Published online Oct 28, 2007. doi: 10.3748/wjg.v13.i40.5384
Revised: August 12, 2007
Accepted: August 24, 2007
Published online: October 28, 2007
AIM: To investigate the potential protective effect of HO-1 on cirrhotic liver cells in rats.
METHODS: Male Wistar rats included in the current study were randomly divided into 5 groups as follows: normal (N) group; liver cirrhotic (LC) group; sham (S) group; I/R group and I/R + hemin group. The model for inducing liver cirrhosis in rats was established according to a previously published protocol. Following this the segmental hepatic ischemia reperfusion operation was carried out. The rats were treated with 30 μmol/kg hemin (HO-1 inducer, ferric protoporphyrin IX chloride) i.p. or 0.9% NaCl (control) 24 h and 12 h before hepatic ischemia for 30 min or sham laparotomy. Blood was collected for serum enzymatic measurement 6 and 12 h after reperfusion or sham laparotomy. HO-1, NF-κB and caspase-3 expressions were assessed by immunohistochemical analysis.
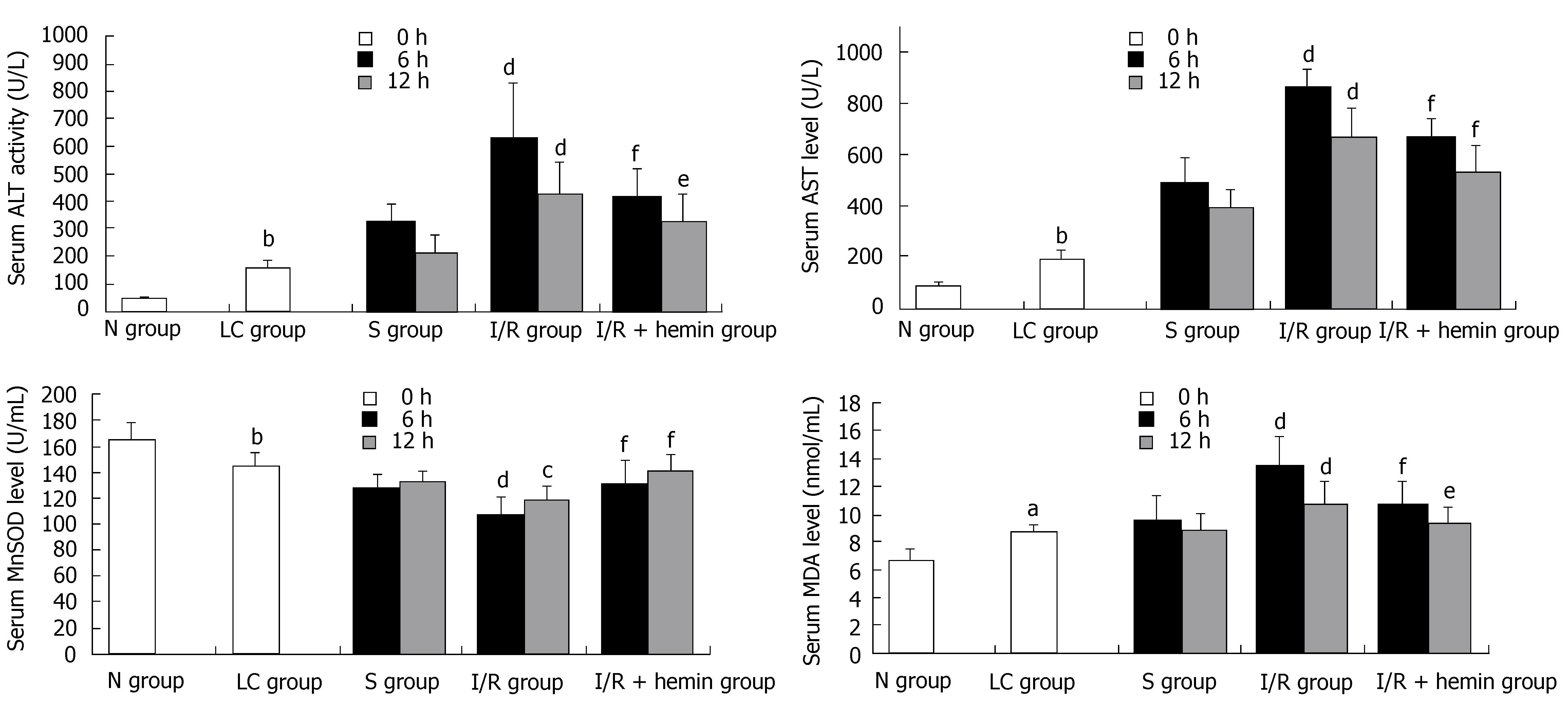
RESULTS: The expressions of proteins are inversely correlated to the gray values. HO-1 expression in the I/R + hemin group was increased significantly than I/R group at 6 h and 12 h after hepatic I/R (6 h: 112.0 ± 8.3 vs 125.1 ± 5.7, P < 0.01; 12 h: 120.8 ± 11.0 vs 132.4 ± 6.2, P < 0.01). Hemin improved serum manganese superoxide dismutase (MnSOD) (6 h: 131.3 ± 17.6 vs 107.0 ± 13.9, P < 0.01; 12 h: 141.4 ± 12.5 vs 118.3 ± 10.2, P < 0.01), lessened liver cell injury, decreased caspase-3(6 h: 166.7 ± 8.1 vs 145.5 ± 14.6, P < 0.01; 12 h: 172.8 ± 3.8 vs 148.0 ± 6.5, P < 0.01) and NF-κB expression (6 h: 150.2 ± 8.6 vs 139.7 ± 6.0, P < 0.01; 12 h: 151.1 ± 5.9 vs 148.1 ± 5.3, P > 0.05) and serum alanine aminotransferase (ALT) (6 h: 413.3 ± 104.1 vs 626.8 ± 208.2, P < 0.01; 12 h: 322.2 ± 98.8 vs 425.8 ± 115.4, P < 0.05), aspartate aminotransferase (AST) (6 h: 665.2 ± 70.1 vs 864.3 ± 70.4, P < 0.01; 12 h: 531.1 ± 98.6 vs 664.4 ± 115.6, P < 0.01), malondialdehyde (MDA) levels (6 h: 11.1 ± 2.17 vs 13.5 ± 2.01, P < 0.01; 12 h: 9.36 ± 1.10 vs 10.8 ± 1.62, P < 0.05) in the I/R + hemin group when compared with the I/R group.
CONCLUSION: These results suggest that HO-1 plays an important role in protecting liver cells from hepatic I/R injury in cirrhotic rats by decreasing oxidative stress, apoptosis and inflammation.
- Citation: Xue H, Guo H, Li YC, Hao ZM. Heme oxygenase-1 induction by hemin protects liver cells from ischemia/reperfusion injury in cirrhotic rats. World J Gastroenterol 2007; 13(40): 5384-5390
