Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5571
Revised: August 28, 2007
Accepted: September 4, 2007
Published online: November 14, 2007
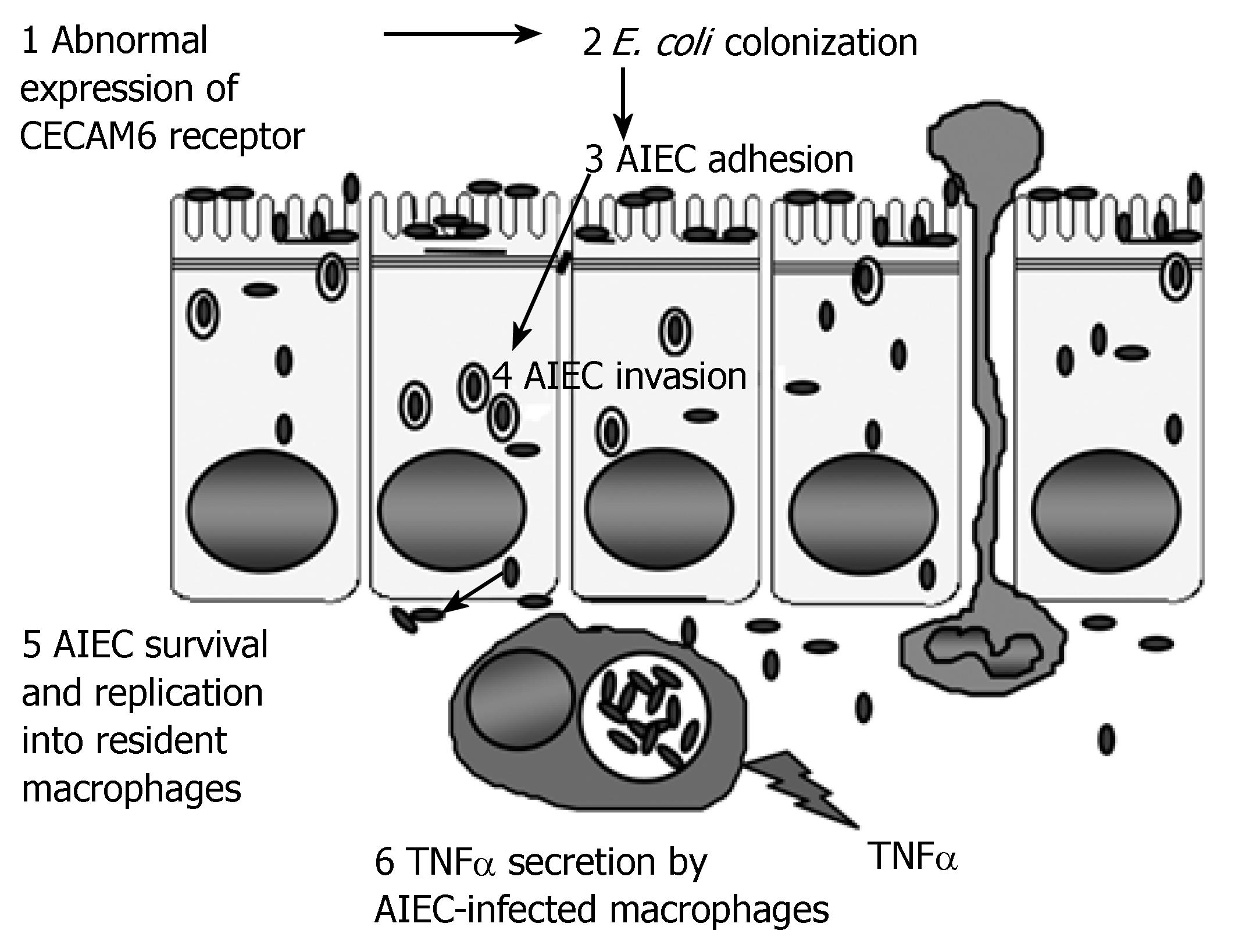
Increased numbers of mucosa-associated Escherichia coli are observed in both of the major inflammatory bowel diseases, Crohn's disease (CD) and ulcerative colitis (UC). A potential pathophysiological link between the presence of pathogenic invasive bacteria and genetic host susceptibility of patients with ileal CD is suspected. In CD patients, with increased ileal expression of the CEACAM6 molecule acting as a receptor recognized by type 1 pilus bacterial adhesin, and with the identification of mutations in the NOD2-encoding gene, the presence of pathogenic invasive bacteria could be the link between abnormal ileal bacterial colonization and innate immune responses to invasive bacteria. In a susceptible host, the sequential etiological steps of the disease induced by adherent-invasive E. coli (AIEC) are: (1) abnormal colonization via binding to the CEACAM6 receptor, which is overexpressed in the ileal mucosa of CD patients; (2) ability to adhere to and to invade intestinal epithelial cells, which allows bacteria to cross the mucosal barrier; (3) survival and replication within infected macrophages in the lamina propria; and (4) induction of tumor necrosis factor-α secretion and granuloma formation.
