Published online Nov 7, 2007. doi: 10.3748/wjg.v13.i41.5501
Revised: May 29, 2007
Accepted: August 24, 2007
Published online: November 7, 2007
AIM: To investigate the regulation of activin receptor-interacting protein 2 (ARIP2) expression and its possible relationships with collagen type IV (collagen IV) in mouse hepatoma cell line Hepal-6 cells.
METHODS: The ARIP2 mRNA expression kinetics in Hepal-6 cells was detected by RT-PCR, and its regulation factors were analyzed by treatment with signal transduction activators such as phorbol 12-myristate 13-acetate (PMA), forskolin and A23187. After pcDNA3-ARIP2 was transfected into Hepal-6 cells, the effects of ARIP2 overexpression on activin type II receptor (ActRII) and collagen IV expression were evaluated.
RESULTS: The expression levels of ARIP2 mRNA in Hapel-6 cells were elevated in time-dependent manner 12 h after treatment with activin A and endotoxin LPS, but not changed evidently in the early stage of stimulation (2 or 4 h). The ARIP2 mRNA expression was increased after stimulated with signal transduction activators such as PMA and forskolin in Hepal-6 cells, whereas decreased after treatment with A23187 (25.3% ± 5.7% vs 48.1% ± 3.6%, P < 0.01). ARIP2 overexpression could remarkably suppress the expression of ActRIIA mRNA in dose-dependent manner, but has no effect on ActRIIB in Hepal-6 cells induced by activin A. Furthermore, we have found that overexpression of ARIP2 could inhibit collagen IV mRNA and protein expressions induced by activin A in Hapel-6 cells.
CONCLUSION: These findings suggest that ARIP2 expression can be influenced by various factors. ARIP2 may participate in the negative feedback regulation of signal transduction in the late stage by affecting the expression of ActRIIA and play an important role in regulation of development of liver fibrosis induced by activin.
- Citation: Zhang HJ, Tai GX, Zhou J, Ma D, Liu ZH. Regulation of activin receptor-interacting protein 2 expression in mouse hepatoma Hepa1-6 cells and its relationship with collagen type IV. World J Gastroenterol 2007; 13(41): 5501-5505
- URL: https://www.wjgnet.com/1007-9327/full/v13/i41/5501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i41.5501
Activin is a multifunctional growth and differentiation factor of transforming growth factor-beta (TGF-β) superfamily[1,2]. As an important regulator, activin is involved in the acute phase response of inflammatory diseases and tissue repair, and also play an important role in inducing liver fibrosis[3-5]. The actions of activin on target cells are tissue-specific, which associate with the difference of activin receptor signal transduction. It has been found that the tissue-specificity might depend on a new group of intracellular signal proteins, activin receptor-interacting proteins (ARIPs)[6-8]. ARIPs have four forms at least, all of which can specifically interact with activin type II receptor (ActRII) and regulate intracellular signal transduction induced by activin[6-10]. It has been demonstrated that not only the expression and distribution but also the biological activities of ARIPs were obviously different in various tissues. ARIP2 can enhance ActRII endocytosis and reduce ActRIIA receptor expression on cell membranes via Ral/RalBP1-depending pathway, and has a capability of suppressing activin-induced signal transduction. There was high expression of ARIP2 mRNA in liver tissues tested by Northern blot[7]. Therefore, we reason out that ARIP2 may participate in the functional regulation of hepatocytes treated by activin.
Since ARIP2 has only been recently discovered, the mode of expression regulation and function of it have not been well characterized. In this study, we have explored regulation of ARIP2 expression and its effects on the expression of collagen type IV (collagen IV) which is component of extracellular matrix (ECM), using mouse Hepal-6 cells, which were obtained from mouse hepatoma cell line and had functions of hepatic parenchymal cells[11].
Lipopolysaccharide (LPS, from E.coli 0111:B4), A23187, phorbol 12-myristate 13-acetate (PMA) and forskolin were obtained from Sigma. AMV Reverse Transcriptase was purchased from Promega. ExTaq was obtained from Takara Biotechnology Co (Kyoto, Japan). Dulbecco's modified Eagle's medium (DMEM) was purchased from GIBCO. Trizol reagent was obtained from Invitrogen. Activin A was provided by Dr. Eto T (Ajinomoto Central Research Laboratories, Japan).
Hepa1-6 cells from mouse hepatoma cell line were provided by Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China) and were maintained in DMEM medium supplemented with 10% fetal calf serum (FCS) at 37°C in a 5% CO2 humidified incubator.
The vector construction has been described previously[8]. The set of primers was designed as follows. The sense primer was 5'-GGAATTCATGAACGGACGGGTGGATTA-3', which introduced an EcoRIsite, and the anti-sense primer 5'-GCTCGAGTCATTGTCTGCACAATAAACA-3', which introduced an XholIsite. cDNA fragments encoding full-length ARIP2 (1-153 amino acid residues) were amplified by PCR. The amplified cDNA fragments were inserted into plasmid pMD18-T, and were then subcloned into eukaryotic expression vector pcDNA3. The reconstructed plasmid was named as pcDNA3-ARIP2.
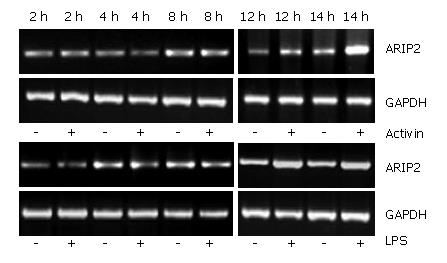
Hepal-6 cells were plated into 12-well tissue culture plates at a density of 2 × 105 cells/mL and incubated in 10% FCS-DMEM at 37°C, 5% CO2 over night. The cells were cultured in 2% FCS-DMEM in the presence or absence of activin A (5 ng/mL) and LPS (2.5 μg/mL), respectively. After 2, 4, 8, 12 and 24 h, the cells were harvested respectively and total RNA was extracted by using the TRIzol reagent according to the manufacturer's protocol (Invitrogen). The mRNA expression of ARIP2 was examined by RT-PCR, and GAPDH was considered as inner control. PCR was performed for 30 cycles. Amplified PCR products were subjected to 1.5% agarose gel electrophoresis, and stained with ethidium bromide for detection. Specific bands were analyzed using ImageMaster VDS (Pharmacia Biotech Company, Sweden). The primer sequences were shown at Table 1.
| Target | Primers | Sequences | Products size (bp) | Genbank No. |
| GAPDH | Sense | 5'-GATTGTTGCCATCAACGACC-3' | 371 | BC083149 |
| Antisense | 5'-GTGCAGGATGCATTGCTGAC-3' | |||
| ARIP2 | Sense | 5'-GTCAGCCGTATCAAAGAGGATG-3' | 371 | AY157057 |
| Antisense | 5'-CTTGTGGCAATACTTCTCTGGTG-3' | |||
| ActRIIA | Sense | 5'-ATTGGCCAGCATCCATCTCTTG-3' | 296 | XM_123799 |
| Antisense | 5'-TGCCACCATCATAGACTAGATTC-3' | |||
| ActRIIB | Sense | 5'-TGCTGAAGAGCGACCTCAC-3' | 544 | NM_007397 |
| Antisense | 5'-AGCAGGTCCACATTGGTGAC-3' | |||
| Collagen IV | Sense | 5'-GCCTGCTCAAGGAGAAGACA-3' | 380 | NM_007734 |
| Antisense | 5'-GATCCATAGGAGTCTCCAGGT-3' |
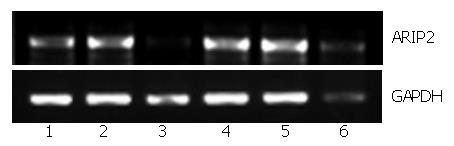
To further study the regulation elements of ARIP2 expression, the Hepal-6 cells plated into 12-well tissue culture plates were cultured in 2% FCS-DMEM in the presence or absence of activin A (5 ng/mL), A23187 (200 nmol/L), PMA (20 nmol/L), forskolin (50 μmol/L) and LPS (2.5 μg/mL), respectively. After 24 h, the cells were harvested respectively and total RNA was extracted by using the TRIzol reagent. The expression of ARIP2 mRNA was examined by RT-PCR.
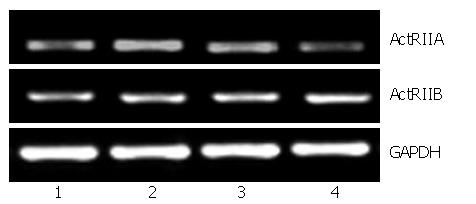
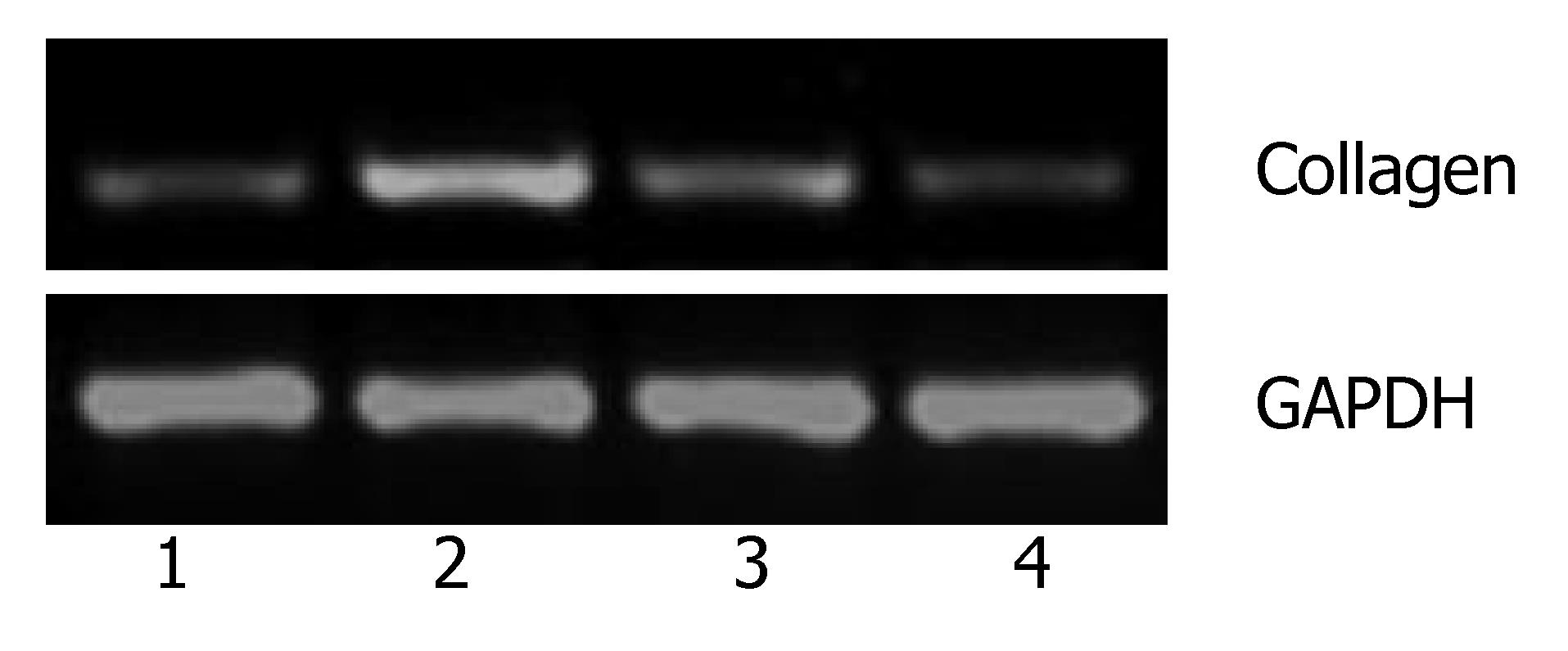
To determine possible bioactivity of ARIP2, effects of ARIP2 on the mRNA expressions of ActRIIA, ActRIIB and collagen type IV were analyzed by RT-PCR. The Hepal-6 cells were washed once with serum-free DMEM, and were then transfected with pcDNA3-ARIP2 (0.1, 0.3 μg) and pcDNA3 (0.3 μg) by using Lipofectamine 2000 reagent according to the manufacturer's protocol (Invitrogen), respectively. The transfected cells were incubated in the presence or absence of activin A (5 ng/mL) overnight. The cultured cells were harvested and total RNA was extracted by using the TRIzol reagent. RT-PCR was performed for detecting ActRIIA, ActRIIB and type IV collagen mRNA expressions. The primer sequences were shown at Table 1.
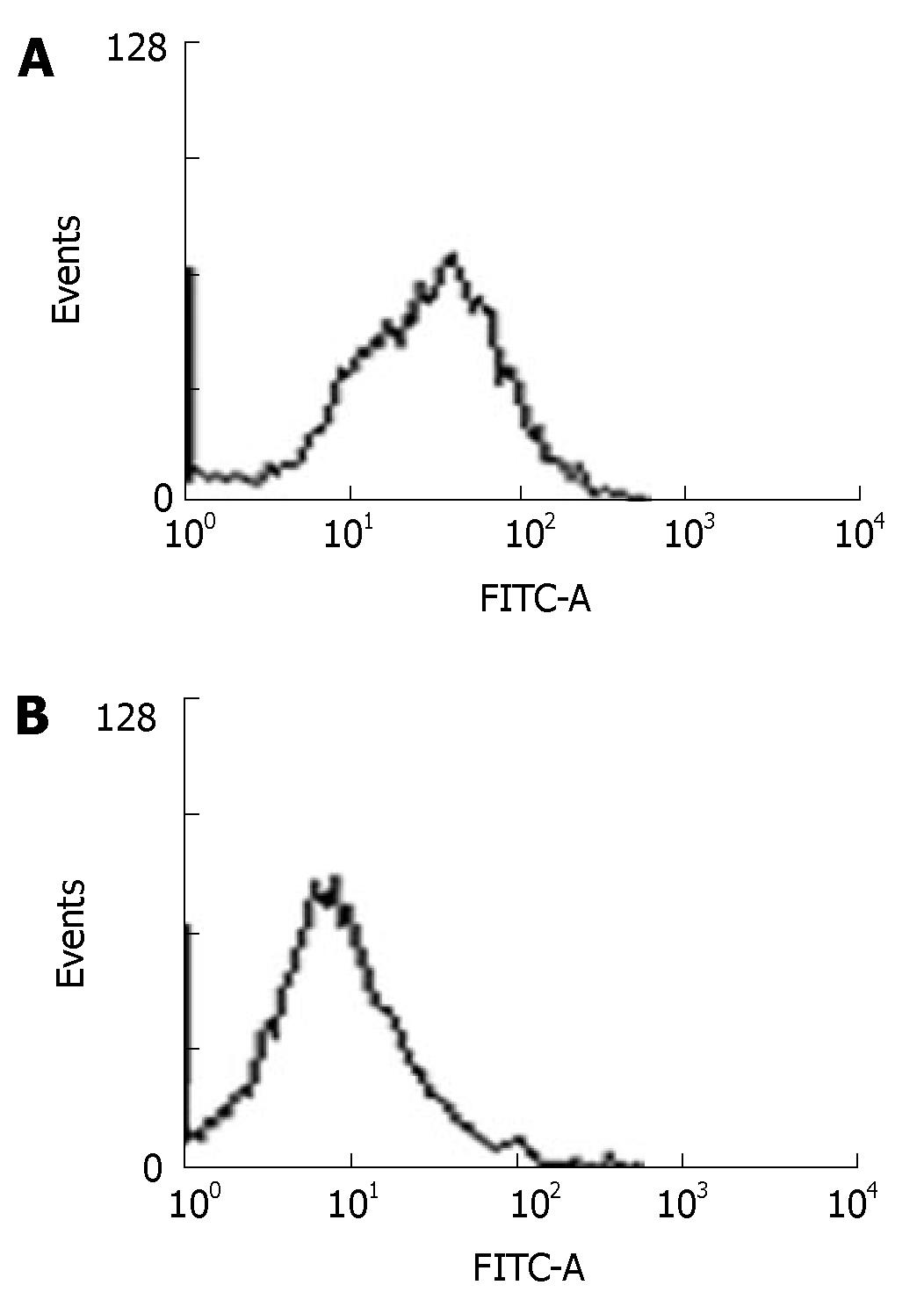
Hepal-6 cells were collected 24 h after transfected with pcDNA3-ARIP2. The expression of type IV collagen proteins were assessed by flow cytometry (FACSort Vantage; BD, Franklin Lakes, NJ ) using anti-mouse type IV collagen antibodies. The data were collected and analyzed on computer (Cell Quest software; BD Biosciences), to assess the percentage of positive fluorescence cells. A representative experiment of the two performed was shown.
As a regulation protein of activin signal pathway, the expression of ARIP2 mRNA could be increased by stimulation with activin A. In this study, the levels of ARIP2 mRNA expression were time-dependently up-regulated 12 h after treatment with activin A in Hepal-6 cells, but not obviously changed at 2-4 h after being treated with activin A. Endotoxin LPS as inflammatory factor can bind with Toll-like receptor 4 on hepatocytes. We found that ARIP2 mRNA expression in Hepal-6 cells was remarkably promoted by LPS treatment, and the expression levels were time-dependently up-regulated 12 h after treatment with LPS in Hepal-6 cells (Figure 1). These data suggested that the expression of ARIP2 was increased in the late stage of activin A and LPS treatment, and ARIP2 might participate in the negative regulation of the late stage signal transduction in Hepal-6 cells.
PMA is the activator of protein kinase C (PKC)[12], A23187 is the calcium ion vector[13], forskolin is the kinetin of cAMP-dependent protein kinase A (PKA)[14] and endotoxin LPS can bind with Toll-like receptor 4 on the surface of hepatocytes to stimulate cellular activities non-specifically[15]. To further study the regulation factors of ARIP2 expression, we used all of the above signal transduction activators to stimulate Hepal-6 cells and observed the expression of ARIP2 mRNA. The results showed that activin A (ARIP2 mRNA content relative to GAPDH, 66.2% ± 4.9%), LPS (76.5% ± 5.7%), PMA (72.3% ± 5.2%) and forskolin (79.8% ± 6.6%) could promote the expressions of ARIP2 mRNA (untreated control group, 48.1% ± 3.6%), whereas A23187 (25.3% ± 5.7%) could suppress it markedly (Figure 2), 25.3% ± 5.7% vs 48.1% ± 3.6%, P < 0.01. These data indicated that activators of the PKC, PKA signal transduction pathways and LPS via Toll-like receptor 4 could up-regulated the expression of ARIP2 mRNA.
To investigate the biological activities of ARIP2 expression in Hepal-6 cells, expression vectors pcDNA3-ARIP2 were transfected into Hepal-6 cells and the effects of ARIP2 overexpression on ActRIIA and ActRIIB expression were observed in Hepal-6 cells. In this study, we found that ARIP2 overexpression could obviously suppress the expression of ActRIIA mRNA in Hepal-6 cells induced by activin A in dose-dependent manner, but has no effect on ActRIIB (Figure 3). These findings indicated that ARIP2 might down-regulated the expression of ActRIIA to suppress activin signal transduction in hepatocytes.
The previous studies showed that activin A could induce liver fibrosis and stimulate excess secretion of ECM components, for example, collagen and fibronectin[3,16]. As an inhibitor of activin signal transduction, ARIP2 maybe influence the collagen production in hepatocytes induced by activin A. In this study, activin A could obviously stimulate the expression of type IV collagen mRNA in Hepal-6 cells. Whereas, after transfecting pcDNA3-ARIP2 into Hepa1-6 cells for 24 h, the ARIP2 overexpression could significantly suppress the expressions of type IV collagen mRNA induced by activin A in dose-dependent manner (Figure 4). To further determine the type IV collagen protein expression, the mature type IV collagen protein levels in Hepa1-6 cells were examined by flow cytometry. The results showed that ARIP2 overexpression could remarkably inhibit the expression levels of type IV collagen proteins in Hepa1-6 cells induced by activin A (the percent of positive fluorescence cells, 2% vs 16%) (Figure 5), which results were the same with that of type IV collagen mRNA by RT-PCR. These findings suggested that high level expression of ARIP2 might influence the expression of ECM components in hepatocytes and down-regulate the development of liver fibrosis induced by activin.
ARIPs have obvious expression and distribution diversity, which are key factors to the histological specificity of activin action[6-8]. It has been demonstrated that both ARIP1 and ARIP2 are inhibitors of activin signal transduction. However, ARIP1 mainly distributed in nerve tissues, ARIP2 widely existed in tissues. The high expression of ARIP2 mRNA could be detected in liver tissue by Northern blot[7]. In order to investigate the kinetic changes of ARIP2 expression, we used activin A and endotoxin LPS to stimulate Hepal-6 cells, and then examined the expression of ARIP2 mRNA. The results showed that the expression levels of ARIP2 mRNA were not changed obviously after being treated by activin in the early stage, but up-regulated depending on time 12 to 24 h after treatment (Figure 1). Stimulated by LPS, ARIP2 expression is also up-regulated evidently 12 h after treatment. In the present study, we further examined the effects of signal transduction activators PMA, A23187 and forskolin on the expression of ARIP2 in Hepal-6 cells (Figure 2). These data indicated that activin A, LPS, PMA and forskolin could promote the expression of ARIP2 mRNA in Hepal-6 cell, whereas calcium ion vector-A23187 could inhibit the expression of ARIP2 mRNA. These findings suggested that ARIP2 expression could be influenced by various factors and might participate in the regulation of signal transduction in the late stage in Hepal-6 cells.
Activin receptors are the members of serine/threonine kinase receptors[9,10]. Activin binds to receptor type II to form a complex primarily. The complex interacts with the receptor typeIand makes it phosphorylated, then activates endocellular Smad2/3 protein binding to receptor typeI. Finally, it transduces signal into nucleus mediated by Smad4. Therefore, ActRIIs are the crucial receptors of activin signal transduction. In this study, we found that both ActR IIA and ActR IIB could be expressed in heapl-6 cells. To investigate the biological activities of ARIP2 expression in Hepal-6 cells, we transfected Hepal-6 cells with pcDNA3-ARIP2 and observed the effect of ARIP2 overexpression on ActRIIA and ActRIIB expressions in Hepal-6 cells. The results showed that ARIP2 overexpression could obviously suppress the expression of ActRIIA mRNA in Hepal-6 cells induced by activin A, but had no effect on ActRIIB (Figure 3). All the above data indicated that ARIP2 could down-regulated the expression of ActRIIA and participate in the process of negative feedback regulation of activin signal in hepatocytes.
Activin not only play an important role in regulating secretion of hormone, but also serves as autocrine and paracrine factors to regulate the differentiation, proliferation, apoptosis of cells and embryonic development[17-21]. The latest studies have reported that as an important regulator, activin also has effects on inducing liver fibrosis, suppressing hepatocyte growth and so on[21-26]. It has been demonstrated that activin was produced by hepatocyte and hepatic stellate cell (HSC) and could promote HSC activation and stimulate excess production of ECM components, for example, collagen and fibronectin[3,16,25]. It has been reported that activin A could be expressed positively in fibrotic hepatocytes, and it also could take actions by autocrine[3,26]. In this study, we found that activin A could stimulate the expression of type IV collagen mRNA, whereas, the ARIP2 overexpression could remarkably suppress the mRNA expression of type IV collagen in Hepal-6 cells induced by activin A (Figure 4) and decrease the protein expression levels of type IV collagen. As a kind of collagen composed ECM, type IV collagen could co-deposited in Diss with fibronectin in the early stage of hepatic injury and take part in the formation of liver fibrosis. These findings suggested that ARIP2 overexpression might influence the synthesis of ECM in hepatocyte and negatively regulate the formation and development of liver fibrosis induced by activin A.
In conclusion, ARIP2 can be up-expressed in Hepal-6 cells by inducement with various factors and may participate in the regulation of signal transduction in the late stage. We may release or restraint liver diseases induced by activin and achieve the goal of treatment if ARIP2 expression can be elevated in hepatocytes to inhibit the effects of activin.
The authors thank Dr. Eto T (Ajinomoto Central Research Laboratories, Japan) for the kind gift of activin A, Dr. Tsuchida K (Institute for Comprehensive Medical Science, Fujita Health University, Toyoake, Japan) and Sugino H (Institute for Enzyme Research, The University of Tokushima, Tokushima 770-8503, Japan) for his help in field work.
Activin A is involved in hepatic fibrosis formation. However, the mechanism of fibrotic process is not well understood. In this study, effects of anti-fibrosis by ARIP2 are investigated in mouse Hepal-6 cells.
ARIP2 is a regulator of activin signaling pathway, but studies about its regulation in production of component of extracellular matrix (ECM)s are not reported.
Since ARIP2 has only been recently discovered, the mode of expression regulation and function of it have not been well characterized. We designed this experiment to investigate ARIP2 expression and its effects on the expression of collagen type IV by using Hepal-6 cells.
No ideal drug is available so far for the therapy of hepatic fibrosis. ARIP2 may be play an important role in regulation of development of liver fibrosis induced by activin.
Activin receptor-interacting protein2 (ARIP2) can specifically interact with activin type II receptor (ActRII) and down-regulate intracellular signal transduction induced by activin.
The topic is of interest for that up to now no antifibrotic therapy is available in patients with hepatic fibrosis. The negative effect of ARIP2 on production of component of extracellular matrix described in this paper shows that ARIP2 might be a potential treatment option.
S- Editor Zhu LH L- Editor Li M E- Editor Yin DH
| 1. | Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 733] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1385] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Wada W, Kuwano H, Hasegawa Y, Kojima I. The dependence of transforming growth factor-beta-induced collagen production on autocrine factor activin A in hepatic stellate cells. Endocrinology. 2004;145:2753-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Endo D, Maku-Uchi M, Kojima I. Activin or follistatin: which is more beneficial to support liver regeneration after massive hepatectomy? Endocr J. 2006;53:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 2006;17:157-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Shoji H, Tsuchida K, Kishi H, Yamakawa N, Matsuzaki T, Liu Z, Nakamura T, Sugino H. Identification and characterization of a PDZ protein that interacts with activin type II receptors. J Biol Chem. 2000;275:5485-5492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Matsuzaki T, Hanai S, Kishi H, Liu Z, Bao Y, Kikuchi A, Tsuchida K, Sugino H. Regulation of endocytosis of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem. 2002;277:19008-19018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Liu ZH, Tsuchida K, Matsuzaki T, Bao YL, Kurisaki A, Sugino H. Characterization of isoforms of activin receptor-interacting protein 2 that augment activin signaling. J Endocrinol. 2006;189:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Tsuchida K, Matsuzaki T, Yamakawa N, Liu Z, Sugino H. Intracellular and extracellular control of activin function by novel regulatory molecules. Mol Cell Endocrinol. 2001;180:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Tsuchida K, Nakatani M, Matsuzaki T, Yamakawa N, Liu Z, Bao Y, Arai KY, Murakami T, Takehara Y, Kurisaki A. Novel factors in regulation of activin signaling. Mol Cell Endocrinol. 2004;225:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Darlington GJ. Liver cell lines. Methods Enzymol. 1987;151:19-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Yokoyama G, Fujii T, Tayama K, Yamana H, Kuwano M, Shirouzu K. PKCdelta and MAPK mediate G(1) arrest induced by PMA in SKBR-3 breast cancer cells. Biochem Biophys Res Commun. 2005;327:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Shaposhnikova VV, Egorova MV, Kudryavtsev AA, Levitman MKh. The effect of melittin on proliferation and death of thymocytes. FEBS Lett. 1997;410:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Hoshi T, Garber SS, Aldrich RW. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988;240:1652-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-3752. [PubMed] |
| 16. | Date M, Matsuzaki K, Matsushita M, Tahashi Y, Sakitani K, Inoue K. Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury. J Hepatol. 2000;32:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton DA. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 452] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Liu ZH, Shintani Y, Sakamoto Y, Harada K, Zhang CY, Fujinaka Y, Abe M, Goto T, Saito S. Effects of LHRH, FSH and activin A on follistatin secretion from cultured rat anterior pituitary cells. Endocr J. 1996;43:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Zhang XJ, Li Y, Tai GX, Xu GY, Zhang PY, Yang Y, Lao FX, Liu ZH. Effects of activin A on the activities of the mouse peritoneal macrophages. Cell Mol Immunol. 2005;2:63-67. [PubMed] |
| 20. | Takamura K, Tsuchida K, Miyake H, Tashiro S, Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. J Surg Res. 2005;126:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Chen W, Woodruff TK, Mayo KE. Activin A-induced HepG2 liver cell apoptosis: involvement of activin receptors and smad proteins. Endocrinology. 2000;141:1263-1272. [PubMed] |
| 22. | Kanamaru C, Yasuda H, Fujita T. Involvement of Smad proteins in TGF-beta and activin A-induced apoptosis and growth inhibition of liver cells. Hepatol Res. 2002;23:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Yuen MF, Norris S, Evans LW, Langley PG, Hughes RD. Transforming growth factor-beta 1, activin and follistatin in patients with hepatocellular carcinoma and patients with alcoholic cirrhosis. Scand J Gastroenterol. 2002;37:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Hughes RD, Evans LW. Activin A and follistatin in acute liver failure. Eur J Gastroenterol Hepatol. 2003;15:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | De Bleser PJ, Niki T, Xu G, Rogiers V, Geerts A. Localization and cellular sources of activins in normal and fibrotic rat liver. Hepatology. 1997;26:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Huang X, Li DG, Wang ZR, Wei HS, Cheng JL, Zhan YT, Zhou X, Xu QF, Li X, Lu HM. Expression changes of activin A in the development of hepatic fibrosis. World J Gastroenterol. 2001;7:37-41. [PubMed] |