- URL: https://www.wjgnet.com/1007-9327/full/v13/i40/5384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i40.5384
Gastrointestinal bleeding, liver surgery and liver transplantation may lead to hepatic ischemia/reperfusion (I/R) injury. Previous studies have shown that pro-inflammatory, apoptosis, oxidative stress and microcirculation dysfunction are strongly correlated with hepatic I/R[1,2]. Many patients presenting with hepatic ischemia have liver cirrhosis disease. Some studies have shown that cells from a cirrhotic liver are more fragile than the cells from a normal liver. Similarly, other studies have found that the anti-oxidative capacity during stress decreases in patients with hepatic decompensated cirrhosis[3,4], and further, the tolerance time of ischemia is shorter in cirrhotic rats than in normal rats[5].
Heme oxygenases (HOs), the rate-limiting enzymes in heme catabolism, catalyzes the oxidative degradation of heme into carbon monoxide (CO), free iron, and biliverdin. Three isoforms of the enzyme have been identified: The inducible HO-1, also known as heat shock protein 32, the constitutive HO-2, and a not fully defined HO-3. It has been found that HO-1 serves as a protective gene by virtue of anti-inflammatory, anti-apoptotic and anti-oxidative actions and improves microcirculation in many cell types[6,7].
Amersi et al[8] reported that up-regulation of HO-1 protects genetically fat Zucker rat livers from I/R injury. Some researchers have demonstrated that HO-1 has potent protecting effects against I/R injury in various organ systems[9,10]. Kaizu et al[11] reported that prior induction of the HO-1 protein may lead to anti-inflammatory and anti-apoptotic effects on warm renal I/R injury.
HO-1 has been shown to protect liver cells from I/R injury[12,13]. However, there are few reports regarding the protective effect of HO-1 on liver cells affected by the pathological condition of cirrhosis. Investigations into the role of HO-1 in protecting liver cells from hepatic I/R injury in cirrhotic rats and the mechanism by which it achieves this protective effect have great significance. Thus, the objective of the present study was to investigate whether upregulating HO-1 would result in reduced damage to liver cells as a result of hepatic I/R in a liver cirrhotic rat model. Secondly, we investigated the possible mechanism/s by which HO-1 exerts its protective effect.