- Citation: Barnich N, Darfeuille-Michaud A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol 2007; 13(42): 5571-5576
- URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5571
Idiopathic inflammatory bowel diseases (IBDs), which include Crohn's disease (CD) and ulcerative colitis (UC), are chronic disorders of the gastrointestinal tract that have a combined prevalence of approximate 150-200 cases per 100000 population in Western countries[1]. Several lines of evidence suggest that bacteria play a role in the onset and perpetuation of IBD[2-6]. Intestinal bacteria are essential for the development of intestinal inflammation, and are required for the onset of inflammation in numerous knockout models of IBD[7-9]. The pathogenesis of CD is complex and consists of three interacting elements: genetic susceptibility factors such as NOD2/CARD15 and ileal CEACAM6 expression; priming by enteric microflora; and immune-mediated tissue injury[4,6,10-13]. The role of luminal bacteria in the pathogenesis of CD is strongly supported by observations showing that clinical symptoms of CD improve when luminal bacterial levels decrease following intestinal washes and antibacterial drug administration[14-16]. In addition, postoperative exposure of the terminal ileum to luminal contents is associated with increased inflammation in CD, and diversion of the fecal stream is associated with improvement[17].
Studies of luminal bacterial composition in patients with IBD, using culture and molecular biology techniques, have shown a decrease in the number of beneficial bacteria such as Bifidobacterium and Lactobacillus spp. and an increase in pathogenic bacteria such as Bacteroides and Escherichia coli (E. coli)[18-20]. Such dysbiosis induces a breakdown in the balance between putative species of protective vs harmful intestinal bacteria, and may promote inflammation[21,22]. Patients with IBD have higher numbers of mucosa-associated bacteria than control patients[18], and the generalized or localized dysbiosis observed is due to the presence of low numbers of normal bacteria, high numbers of unusual bacteria, and sometimes, a reduction in biodiversity. CD has features that might be the result of a microbial process in the gut. These include onset of infection in Peyer's patches and lymphoid aggregates, and the presence of ulceration, micro-abscesses, fissures, fistulas, granulomas and lymphangitis. Interestingly, the earliest lesions are aphthous ulcers in the intestine, which also occur in some viral and bacterial infections.
Although a number of organisms have been implicated in CD, only two agents, Mycobacterium paratuberculosis and E. coli, are presently being actively investigated. The theory that M. paratuberculosis has a role in CD has some attractive features[23]. Indeed, there are clinical similarities between Johne's disease, a spontaneous M. paratuberculosis infection in ruminants, and CD. M. paratuberculosis is detected at a greater frequency in CD than in control patients (UC patients and healthy subjects), by culture and polymerase chain reaction (PCR). This organism has been detected in blood and breast milk of patients with CD[24]. The high levels of E. coli colonizing the intestinal mucosa in CD patients strongly suggest that it plays a role in the etiopathogenesis of CD.
Bacterial adhesion to intestinal epithelial cells is the first step in the pathogenicity of many organisms involved in infectious diseases of the gut. Adhesion enables the bacteria to colonize the mucosa and to resist mechanical removal from the intestine. Studies on the adherence properties of E. coli in CD have yielded the general conclusion that E. coli strains are able to adhere to various human cells or cell lines. Fifty-three to 62% of E. coli strains isolated from feces of CD patients were able to adhere to buccal cells, compared to only 5%-6% of those isolated from control subjects[25,26]. The comparison of the adhesive properties of E. coli strains isolated from the ileum of CD patients and controls has revealed that 80% of E. coli strains associated with the ileal mucosa of CD patients preferentially adhered to differentiated Caco-2 cells, which mimic a mature intestinal cell model[20]. This is consistent with the finding that in patients with CD, crypt epithelial cells, which correspond to immature cells, are rarely involved in early lesions[27]. In addition, a correlation between bacterial adhesion to intestinal cells and intestinal colonization has been observed[20]. The presence of high levels of bacteria creates a biofilm on the surface of the gut mucosa in patients with CD and UC[18]. When bacteriologic samples were taken during surgery for CD, E. coli was isolated more frequently from the intestinal serosa and mesenteric nodes of CD patients (27% and 33%, respectively) than from those of control subjects[28,29]. Increased numbers of mucosa-associated E. coli are observed in CD and UC[18-20,30-33]. Rectal mucosa-associated E. coli counts were also higher in active than in inactive UC and CD and controls, and clusters of E. coli were observed in the lamina propria in UC and CD specimens, but not in controls[34]. In a study to assess the predominance of E. coli strains associated with the ileal mucosa of CD patients, E. coli was recovered from 65% of chronic lesions (resected ileum) and from 100% of the biopsies of early lesions (postoperative endoscopic recurrence)[20]. E. coli was abnormally predominant (between 50 and 100% of the total number of aerobes and anaerobes) in early and chronic ileal lesions of CD patients[20]. Moreover, in any given patient, healthy and ulcerated mucosa are colonized by E. coli strains having the same ribotype profile, which is indicative of uniform colonization, regardless of the inflammatory state of the mucosa[35].
Abnormal colonization of the ileal mucosa is due to increased expression of CEACAM6, a receptor for adherent–invasive E. coli (AIEC)[13]. These bacteria have been isolated from ileal lesions of CD patients, and express the type 1 pilus variant, as opposed to the type 1 pilus expressed by E. coli MG1655[36]. CD-associated AIEC strains adhere to the brush border of primary ileal enterocytes isolated from CD patients, but not from control patients without IBD. AIEC adhesion is dependent on type 1 pilus variant expression on the bacterial surface[36] and on abnormal CEACAM6 expression on ileal epithelial cells in CD patients[13]. The significantly increased ileal CEACAM6 expression in the uninvolved ileal mucosa of CD patients compared to that in controls without IBD, suggests that patients expressing a basal level of CEACAM6 are genetically predisposed to express that molecule. Additionally, CEACAM6 expression in cultured intestinal epithelial cells is increased after interferon (IFN)-γ or tumor necrosis factor (TNF)-α stimulation, and after infection with AIEC bacteria, which indicates that AIEC can promote its own colonization in CD patients[13]. Accordingly, in patients expressing a basal level of CEACAM6, the presence of AIEC bacteria and the secretion of IFN-γ and TNF-α lead to amplification of colonization and inflammation.
Analysis of E. coli strains isolated from early or chronic ileal lesions of patients with CD has revealed the presence of true invasive pathogens, since CD-associated bacteria efficiently invade a wide range of human epithelial cell lines, including Hep-2 cells and the intestinal cell lines Intestine-407, Caco-2 and HCT-8[37]. Their uptake is dependent on functioning host-cell actin microfilaments and microtubules[37]. Electron microscopy of epithelial cells infected with CD-associated bacteria has revealed a macropinocytosis-like process of entry, characterized by elongation of the membrane extensions, which surround bacteria at the sites of contact between entering bacteria and epithelial cells. Inside the host cells, CD-associated bacteria survive and replicate in the cytoplasm after lysis of the endocytic vacuole. The invasive process of CD-associated bacteria is unique since it does not possess any of the known genetic invasive determinants described for enteroinvasive, enteropathogenic, and enterotoxigenic E. coli, and Shigella strains. The major virulence factors of CD-associated AIEC that play a role in their invasive ability are type 1 pili that induce membrane extensions[36], flagella that confer bacterial mobility and down-regulate the expression of type 1 pili[38], outer membrane vesicles that deliver bacterial effector molecules to host cells[39], and outer membrane protein C (OmpC), which regulates the expression of several virulence factors via the sigma(E) regulatory pathway[40]. Interestingly, among these virulence factors, the outer membrane vesicles of H pylori and Pseudomonas aeruginosa have been reported to induce pro-inflammatory responses[41,42], and bacterial flagellin can interact with Toll-like receptor (TLR) 5 to activate an innate immune response.
The invasive ability of AIEC strains can allow bacteria to translocate across the human intestinal barrier and move into the deep tissues. Consequently, AIEC can interact with resident macrophages and continuously activate immune cells. In addition, patients with CD are more likely to be sensitive to AIEC infection. Indeed, the NOD2 gene, located on chromosome 16q12, has been identified as the first susceptibility gene for CD[11,12]. NOD2-deficient mice show loss of protective immunity in response to bacterial muramyl dipeptide, and mice are susceptible to Listeria infection via the oral route[43]. The 3020insC mutant of NOD2 associated with CD has impaired function as a defensive factor against intracellular bacteria in intestinal epithelial cells[44]. Thus, patients carrying NOD2 mutations are unable to control bacterial infections. The mutated NOD2 receptor does not contribute to pro-inflammatory gene transcription in response to bacteria, which results in an inadequate innate response to bacterial invasion and enables bacteria to accumulate. Such a poor innate response can lead to the formation of granulomas and thus, to the activation and perpetuation of a deregulated secondary adaptive response.
The search for infectious agents likely to cause CD has focused mainly on intracellular pathogens that have evolved to resist phagocytosis and to persist within macrophages, and which may be involved in chronic antigenic stimulation leading to T-cell and macrophage activation. AIEC strains isolated from CD patients are able to survive and replicate extensively within murine macrophages[45]. At 48 h post-infection, the number of intracellular AIEC bacteria can increase up to 74-fold compared to the initial infection. In contrast to its behavior within intestinal epithelial cells[37], CD-associated bacterial replication does not require bacterial escape into the cytoplasmic compartment[45]. Within J774-A1 macrophages, AIEC bacteria induce the formation of a single spacious vacuole by fusion of initial phagosomes. The behavior of the AIEC strains within macrophages is different from that of other invasive bacteria. In contrast to most invasive bacteria that induce death of infected macrophages[46], no necrosis or apoptosis of AIEC-infected J774-A1 macrophages is observed even after 24 h post-infection[45]. Moreover, in contrast to many pathogens that escape from the normal endocytic pathway, AIEC bacteria are taken up by macrophages within phagosomes, which mature without diverting from the classical endocytic pathway, and share features with phagolysosomes[47]. To survive and replicate in the harsh environment encountered inside these compartments, including acid pH and proteolytic activity of cathepsin D, AIEC have elaborate adaptation mechanisms, for which acidity constitutes a crucial signal, to activate the expression of virulence genes[48]. The major virulence factors of CD-associated AIEC that have a role in their ability to survive and replicate within macrophages are the htrA gene that encodes the stress protein HtrA, essential for intracellular replication within macrophages[48], and the dsbA gene that encodes the periplasmic oxidoreductase DsbA, essential for AIEC LF82 to survive within macrophages, irrespective of the loss of flagellum and type 1 pilus expression[49]. LF82-infected macrophages release large amounts of TNF-α[45]. This result is in accordance with the fact that several studies have shown that T helper (Th)1 cytokines, such as IFN-γ, TNF-α, and interleukin (IL)-12, are secreted in excess in CD whereas in UC, an atypical Th2 immune response with secretion of IL-4 or transforming growth factor (TGF)-β was observed[50]. Continuous macrophage activation and TNF-α release in CD patients may be due to the sustained multiplication of intracellular AIEC bacteria within phagosomes, and may be involved in the formation of granulomas. Granulomatous inflammation is a histological hallmark of CD and infection with some intracellular bacteria. E. coli DNA is present in 80% of microdissected granulomas in CD patients[51]. Granulomatous responses to E. coli have been reported in animals, such as granulomatous colitis of boxer dogs or Hjarre's disease in chickens and turkeys. E. coli strains were isolated from 100% of granulomas in boxer dogs with colitis[52], and these bacteria resembled CD-associated AIEC in phylogeny and virulence gene profile[53]. In Hjarre's disease, mucoid E. coli has been isolated from tuberculoid lesions of the cecum and liver of chickens and turkeys, while intramuscular inoculation of pure bacterial cultures or triturated diseased tissues reproduced the disease[54-56]. Using an in vitro model of human granuloma[57], CD-associated AIEC LF82 were reported to induce aggregation of infected macrophages, some of which fused to form multinucleated giant cells and subsequent recruitment of lymphocytes. Analysis of the cell aggregates indicated that they are very similar to the early stages of epithelioid granulomas[58].
AIEC strains have been found to be highly associated with ileal mucosa in CD patients[56]. Such pathogenic strains were isolated from ileal specimens of 36.4% of CD patients vs 6% of controls. In colonic specimens, AIEC strains were found in 3.7% of CD patients, 0% of UC patients, and 1.9% of controls. These strains are preferentially found in early recurrent lesions after surgery, thus indicating their role in the initiation of inflammation, and not just as secondary invaders. Another study has shown that mucosa-associated E. coli, which accounted for 53% of isolates, were more common in CD (43%) than in non-inflamed control patients (17%), while intramucosal E. coli were found in 29% of CD patients vs 9% of controls[30]. These studies support a central role for mucosa-associated AIEC in the pathogenesis of CD[30,56], since the translocation of these pathogenic bacteria through the intestinal mucosa may be a crucial step in the propagation of the inflammatory process.
Various factors lend credence to the theory that AIEC is intimately linked to the etiopathogenesis of ileal CD. The high prevalence of AIEC in patients with ileal CD may be the first step in the establishment of a modified Koch's postulate that takes into account the genetic susceptibility of the host[30,56]. A possible role for AIEC in the etiopathogenesis of CD in susceptible hosts is summarized in Figure 1. The sequential steps involved in the induction of disease by the bacteria are: (1) abnormal colonization via binding to the CEACAM6 receptor, which is overexpressed in the ileal mucosa of CD patients[13]; (2) ability to adhere to and to invade intestinal epithelial cells, which allows bacteria to cross the mucosal barrier[37]; (3) survival and replication within infected macrophages in the lamina propria; and (4) induction of TNF-α secretion[45] and granuloma formation[58].
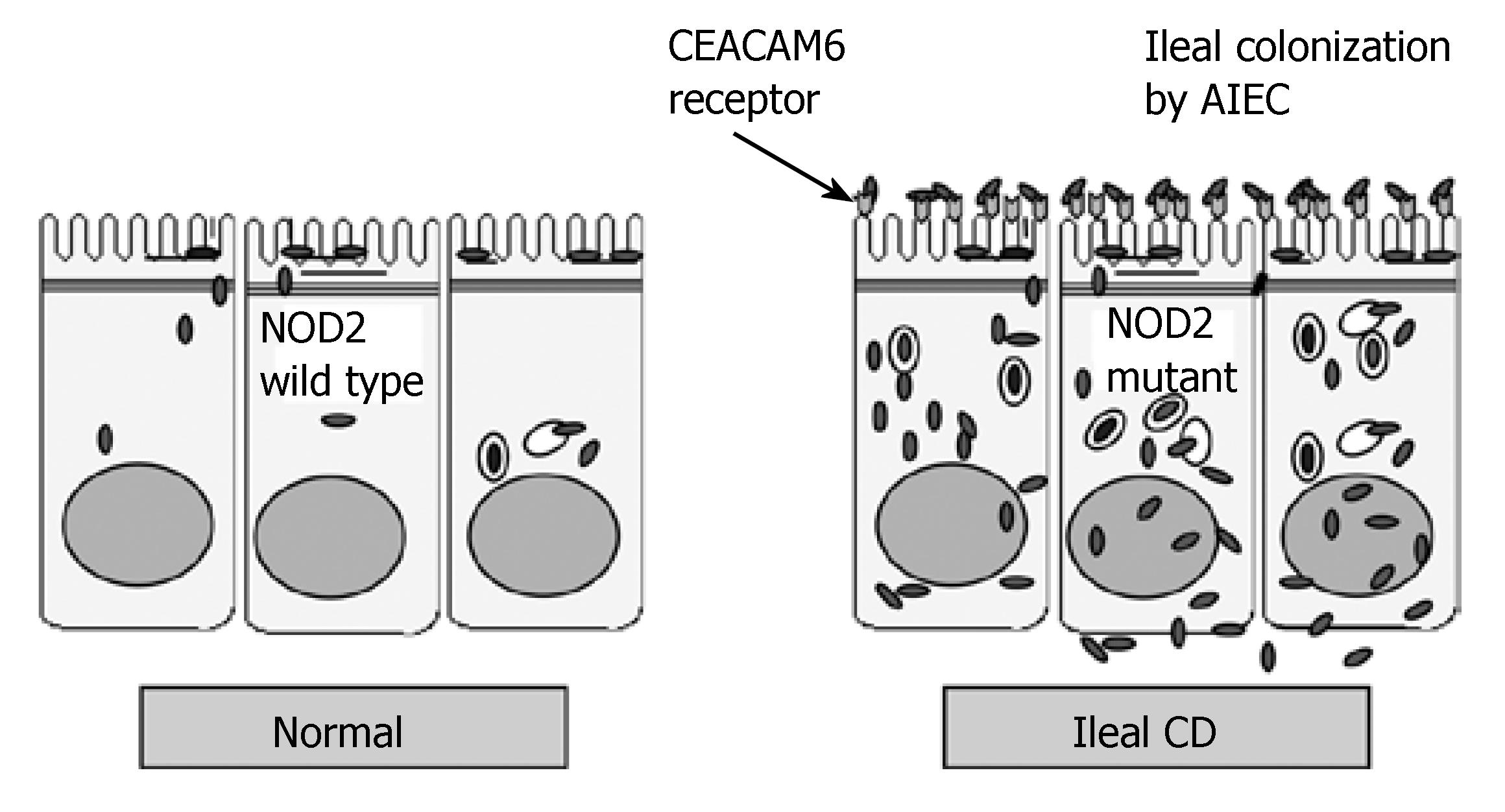
AIEC strains could colonize the ileal mucosa of CD patients by binding to CEACAM6, translocate across the human intestinal barrier to move into deep tissues, and once there, continuously activate immune cells. Patients having a high risk for developing severe ileal CD may be those who, in addition to expressing a variant of the NOD2 intracytoplasmic receptor[11,12], overexpress CEACAM6 at the surface of the ileal mucosa[13] (Figure 2). Host innate immune receptors that can be activated by AIEC components are mainly the transmembrane receptor TLR2 and the intracellular receptor NOD2. NOD2 is a negative regulator of the TLR2-mediated Th1 response, while the NOD2 3020insC mutation associated with CD is unable to inhibit TLR2 signaling, which skews the system toward an overactive Th1-mediated response[59]. This result provides a compelling explanation for why people carrying the NOD2 mutation might develop CD in response to abnormal colonization by AIEC[60]. The treatment of severe ileal CD could evolve from being almost exclusively surgical to management that places much greater emphasis on medical therapy, such as immunomodulators and anti-TNF-α agents, and also on antibiotic or probiotic treatments.
S- Editor Ma N L- Editor Kerr C E- Editor Yin DH
| 1. | Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 433] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S-11S. |
| 3. | Sartor RB. Intestinal microflora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2746] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 5. | Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:41-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Shanahan F. Host-flora interactions in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10 Suppl 1:S16-S24. [PubMed] |
| 7. | Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224-5231. [PubMed] |
| 8. | Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 604] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91-97. [PubMed] |
| 10. | Elson CO. Commensal bacteria as targets in Crohn's disease. Gastroenterology. 2000;119:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3901] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 12. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3474] [Article Influence: 144.8] [Reference Citation Analysis (1)] |
| 13. | Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 433] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 14. | Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995;108:1617-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 489] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 332] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Ursing B, Kamme C. Metronidazole for Crohn's disease. Lancet. 1975;1:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 106] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, Aerts R, Kerremans R, Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 509] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 957] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 19. | Neut C, Bulois P, Desreumaux P, Membré JM, Lederman E, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel JF. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol. 2002;97:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 638] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, Pochart P, Doré J, Marteau P. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 519] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 23. | Sartor RB. Does Mycobacterium avium subspecies paratuberculosis cause Crohn's disease? Gut. 2005;54:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Burke DA, Axon AT. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ. 1988;297:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Giaffer MH, Holdsworth CD, Duerden BI. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut. 1992;33:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Sankey EA, Dhillon AP, Anthony A, Wakefield AJ, Sim R, More L, Hudson M, Sawyerr AM, Pounder RE. Early mucosal changes in Crohn's disease. Gut. 1993;34:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Laffineur G, Lescut D, Vincent P, Quandalle P, Wurtz A, Colombel JF. Bacterial translocation in Crohn disease. Gastroenterol Clin Biol. 1992;16:777-781. [PubMed] |
| 29. | Ambrose NS, Johnson M, Burdon DW, Keighley MR. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br J Surg. 1984;71:623-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004;127:80-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 558] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 31. | Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut. 2007;56:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 32. | Lederman E, Neut C, Desreumaux P, Klein O, Gambiez L, Cortot A, Quandalle P, Colombel JF. Bacterial overgrowth in the neoterminal ileum after ileocolonic resection for Crohn's disease. Gastroenterology. 1997;112:1023. |
| 33. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Mylonaki M, Rayment NB, Rampton DS, Hudspith BN, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Masseret E, Boudeau J, Colombel JF, Neut C, Desreumaux P, Joly B, Cortot A, Darfeuille-Michaud A. Genetically related Escherichia coli strains associated with Crohn's disease. Gut. 2001;48:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2001;39:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499-4509. [PubMed] |
| 38. | Barnich N, Boudeau J, Claret L, Darfeuille-Michaud A. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol Microbiol. 2003;48:781-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. Strong decrease in invasive ability and outer membrane vesicle release in Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol. 2005;187:2286-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Rolhion N, Carvalho FA, Darfeuille-Michaud A. OmpC and the sigma(E) regulatory pathway are involved in adhesion and invasion of the Crohn's disease-associated Escherichia coli strain LF82. Mol Microbiol. 2007;63:1684-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Ismail S, Hampton MB, Keenan JI. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun. 2003;71:5670-5675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 43. | Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1325] [Article Influence: 66.3] [Reference Citation Analysis (2)] |
| 44. | Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 45. | Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529-5537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Bringer MA, Glasser AL, Tung CH, Méresse S, Darfeuille-Michaud A. The Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol. 2006;8:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Bringer MA, Barnich N, Glasser AL, Bardot O, Darfeuille-Michaud A. HtrA stress protein is involved in intramacrophagic replication of adherent and invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect Immun. 2005;73:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Bringer MA, Rolhion N, Glasser AL, Darfeuille-Michaud A. The oxidoreductase DsbA plays a key role in the ability of the Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 to resist macrophage killing. J Bacteriol. 2007;189:4860-4871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Peluso I, Pallone F, Monteleone G. Interleukin-12 and Th1 immune response in Crohn's disease: pathogenetic relevance and therapeutic implication. World J Gastroenterol. 2006;12:5606-5610. [PubMed] |
| 51. | Ryan P, Kelly RG, Lee G, Collins JK, O'Sullivan GC, O'Connell J, Shanahan F. Bacterial DNA within granulomas of patients with Crohn's disease--detection by laser capture microdissection and PCR. Am J Gastroenterol. 2004;99:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Van Kruiningen HJ, Civco IC, Cartun RW. The comparative importance of E. coli antigen in granulomatous colitis of Boxer dogs. APMIS. 2005;113:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Simpson KW, Dogan B, Rishniw M, Goldstein RE, Klaessig S, McDonough PL, German AJ, Yates RM, Russell DG, Johnson SE. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778-4792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | SCHOFIELD FW. Hjarre and Wramby disease in turkeys (coli-granuloma). Can J Comp Med Vet Sci. 1947;11:141-143. [PubMed] |
| 55. | Morishita TY, Bickford AA. Pyogranulomatous typhlitis and hepatitis of market turkeys. Avian Dis. 1992;36:1070-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1122] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 57. | Puissegur MP, Botanch C, Duteyrat JL, Delsol G, Caratero C, Altare F. An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol. 2004;6:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Meconi S, Vercellone A, Levillain F, Payré B, Al Saati T, Capilla F, Desreumaux P, Darfeuille-Michaud A, Altare F. Adherent-invasive Escherichia coli isolated from Crohn's disease patients induce granulomas in vitro. Cell Microbiol. 2007;9:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 602] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 60. | Bamias G, Sugawara K, Pagnini C, Cominelli F. The Th1 immune pathway as a therapeutic target in Crohn's disease. Curr Opin Investig Drugs. 2003;4:1279-1286. [PubMed] |