in rat and human. Drug Metab Dispos. 1996;24:1134-1138. [PubMed] |
| 65. | Hedaya MA, El-Afify DR, El-Maghraby GM. The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers. Biopharm Drug Dispos. 2006;27:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Herrlin K, Segerdahl M, Gustafsson LL, Kalso E. Methadone, ciprofloxacin, and adverse drug reactions. Lancet. 2000;356:2069-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Hoey LL, Lake KD. Does ciprofloxacin interact with cyclosporine? Ann Pharmacother. 1994;28:93-96. [PubMed] |
| 68. | Borrás-Blasco J, Conesa-García V, Navarro-Ruiz A, Marín-Jiménez F, González-Delgado M, Gomez-Corrons A. Ciprofloxacin, but not levofloxacin, affects cyclosporine blood levels in a patient with pure red blood cell aplasia. Am J Med Sci. 2005;330:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | McLellan RA, Drobitch RK, McLellan H, Acott PD, Crocker JF, Renton KW. Norfloxacin interferes with cyclosporine disposition in pediatric patients undergoing renal transplantation. Clin Pharmacol Ther. 1995;58:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Raaska K, Neuvonen PJ. Ciprofloxacin increases serum clozapine and N-desmethylclozapine: a study in patients with schizophrenia. Eur J Clin Pharmacol. 2000;56:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Batty KT, Davis TM, Ilett KF, Dusci LJ, Langton SR. The effect of ciprofloxacin on theophylline pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 1995;39:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Markowitz JS, DeVane CL. Suspected ciprofloxacin inhibition of olanzapine resulting in increased plasma concentration. J Clin Psychopharmacol. 1999;19:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Kunii T, Fukasawa T, Yasui-Furukori N, Aoshima T, Suzuki A, Tateishi T, Inoue Y, Otani K. Interaction study between enoxacin and fluvoxamine. Ther Drug Monit. 2005;27:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Liu YN. Effect of enoxacin on theophylline pharmacokinetics. Zhonghua Jiehe He Huxi Zazhi. 1992;15:106-108. [PubMed] |
| 76. | Ivashkin VT, Lapina TL, Bondarenko OY, Sklanskaya OA, Grigoriev PY, Vasiliev YV, Yakovenko EP, Gulyaev PV, Fedchenko VI. Azithromycin in a triple therapy for H.pylori eradication in active duodenal ulcer. World J Gastroenterol. 2002;8:879-882. [PubMed] |
| 77. | Zhang L, Shen L, Ma JL, Pan KF, Liu WD, Li J, Xiao SD, Lin SR, Classen M, You WC. Eradication of H pylori infection in a rural population: one-day quadruple therapy versus 7-day triple therapy. World J Gastroenterol. 2006;12:3915-3918. [PubMed] |
| 78. | Xia HH, Yu Wong BC, Talley NJ, Lam SK. Alternative and rescue treatment regimens for Helicobacter pylori eradication. Expert Opin Pharmacother. 2002;3:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Berstad A, Berstad K, Wilhelmsen I, Hatlebakk JG, Nesje LB, Hausken T. Spiramycin in triple therapy of Helicobacter pylori-associated peptic ulcer disease. An open pilot study with 12-month follow-up. Aliment Pharmacol Ther. 1995;9:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Liu WZ, Xiao SD, Hu PJ, Lu H, Cui Y, Tytgat GN. A new quadruple therapy for Helicobacter pylori using tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment Pharmacol Ther. 2000;14:1519-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Dhir R, Richter JE. Erythromycin in the short- and long-term control of dyspepsia symptoms in patients with gastroparesis. J Clin Gastroenterol. 2004;38:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Xu CT, Pan BR. Effect of erythromycin on gastric emptying. China Nati J New Gastroenterol. 1996;2:53-57. |
| 83. | Rabine JC, Barnett JL. Management of the patient with gastroparesis. J Clin Gastroenterol. 2001;32:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 85. | Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic drug interactions of macrolides. Clin Pharmacokinet. 1992;23:106-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 219] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 86. | von Rosensteil NA, Adam D. Macrolide antibacterials. Drug interactions of clinical significance. Drug Saf. 1995;13:105-122. [PubMed] |