Adult male Wistar rats (weighting 200-250 g) were obtained from the experimental Animal Center of Xi'an Jiaotong University. Ethical approval for this study was obtained from the Ethical Committee on Animal Experiments at the Medical College of Xi'an Jiaotong University. The rats had free access to food and water, were kept in an air-conditioned room at 23°C, with a 12 h/12 h light/dark cycle, and were handled humanely. Liver cirrhosis was induced by subcutaneous injection of 400 mL/L CCl4-olive oil solution twice a week at an initial dose of 5 mL/kg. Subsequent doses were adjusted to body mass changes at a dose of 3 mL/kg for 11 wk as described previously[14,15]. The rats in the normal group received the same dose of pure olive oil only. The I/R operation was carried out in wk 12.
All 91 animals were randomly divided into 5 groups as follows: Normal (N) group; liver cirrhotic (LC) group; sham (S) group (cirrhotic group operated); I/R group and I/R + hemin group. The last three groups were divided into two subgroups for sampling at different time-points. The I/R + hemin group was administered 30μmol/kg hemin[16] (HO-1 inducer, Sigma chemical Co., USA) 24 h and 12 h before hepatic I/R. Hemin solution was prepared under subdued lighting by dissolving the compound in 1 mL of 0.2 mol/L NaOH, adjusting the pH to 7.4 with 1 mol/L HCl, and diluting the solution with 9 g/L NaCl[11]. The stock concentration was 5 g/L. The solution was kept in the dark and used within 1 h. The concentration dose of hemin used in the present study was selected according to previous reports.
Rats were anaesthetized using ketamine hydrochloride(800 mg/kg) intraperitoneally. The laparotomy was performed through a midline abdominal incision. Partial (70%) hepatic ischemia of the median and the left lobes was induced by placing non-crushing microvascular clamps around the appropriate branches of the portal vein and hepatic artery for 30 min. The portal branches to the right and caudate lobes were left open to prevent mesenteric venous congestion[17]. Reperfusion was initiated by removal of the clamps. Hepatic I/R injury was induced by 30 min of ischemia followed by 6 h and 12 h of reperfusion. After 30 min of ischemia, the clamps were removed allowing the liver to reperfuse, and the wound was closed with 3-0 silk. Sham operated rats underwent isolation of the portal vein and hepatic artery without occlusions. At the indicated time after the start of reperfusion, rats were anesthetized and sacrificed. Blood was collected from the portal vein and liver samples were taken from the left lobe. The blood samples were centrifuged to obtain the serum for the biochemical analyses, and the left lobe liver tissue samples(0.8 cm × 0.4 cm × 0.4 cm) were fixed in 40 g/L paraformaldehyde for immunohistochemical analysis.
Serial 5μmol/L sections were prepared after the samples had been dehydrated in graded ethanol solutions, cleared in chloroform and embedded in paraffin. Immunohistochemical staining was performed to detect expression of NF-κB(diluted 1:300; Boster, China), caspase-3 (diluted 1:100; Boster, China), and HO-1 (diluted 1:100; Boster, China) using the MaxVisionTM kit-5004 (Maxin Biotechnology Co., Fujian, China). Sections were boiled in 0.01 mol/L citrate buffer (pH 6.0) to retrieve the antigens. Endogenous peroxidases were inactivated by immersing the sections in 30 mL/L hydrogen peroxide for 20 min. The sections were then incubated overnight at 4°C with the relevant primary rabbit anti-rat polyclonal antibodies. After washing, the sections were overlaid with peroxidase-conjugated goat anti-rabbit secondary antibodies (MaxVisionTM kit-5004, Maxin Biotechnology Co., Fujian, China) for 15 min. The chromogenic reaction was developed with diaminobenzidine. Some sections were counterstained with hematoxylin. The immunohistochemical signal was analyzed using an image acquiring and analysis system (QWin500CW, Leica, Germany). At least four random fields of each section were examined at a magnification of × 400, and analyzed using a computer image analysis system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). The expressions of proteins are inversely correlated to the gray values.
For the assessment of hepatic injury, serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) activities were measured using an automatic biochemical analyzer (Vitros 250, Johnson & Johnson Co., USA). Serum Malondialdehyde (MDA) and superoxide dismutase (SOD) activity were measured with a commercially available kit (Nanjing Jiancheng Bioengineering Institute, China, NJBI). Total SOD and Cu/Zn-SOD activities were measured. Mn-SOD activity was calculated as the difference between total SOD and Cu/Zn-SOD activity.
All results were expressed as mean ± SD. The statistical analysis was performed using a one-way analysis of variance. The results were considered statistically significant if P < 0.05.
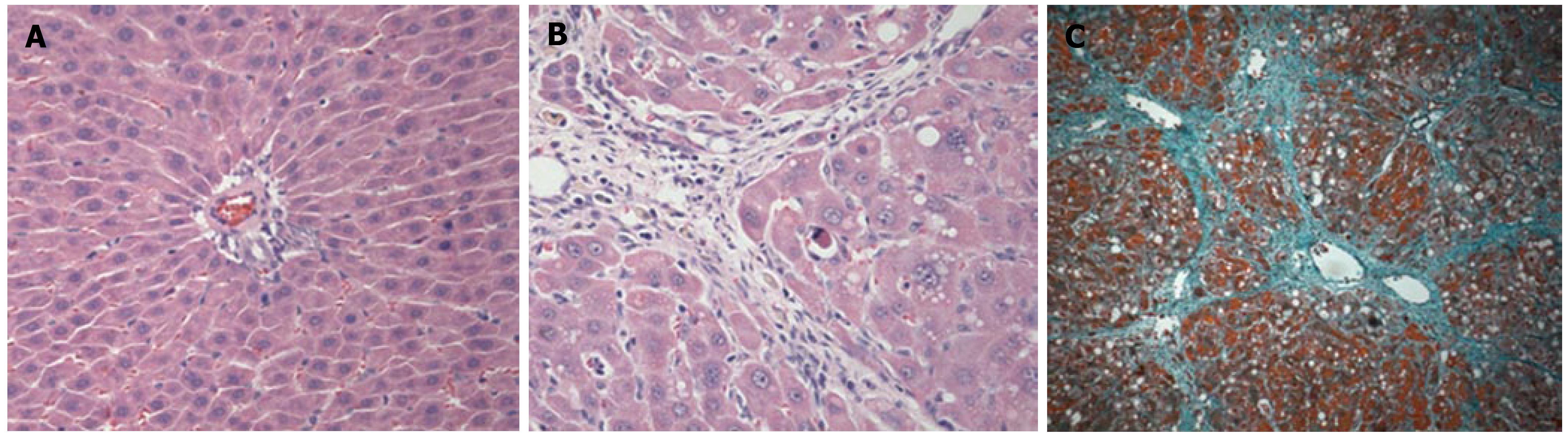
To confirm the occurrence of liver cirrhosis, the liver tissues were examined following hematoxylin and eosin staining (HE staining) and Masson's trichrome staining (Figure 1). Biochemical analyses of the serum and immunohistochemical analysis of the liver samples were also performed. The levels of serum ALT and AST activities are markers for hepatic injury, MDA and MnSOD reflect the anti-oxidative ability. Following the administration of CCl4 for 11 wk, the activities of ALT, AST and MDA in the LC group were increased when compared with those observed in the N group. The MnSOD in the LC group was decreased when compared with the N group (Figure 2).
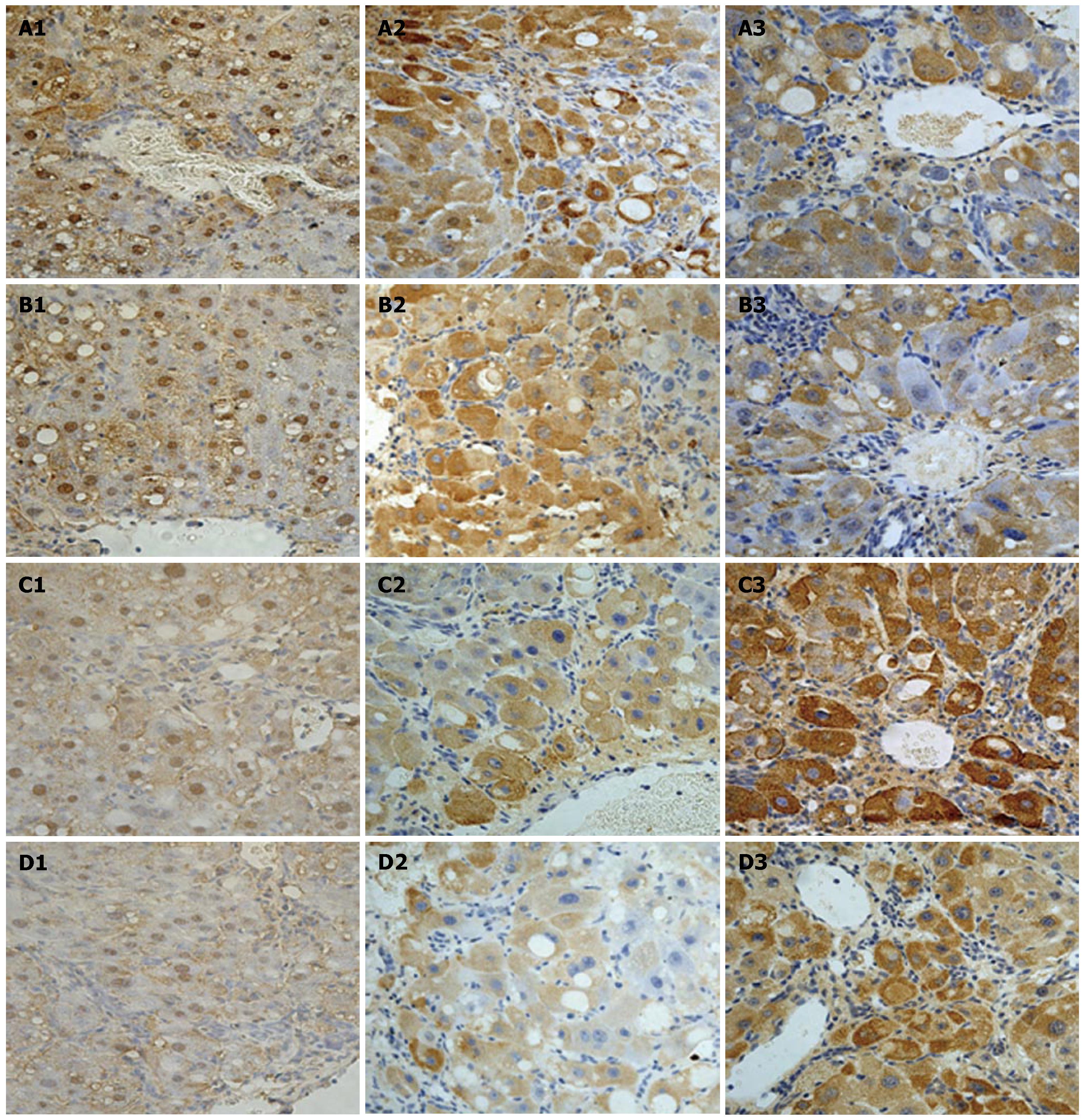
Animals in the I/R + Hemin group were given hemin prior to the induction of I/R. We examined the changes in HO-1 expression in liver tissues after I/R using the gray value method. HO-1 expression in the I/R + hemin group was increased significantly, especially 6 h after reperfusion (Table 1 and Figure 3). Hepatic I/R resulted in an increase in serum ALT, AST and MDA levels in the I/R group compared with the S group, especially at 6 h after reperfusion. In contrast, the I/R + hemin group had significantly lower serum ALT, AST and MDA levels at both time-points relative to the I/R group (Figure 2). MnSOD levels were markedly decreased in the I/R group at 6 h and 12 h after hepatic I/R compared with that of the S group. However, hemin administration increased MnSOD levels in the I/R+hemin group at both time-points (Figure 2).
| Group | n | NF-κB | Caspase-3 | HO-1 | |
| Normal | 6 | 172.0 ± 1.7 | 188.3 ± 3.5 | 158.9 ± 2.6 | |
| Liver cirrhosis | 10 | 164.1 ± 3.5b | 176.8 ± 4.9b | 148.1 ± 3.7b | |
| Sham | 6 h | 10 | 159.4 ± 4.7d | 177.2 ± 2.7d | 145.4 ± 2.9d |
| 12 h | 10 | 159.6 ± 4.2d | 178.5 ± 3.9d | 146.8 ± 3.8d | |
| I/R | 6 h | 10 | 139.7 ± 6.0 | 145.5 ± 14.6 | 125.1 ± 5.7 |
| 12 h | 9 | 148.1 ± 5.3 | 148.0 ± 6.5 | 132.4 ± 6.2 | |
| I/R+hemin | 6 h | 10 | 150.2 ± 8.6d | 166.7 ± 8.1d | 112.0 ± 8.3d |
| 12 h | 9 | 151.1 ± 5.9c | 172.8 ± 3.8d | 120.8 ± 11.0d |
Caspase-3 and NF-κB immunohistochemical staining
To examine the protective effects of elevated HO-1 expression on apoptosis and inflammation in the liver following hepatic I/R, caspase-3 and NF-κB expression in the liver were assessed by immunohistochemical staining. Most positive NF-κB expression was observed in the nucleus of liver cells, while caspase-3 expression was localized in the cytoplasm of hepatocytes. After hepatic I/R, the expression of NF-κB and caspase-3 was significantly stronger with a lower gray value in the I/R group compared with the S group (P < 0.01). After giving hemin to upregulate HO-1, caspase-3 expression in the I/R + hemin group was decreased 6 h and 12 h after reperfusion and the gray values were increased (P < 0.01). Following upregulation of HO-1, the expression of NF-κB in the I/R + hemin group was lower 6 h after reperfusion than in the I/R group (P < 0.01), but there was no difference 12 h after reperfusion between the two groups (P > 0.05, Figure 3).
In this study, we have shown that HO-1 upregulation following hemin administration protects cirrhotic rats from hepatic I/R injury. Hepatic injury induced by I/R has been proposed as a key clinical problem associated with liver upper gastrointestinal bleeding, liver surgery or liver transplantation. There are two distinct phases of liver injury after I/R. The initial phase (< 2 h after reperfusion) is characterized by oxidant stress, where production and release of reactive oxygen species (ROS) appears to directly result in hepatocellular injury. The late phase of liver injury, from 6 h to 48 h after hepatic reperfusion, is an inflammatory disorder mediated by recruited neutrophils[18-20]. Our results regarding the mechanism leading to I/R injury will undoubtedly provide important clues for developing an approach to decreasing liver cell damage caused by I/R. Protection against hepatic I/R injury is, clinically, one of the most critical problems in liver cirrhotic patients.
Recently, it was demonstrated that HO-1 upregulation ameliorates hepatic I/R injury[12,13]. In addition, Coito et al[21] reported that HO-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from I/R injury. In the present study, our data support the hypothesis that HO-1 provides a protective effect against I/R injury in cirrhotic rats via anti-oxidative, anti-apoptotic and anti-inflammatory actions. Our results also highlight that administration of hemin may be an important new therapy for the treatment of hepatic I/R. Although these mechanisms are complicated and largely unknown, our findings suggest the possibility that upregulation of HO-1 may reduce the potential risk in hepatocellular I/R complicated with liver cirrhosis.
HO-1 is one of the most critical of the cytoprotective mechanisms activated during cellular stress, exerting anti-oxidative and anti-inflammatory functions, modulating the cell cycle, and maintaining the microcirculation. The upregulation of HO-1 is thought to be a protective response from cellular stress following ischemia, inflammation and radiation. Given the multi-factorial cytoprotective properties of HO-1, it is used as a novel strategy to prevent I/R injury[22]. In line with this, we have found that hemin administration significantly protects the cells in the hepatic I/R as compared with control treatment.
The role of SOD in hepatic I/R has received more attention. There are three isoforms of SOD. The Cu/ZnSOD is located in the cytosol, the MnSOD is primarily a mitochondrial enzyme, and extracellular SOD is usually found on the outside of the plasma membrane interacting with matrix component. Marczin et al[23] reported that, by utilizing adenoviral gene transfer, overexpression of MnSOD may reduce the extent of in vivo regional I/ R injury in the rat heart. Similarly, in the current study we found that MnSOD levels were increased, and the injury due to I/R was lessened, after giving hemin to induce HO-1 expression. Based on this, we suggest that HO-1 has an anti-oxidant function during hepatic I/R.
MDA is the main product of lipid peroxidation, and is reactive with thiobarbituric acid (TBA). MDA concentration is therefore generally presented as the total level of lipid peroxidation products[24]. As the end product of lipid peroxidation MDA can produce ozone, which reacts rapidly with cellular structures, generates hydrogen peroxide and other active oxygen species and causes peroxidation and denaturation of membranes[25]. Our findings suggest that HO-1, induced by hemin administration, decreases the serum MDA level after hepatic I/R. Therefore, we speculate that high HO-1 expression induced by hemin may decrease the lipid peroxidation and play an anti-oxidative role in hepatic I/R.
Apoptosis plays a vital role in lethal hepatic I/R injury as indicated by many studies in various animal models[2,26]. There are two major pathways of apoptosis, and caspase-3 is the final executor of apoptosis[27]. Although the TUNEL assay is a very sensitive and widely used method[28,29], data is also available suggesting that caspase-3 immunohistochemistry is an easy, sensitive, and reliable method for detecting and qualifying apoptosis in tissue sections[30]. In the present study, to determine the effect of HO-1 on apoptosis, we carried out an immunohistochemical analysis of caspase-3. The expression of caspase-3 was elevated following hepatic I/R. However, caspase-3 expression was lower in the I/R + hemin group than in the I/R group. These data suggest that the increase in HO-1 following treatment with hemin results in a decrease in apoptosis during hepatic I/R in liver cirrhotic rats. Our findings in this regard are consistent with other studies[31].
NF-κB is activated during I/R of the liver, and plays an important and complex role in the gene expression of proinflammatory cytokines (TNF-α and IL-1), chemokines and adhesion molecules (ICAM-1 and VCAM-1), which will lead to the tissue injury[32]. In the present study, the level of NF-κB at 6 h after reperfusion was decreased in the I/R + hemin group compared with the I/R group. However, there was no statistical difference 12 h after reperfusion between the I/R group and the I/R + hemin group. We believe that high levels of HO-1 expression may decrease NF-κB expression to lessen the inflammatory reaction at 6 h after reperfusion.
Hepatic I/R injury may cause more severe tissue damage in cirrhotic liver than in normal livers. Our data show that HO-1 overexpression, induced by hemin, plays a crucial role in protecting liver cells from hepatic I/R injury in cirrhotic rats. Our results also raise the possibility of a new treatment for reducing hepatic I/R injury in the pathological condition of cirrhosis.
Gastrointestinal bleeding, liver surgery and liver transplantation may lead to hepatic ischemia/reperfusion (I/R) injury. Previous studies have shown that pro-inflammatory, apoptosis, oxidative stress and microcirculation dysfunction are strongly correlated with hepatic I/R. Many patients presenting with hepatic ischemia have liver cirrhosis disease. Some studies have shown that cells from a cirrhotic liver are more fragile than the cells from a normal liver. Similarly, other studies have found that the anti-oxidative capacity during stress decreases in patients with hepatic decompensated cirrhosis, and further, the tolerance time of ischemia is shorter in cirrhotic rats than in normal rats.
Three isoforms of the Heme oxygenases (HOs) have been identified: The inducible HO-1, also known as heat shock protein 32, the constitutive HO-2, and a not fully defined HO-3. It has been found that HO-1 serves as a protective gene by virtue of anti-inflammatory, anti-apoptotic and anti-oxidative actions and improves microcirculation in many cell types.
Researchers have demonstrated that HO-1 has potent protecting effects against I/R injury in various organ systems. It has also been reported that upregulation of HO-1 protects genetically fat Zucker rat livers from I/R injury and that prior induction of the HO-1 protein may lead to anti-inflammatory and anti-apoptotic effects on warm renal I/R injury. HO-1 has been shown to protect liver cells from I/R injury. Investigations into the role of HO-1 in protecting liver cells from hepatic I/R injury in cirrhotic rats and the mechanism by which it achieves this protective effect have great significance.
There are few reports regarding the protective effect of HO-1 on liver cells affected by the pathological condition of cirrhosis. The objective of the present study was to investigate whether upregulation of HO-1 would lessen the liver cell damage caused by hepatic I/R in a liver cirrhotic rat model and, secondly, to study the possible mechanisms for its protective effect. Our data support the hypothesis that HO-1 has a protective effect from I/R injury in cirrhotic rats. Our data further suggest that this effect is mediated by the anti-oxidative, anti-apoptotic and anti-inflammatory actions of HO-1, and highlight that administration of hemin may be an important new therapy method in hepatic I/R. Although many of these mechanisms are still unknown, our findings suggest the possibility that upregulation of HO-1 may reduce the potential risk in hepatocellular I/R complicated with liver cirrhosis.
Hepatic I/R injury may cause more severe tissue damage in a cirrhotic liver than in a normal liver. Our data have shown that HO-1 over-expression, induced by hemin, plays a crucial role in protecting liver cells from hepatic I/R injury in cirrhotic rats, and raises the possibility of a new treatment in hepatic I/R injury in the pathological condition of cirrhosis.
Heme oxygenases (HOs): Heme oxygenases (HOs) are the rate-limiting enzymes in heme catabolism. HOs catalyse the oxidative degradation of heme into carbon monoxide (CO), free iron, and biliverdin. Three isoforms of the enzyme have been identified: HO-1, HO-2 and HO-3. Superoxide dismutase (SOD): Superoxide dismutase (SOD) is a ubiquitous enzyme with an essential function in protecting cells against oxidative stress by catalytic removal of superoxide radicals and conversion to H2O2 by the dismutation reaction.
In this manuscript, Hui Xu et al have analyzed the beneficial effects of hemin-dependent induction of hepatic heme-oxygenase-1 expression in cirrhotic livers after ischemia/reperfusion which is an interesting paper.
S- Editor Ma N L- Editor Li M E-Editor Li JL
| 1. | Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 432] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Kang KJ. Mechanism of hepatic ischemia/reperfusion injury and protection against reperfusion injury. Transplant Proc. 2002;34:2659-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Koruk M, Aksoy H, Akçay F, Onuk MD. Antioxidant capacity and nitric oxide in patients with hepatic cirrhosis. Ann Clin Lab Sci. 2002;32:252-256. [PubMed] |
| 4. | Rhoden EL, Pereira-Lima L, Kalil AN, Lucas ML, Mauri M, Menti E, Rhoden CR, Pereira-Lima J, Zettler CG, Belló-Klein A. Effects of ischemia and reperfusion on oxidative stress in hepatic cirrhosis induced by carbon tetrachloride in rats. Kobe J Med Sci. 2000;46:171-180. [PubMed] |
| 5. | Figueras J, Farran L, Benasco C, Ribas Y, Ramos E, Borobia FG, Fradera R, Castellví J, Lama C, Jaurrieta E. Vascular occlusion in hepatic resections in cirrhotic rat livers: an experimental study in rats. Liver Transpl Surg. 1997;3:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Yang S, Shih HJ, Chow YC, Tsai PS, Wang TY, Wang PS, Huang CJ. The protective role of heme oxygenase-1 induction on testicular tissues after testicular torsion and detorsion. J Urol. 2007;177:1928-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Wang XH, Wang K, Zhang F, Li XC, Qian XF, Cheng F, Li GQ, Fan Y. Alleviating ischemia-reperfusion injury in aged rat liver by induction of heme oxygenase-1. Transplant Proc. 2004;36:2917-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Katori M, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. A novel strategy against ischemia and reperfusion injury: cytoprotection with heme oxygenase system. Transpl Immunol. 2002;9:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Gandhi CR, Nemoto EM, Watkins SC, Subbotin VM. An endothelin receptor antagonist TAK-044 ameliorates carbon tetrachloride-induced acute liver injury and portal hypertension in rats. Liver. 1998;18:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Xu JW, Gong J, Chang XM, Luo JY, Dong L, Hao ZM, Jia A, Xu GP. Estrogen reduces CCL4- induced liver fibrosis in rats. World J Gastroenterol. 2002;8:883-887. [PubMed] |
| 16. | Datta PK, Duann P, Lianos EA. Long-term effect of heme oxygenase (HO)-1 induction in glomerular immune injury. J Lab Clin Med. 2006;147:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ku Y, Kusunoki N, Shiotani M, Maeda I, Iwasaki T, Tominaga M, Kitagawa T, Fukumoto T, Suzuki Y, Kuroda Y. Stimulation of haematogenous liver metastases by ischaemia-reperfusion in rats. Eur J Surg. 1999;165:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med (Berl). 1999;77:577-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Coito AJ, Buelow R, Shen XD, Amersi F, Moore C, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation. 2002;74:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Marczin N, El-Habashi N, Hoare GS, Bundy RE, Yacoub M. Antioxidants in myocardial ischemia-reperfusion injury: therapeutic potential and basic mechanisms. Arch Biochem Biophys. 2003;420:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Drewa G, Krzyzyńska-Malinowska E, Woźniak A, Protas-Drozd F, Mila-Kierzenkowska C, Rozwodowska M, Kowaliszyn B, Czajkowski R. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med Sci Monit. 2002;8:BR338-BR343. [PubMed] |
| 25. | Ajamieh HH, Menéndez S, Martínez-Sánchez G, Candelario-Jalil E, Re L, Giuliani A, Fernández OS. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia-reperfusion. Liver Int. 2004;24:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Zhao ZQ. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr Opin Pharmacol. 2004;4:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Kam PC, Ferch NI. Apoptosis: mechanisms and clinical implications. Anaesthesia. 2000;55:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Elsässer A, Suzuki K, Schaper J. Unresolved issues regarding the role of apoptosis in the pathogenesis of ischemic injury and heart failure. J Mol Cell Cardiol. 2000;32:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, Kim YM, Chung HT. Overexpression of heme oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radic Biol Med. 2004;36:858-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |