Published online Oct 28, 2007. doi: 10.3748/wjg.v13.i40.5408
Revised: July 28, 2007
Accepted: August 29, 2007
Published online: October 28, 2007
We report a case of acute hepatotoxicity in a 42-year-old woman after administration of clindamycin for a dental infection. After 6 d of treatment, she had fatigue, nausea, vomiting, anorexia, pruritus and jaundice. Her laboratory analysis showed alanine aminotransferase (ALT), 1795 IU/L (normal range 0-40); aspartate aminotransferase (AST), 1337 IU/L (normal range 5-34); alkaline phosphatase (ALP), 339 IU/L (normal range 40-150); γ-glutamyl transpeptidase (GGT), 148 IU/L (normal range 9-64 IU/L); total bilirubin, 4.1 mg/dL; direct bilirubin, 2.9 mg/dL and prothrombin time (PT), 13.5 s, with international normalized ratio (INR), 1.04. She was hospitalized, with immediate drug discontinuation. Her liver biopsy specimen showed mixed-type (both hepatocellular and cholestatic) hepatic injury, compatible with a diagnosis of drug-induced hepatitis. An objective causality assessment using the Naranjo probability scale suggested that clindamycin was the probable cause of the acute hepatitis. In susceptible individuals, clindamycin use may lead to acute mixed-type liver toxicity. Complete recovery may be possible if the drug is discontinued before severe liver injury is established.
- Citation: Aygün C, Kocaman O, Gürbüz Y, Şentürk &, Hülagü S. Clindamycin-induced acute cholestatic hepatitis. World J Gastroenterol 2007; 13(40): 5408-5410
- URL: https://www.wjgnet.com/1007-9327/full/v13/i40/5408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i40.5408
Liver injury may be caused by various drugs and chemicals. The severity of injury may vary from minor non-specific changes in hepatic structure and function to fulminant hepatic failure, cirrhosis and even hepatocellular cancer. The term drug-induced liver disease should be confined to cases in which the nature of the liver injury has been characterized histologically, because biochemical parameters used to detect liver injury may also be elevated as an adaptive response to drugs.
The lincosamide antibiotics seem to be most useful against the bacteria classified as Gram-positive cocci. In addition, they are helpful against protozoa, such as Toxoplasma and Mycoplasma, as well as many anaerobic bacteria[1]. Clindamycin (7-chlorolincomycin), a semisynthetic derivate of lincomycin, has been extensively used in the therapy of obstetric, gynecologic and oral infections for over 20 years. It is more active than erythromycin or clarithromycin against anaerobic bacteria. In humans, absorption of clindamycin is rapid and virtually complete (90%) following oral administration, and is widely distributed throughout the body[2]. We describe herein a case of acute hepatitis following clindamycin use for dental infection.
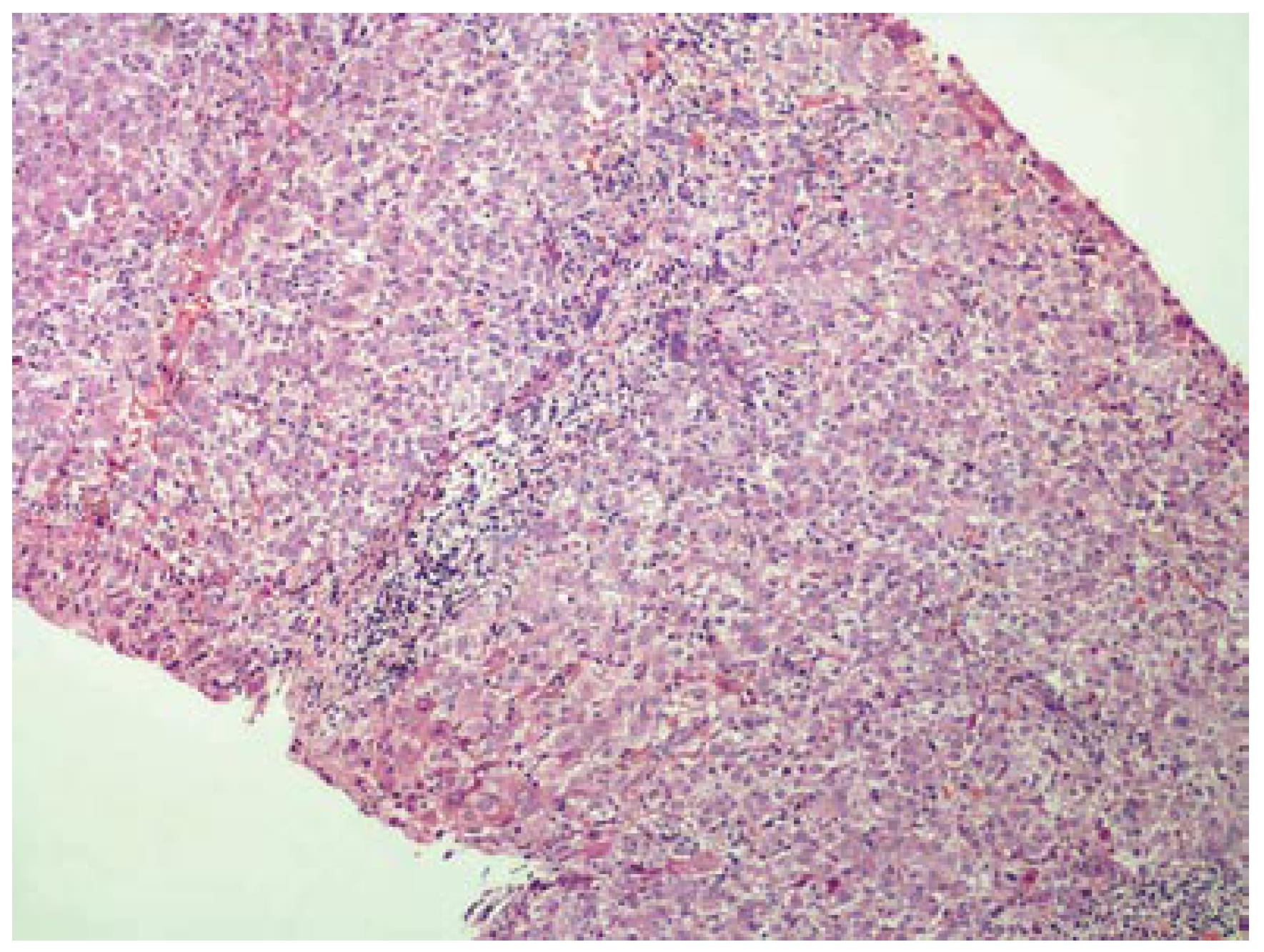
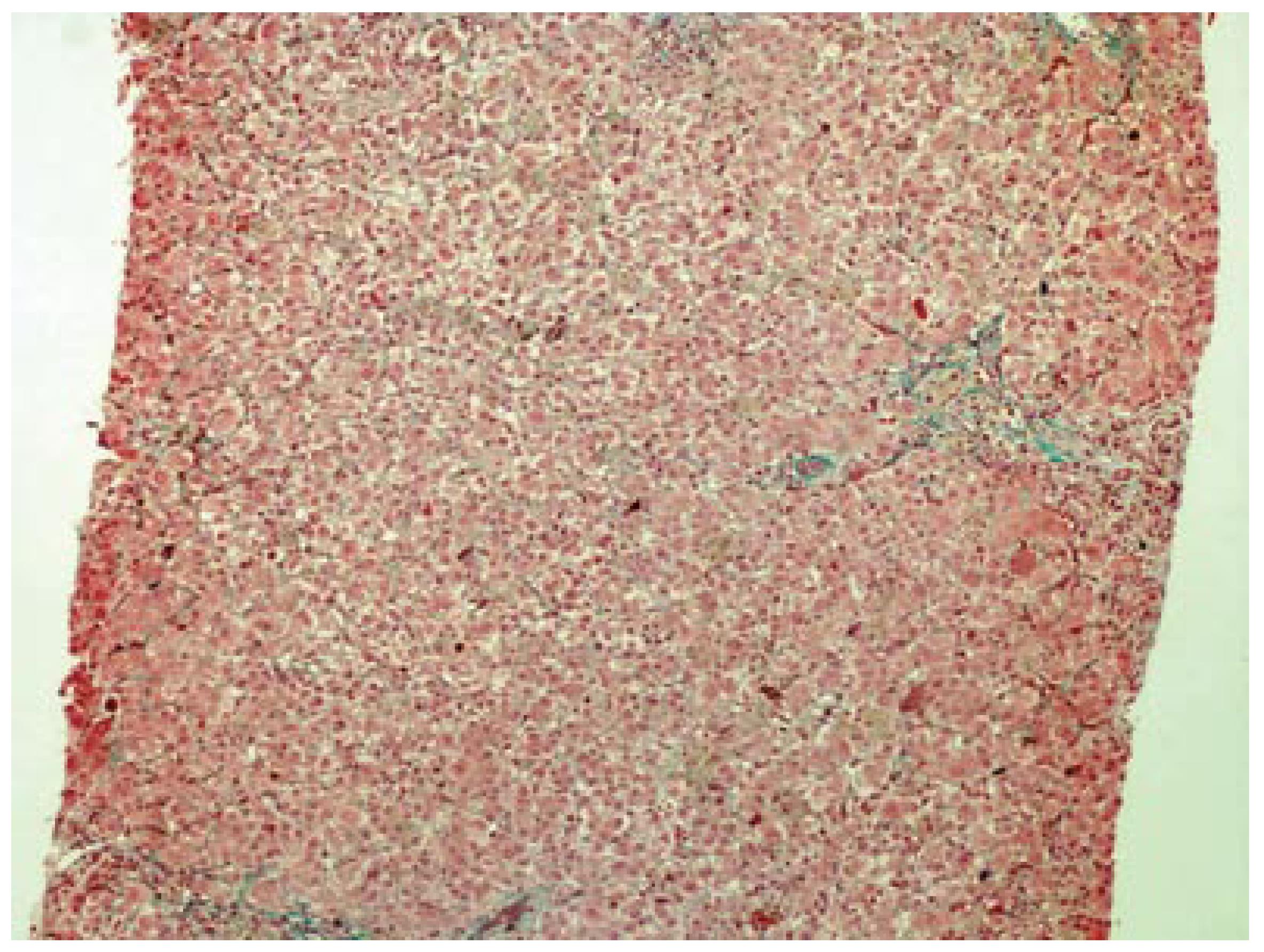
A 42-year-old woman was admitted to hospital for pruritus, jaundice and asthenia. Her medical history included recurrent gingival infections treated with clindamycin on several previous occasions. Six days after the initiation of the last clindamycin (150 mg PO qid) treatment, the patient had fatigue, nausea, vomiting, anorexia, pruritis and jaundice. Emergency laboratory analysis showed the following results: Alanine aminotransferase (ALT), 1795 IU/L (normal range 0-40); aspartate aminotransferase (AST), 1337 IU/L (normal range 5-34); alkaline phosphatase (ALP), 339 IU/L (normal range 40-150); γ-glutamyl transpeptidase (GGT), 148 IU/L (normal range 9-64); total bilirubin, 4.1 mg/dL; direct bilirubin, 2.9 mg/dL and prothrombin time (PT), 13.5 s, with international normalized ratio (INR), 1.04. Blood tests for glucose, blood urea nitrogen (BUN), serum creatinine, total protein, albumin, uric acid, total cholesterol and triglycerides were within normal limits. There was no peripheral eosinophilia. The patient was hospitalized and clindamycin treatment was discontinued. Additional test results for hepatitis A, B and C viruses, cytomegalovirus, and Epstein-Barr virus were negative. Ferritin and ceruloplasmin levels were within normal limits. Autoantibodies (antinuclear, antimitochondrial, anti-smooth-muscle, anti-liver-kidney microsomal enzymes and anti-soluble liver antigen) were also negative. Hepatobiliary imaging with ultrasonography was normal. She denied taking any other medications, or using alcohol or any herbal or folk remedies at any time. There were no known contributing environmental issues. One week after hospitalization, her liver function tests were as follows: ALT, 1579 IU/L; AST, 1031 IU/L; ALP, 345 IU/L; GGT, 112 IU/L; total bilirubin, 3.34 mg/dL; and direct bilirubin, 2.4 mg/dL. Her medical history and laboratory results suggested severe acute hepatitis of mixed-type, with hepatocellular and cholestatic hepatic injury, and liver biopsy was performed. The histopathological samples revealed centrilobular and portal cholestatic hepatitis, without fibrosis or necrosis, highly suggestive of drug-related hepatotoxicity (Figures 1 and 2). Within 3 wk of hospitalization, the patient had significant subjective improvement, and showed improvement of liver function tests (ALT, 754 IU/L; AST, 429 IU/L; ALP, 175 IU/L; GGT, 82 IU/L; total bilirubin, 1.8 mg/dL; and direct bilirubin, 1.26 mg/dL). After 8 wk hospitalization, her liver function test results were within normal limits (ALT, 28 IU/L; AST, 23 IU/L; and total bilirubin, 0.74 mg/dL). The patient was discharged in good condition. The probability that the symptoms of hepatotoxicity that occurred after clindamycin treatment were an adverse drug reaction (ADR) was assessed using the Naranjo ADR probability scale. The total Naranjo score for the patient was 7, which is in the "probable" range[3].
Six days after the start of treatment with clindamycin, our 42-year-old female patient experienced signs of hepatotoxicity with jaundice and asthenia. Laboratory testing showed marked elevations in serum ALT, AST, ALP, GGT and total bilirubin concentrations. Her symptoms, as well as the liver tests, resolved on discontinuation of the drug treatment. The subtype of the hepatotoxicity observed in the patient was considered as mixed hepatocellular and cholestatic. The diagnosis of severe clindamycin-induced hepatotoxicity in our case was suggested by: (1) symptoms such as fatigue, nausea, vomiting, anorexia, pruritis and jaundice 6 d after clindamycin initiation; (2) the chronology between clindamycin introduction and liver test abnormalities; (3) histological findings that were highly suggestive of a toxic mechanism; (4) absence of any other clear etiology; and (5) recovery after clindamycin withdrawal.
Cholestasis with hepatitis is a frequent type of hepatic drug reaction and it is characterized by conspicuous cholestasis with hepatocellular injury. Histological lesions may show lobular and portal tract inflammation, often with neutrophils, eosinophils or mononuclear cells[4]. The clinical spectrum of cholestatic hepatitis was indicated in our case by pruritis and jaundice. It was also reflected by the markedly elevated liver tests, with increased serum bilirubin, GGT and ALP. Cases of mixed cholestasis and hepatitis are considered highly suggestive of a drug reaction[5].
To exclude dilation of large bile ducts produced by biliary obstruction, and hepatic or pancreatic masses, hepatobiliary imaging is essential. Ultrasound is the most preferred method to rule out possible bile duct obstruction. Endoscopic retrograde cholangiopancreatography, percutaneous transhepatic cholangiography or computed tomography may be necessary in difficult cases. In the absence of the symptoms mentioned earlier, drug-induced cholestasis is more likely, and a liver biopsy is often advisable. Certain histological findings, such as lobular and portal tract inflammation, suggest a hepatic drug reaction, whereas others such as edema of the portal tracts suggest biliary obstruction. When the temporal relationship to drug ingestion indicates a high probability of a drug reaction, particularly when the agent is known to be potentially hepatotoxic, it is appropriate to discontinue the incriminated drug and observe whether improvement occurs. Recovery may be seen rapidly if the drug is discontinued before severe liver injury is established[6].
Hepatocytes, because of their capability to metabolize drugs, form minute amounts of drug-protein adducts, for which the immune system normally shows tolerance. Hypersensitivity reactions occur when this tolerance is impaired. Additional signals, such as a concomitant inflammatory reaction, may eventually be needed to break this tolerance. The allergic hepatitis induced by drugs is generally a type IV hypersensitivity reaction involving CD4+, CD8+ cytotoxic lymphocytes, as well as natural killer cells. Antibodies directed to the drug are much less common. Antibodies against cellular components may, however, occur when the sensitization process evolves towards an autoimmune reaction[7,8]. In our patient, autoantibodies were negative and several features, such as the absence of predictable dose-dependent toxicity of clindamycin and pruritis (hypersensitivity manifestations), were consistent with a metabolic type of idiosyncratic toxicity.
Clindamycin is a widely used antibiotic that is available in many countries. Previously, another case of hepatotoxicity associated with use of clindamycin has been reported, in which the clinicopathological spectrum encompassed cholestatic liver disease with portal inflammation, bile duct injury and bile duct paucity (ductopenia). In the same patient, a second biopsy after clinical recovery showed resolution of cholestasis but persistence of duct paucity, whereupon the authors concluded that early onset hepatic injury with cholestasis and duct paucity may occur after clindamycin use, and long term duct paucity may persist on repeat biopsy[9]. Bile duct paucity as a biopsy finding was not significant in our patient.
In summary, with this report, we presented a case of hepatotoxicity after 6 d of oral clindamycin treatment for dental infection. The toxicity appeared to be associated with drug use. In conclusion, although rare, mixed-type (hepatocellular and cholestatic) hepatic injury might be associated with clindamycin use in some cases. Patients may present with fatigue, nausea, vomiting, anorexia, pruritis and jaundice. Complete recovery may be possible if the drug is discontinued before critical liver injury is established.
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
| 1. | Mazur D, Schug BS, Evers G, Larsimont V, Fieger-Büschges H, Gimbel W, Keilbach-Bermann A, Blume HH. Bioavailability and selected pharmacokinetic parameters of clindamycin hydrochloride after administration of a new 600 mg tablet formulation. Int J Clin Pharmacol Ther. 1999;37:386-392. [PubMed] |
| 2. | DeHaan RM, Metzler CM, Schellenberg D, VandenBosch WD, Masson EL. Pharmacokinetic studies of clindamycin hydrochloride in humans. Int J Clin Pharmacol. 1972;6:105-119. [PubMed] |
| 3. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8202] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 4. | Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 819] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 5. | Farrel GC. Liver disease caused by drugs, anesthetics and toxins. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 7th ed. Philadelphia: Saunders 2002; 1403-1437. |
| 6. | Chitturi S, Farrell GC. Drug-Induced Liver Disease. Curr Treat Options Gastroenterol. 2000;3:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 477] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Depta JP, Pichler WJ. Cross-reactivity with drugs at the T cell level. Curr Opin Allergy Clin Immunol. 2003;3:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Altraif I, Lilly L, Wanless IR, Heathcote J. Cholestatic liver disease with ductopenia (vanishing bile duct syndrome) after administration of clindamycin and trimethoprim-sulfamethoxazole. Am J Gastroenterol. 1994;89:1230-1234. [PubMed] |