| 87. | Zhou S, Yung Chan S, Cher Goh B, Chan E, Duan W, Huang M, McLeod HL. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44:279-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 88. | Metz DC, Getz HD. Helicobacter pylori gastritis therapy with omeprazole and clarithromycin increases serum carbamazepine levels. Dig Dis Sci. 1995;40:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Rubinstein E. Comparative safety of the different macrolides. Int J Antimicrob Agents. 2001;18 Suppl 1:S71-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Amsden GW, Kuye O, Wei GC. A study of the interaction potential of azithromycin and clarithromycin with atorvastatin in healthy volunteers. J Clin Pharmacol. 2002;42:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Apseloff G, Foulds G, LaBoy-Goral L, Willavize S, Vincent J. Comparison of azithromycin and clarithromycin in their interactions with rifabutin in healthy volunteers. J Clin Pharmacol. 1998;38:830-835. [PubMed] |
| 92. | Yeates RA, Laufen H, Zimmermann T, Schumacher T. Pharmacokinetic and pharmacodynamic interaction study between midazolam and the macrolide antibiotics, erythromycin, clarithromycin, and the azalide azithromycin. Int J Clin Pharmacol Ther. 1997;35:577-579. [PubMed] |
| 93. | Gooderham MJ, Bolli P, Fernandez PG. Concomitant digoxin toxicity and warfarin interaction in a patient receiving clarithromycin. Ann Pharmacother. 1999;33:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Breckenridge A. Clinical significance of interactions with antifungal agents. Br J Dermatol. 1992;126 Suppl 39:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Breckenridge A; Janssen. Prescribing information for SPORANOX® (ITRACONAZOLE) INJECTION. Available from: http: //www.fda.gov/medwatch/SAFETY/2004/jul_PI/SporanoxInj_PI.pdf. |
| 96. | Pfizer Inc. Prescribing information for DIFLUCAN®. Available from: http: //www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4180b_04_05_Diflucan label 10-04 Division.pdf. |
| 97. | Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 542] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 98. | Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 99. | Pfizer Inc. Prescribing information for VFEND® (voriconazole) Tablets/ Injection. Available from: http: //www.fda.gov/medwatch/SAFETY/2003/03MAR_PI/Vfend_PI.pdf. |
| 100. | Donnelly JP, De Pauw BE. Voriconazole-a new therapeutic agent with an extended spectrum of antifungal activity. Clin Microbiol Infect. 2004;10 Suppl 1:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther. 2004;75:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 102. | Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 103. | Kaukonen KM, Olkkola KT, Neuvonen PJ. Fluconazole but not itraconazole decreases the metabolism of losartan to E-3174. Eur J Clin Pharmacol. 1998;53:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Kivistö KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol. 1998;46:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Rendić S. Drug interactions of H2-receptor antagonists involving cytochrome P450 (CYPs) enzymes: from the laboratory to the clinic. Croat Med J. 1999;40:357-367. [PubMed] |
| 106. | Mizuki Y, Fujiwara I, Yamaguchi T. Pharmacokinetic interactions related to the chemical structures of fluoroquinolones. J Antimicrob Chemother. 1996;37 Suppl A:41-55. [PubMed] |
| 107. | Fuhr U, Strobl G, Manaut F, Anders EM, Sörgel F, Lopez-de-Brinas E, Chu DT, Pernet AG, Mahr G, Sanz F. Quinolone antibacterial agents: relationship between structure and in vitro inhibition of the human cytochrome P450 isoform CYP1A2. Mol Pharmacol. 1993;43:191-199. [PubMed] |
| 108. | Lewis RE. Managing drug interactions in the patient with aspergillosis. Med Mycol. 2006;44 Suppl:349-356. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38:111-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |