Published online Oct 28, 2007. doi: 10.3748/wjg.v13.i40.5371
Revised: July 20, 2007
Accepted: August 13, 2007
Published online: October 28, 2007
AIM: To assess Magnetic resonance colonography with fat enema as a method for detection of colorectal neoplasm.
METHODS: Consecutive twenty-two patients underwent MR colonography with fat enema before colonoscopy. T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence was acquired with the patient in the supine position before and 75 s after Gadopentetate Dimelumine administration. Where by, pre and post MR coronal images were obtained with a single breath hold for about 20 s to cover the entire colon. The quality of MR colonographs and patients' tolerance to fat contrast medium was investigated. Colorectal neoplasms identified by MR colonography were compared with those identified on colonoscopy and sensitivity of detecting the lesions was calculated accordingly.
RESULTS: MR colonography with fat enema was well tolerated without sedation and analgesia. 120 out of 132 (90.9%) colonic segments were well distended and only 1 (0.8%) colonic segment was poor distension. After contrast enhancement scan, mean contrast-to-noise ratio (CNR) value between the normal colonic wall and lumen was 18.5 ± 2.9 while mean CNR value between colorectal neoplasm and lumen was 20.2 ± 3.1. By Magnetic resonance colonography, 26 of 35 neoplasms (sensitivity 74.3%) were detected. However, sensitivity of MRC was 95.5% (21 of 22) for neoplasm larger than 10 mm and 55.6% (5 of 9) for 5-10 mm neoplasm.
CONCLUSION: MR colonography with fat enema and T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence is feasible in detecting colorectal neoplasm larger than 10 mm.
- Citation: Zhang S, Peng JW, Shi QY, Tang F, Zhong MG. Colorectal neoplasm: Magnetic resonance colonography with fat enema-initial clinical experience. World J Gastroenterol 2007; 13(40): 5371-5375
- URL: https://www.wjgnet.com/1007-9327/full/v13/i40/5371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i40.5371
Conventional colonoscopy, with its ability to visualize the mucosa directly, is the standard procedure for evaluating the colon[1]. But poor patients' acceptance and high cost limit the utility of the technique for colorectal screening[2]. This catalyzes the investigation for non-invasive methods to visualize the colon. A great number of studies about computed tomography (CT) colonography have been reported in the United States and Europe[3-6]. But CT colonography has its intrinsic limitation of exposing patients to ionizing radiation and magnetic resonance colonography (MRC) has theoretic capacity for originating soft-tissue contrast 10-1000 times greater than that on CT[7,8]. So the future of colorectal screening has shifted to MRC.
To date, most of the MRC research needs administration of a rectal enema containing paramagnetic contrast medium and the acquisition of three-dimensional (3D) gradient-echo pulse sequence[9-12]. This approach directly demonstrates the lumen but not the bowel wall and requires an effective breath hold of up to 30 s. In addition, enema containing paramagnetic contrast medium needs to be compounded by staff themselves and has high cost. These factors may limit its widespread application in the symptomatic and screening population.
Recently, several MRC studies used air, carbon dioxide and water as intraluminal contrast medium which provides negative contrast within bowel lumen and have a high accuracy in diagnosing colorectal neoplasm[13-16]. But there are few patients in the MRC studies with air enema and the data required from MRC with water enema could not provide the magnetic resonance virtual endoscopy (MRVE) mode.
In this paper, we evaluated the feasibility of detection of colorectal neoplasm with fat enema in MRC using T1-weighted 3D fast spoiled gradient-echo with inversion recovery sequence.
The study was approved by the Ethics Committee of the Fudan University Cancer Hospital. All patients who participated in the study were requested to review and sign a written informed consent. No patient has a history of severe arrhythmia and glaucoma. MRC was performed in consecutive 22 patients (9 men, 13 women) aged 46-86 (mean age 58.6) years. Owing to rectal bleeding, positive fecal occult blood test or altered bowel habits, these patients had been referred for conventional colonoscopy.
Exclusion criteria included contraindications to magnetic resonance imaging, such as the presence of a pacemaker, metallic implants in the central nervous system, as well as claustrophobia.
Following a standard bowel preparation (oral ingestion of 0.5 L of 20% mannite and 1 L of 5% glucose sodium chloride solution), MRC was performed with a 1.5T MR system (Signa twinspeed with excite; GE medical system, Milwaukee, Wis). No sedative or analgesic agents were administered. An eight-channel body coil was used for signal transmission and reception to permit coverage of the entire colon. To minimize bowel peristalsis, 20 mg of raceanisodamine hydrochloride (No.1 Biochem & Pharm, Shanghai, China) were injected intramuscularly.
Following placement of a rectal enema tube (202-26, Shuangling Medical Device, China), fat contrast medium (Kangque; Atai medical system, Inner Mongolia, China) were gently insufflated into the colon at up to 1 m of hydrostatic pressure with the patient lying in the prone position. Fat insufflation was stopped when the patient began to feel uncomfortable. Reinsufflation was not a routine procedure. The administered volume was 1200-1500 mL.
MRC was performed using T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence. Coronal images were obtained in the supine position with a single breath hold that lasted about 20 s to cover the entire colon. Imaging parameters included: repetition time 4 ms, echo time 2 ms, flip angle 14°, field of view 38-42 cm, matrix 256 × 256 and section thickness 5 mm. Zero interpolation was applied in coronal plane, rendering an effective section thickness of 2.5 mm and a matrix of 512 × 512. About 68 sections were required. Subsequently, using an automatic injector (Optistar, Mallinckrodt; Tyco, Missouri, USA), gadopentetate dimelumine (Magnevist; Schering, Berlin, Germany) was intravenously administered by the basilic vein. Injection parameters included a dosage of 0.2 mmol/kg and a flow rate of 2.0 mL/s, followed by rapid injection of 10 mL of normal saline at the same rate. After a delay of 75 s, 3D acquisition was repeated with identical imaging parameters with the patient in the supine position.
The 3D data sets were transferred to a post processing workstation (Advantage 4.0; GE Medical System) and evaluated by two radiologists who were blinded to the colonoscopic data. All the data sets were assessed in the multiplanar reformation and MRVE mode. For the purpose of analysis, the entire colon was divided into six segments: cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The colonic distension was classified by scores as follows: score 1 = well distension; score 2 = moderate distension, if it was distended enough to visualize the mucosa and fat interface; and score 3 = poor distension, if it was collapsed. For quantitative assessment, region of interest (ROI) was placed in the lumen, all the colonic neoplasm and the adjacent healthy colonic walls. Image noise, defined as the standard deviation of signal intensities (SI) measured in a ROI placed outside the body, were determined[14]. On the basis of these measurements, contrast-to-noise ratio (CNR) values were calculated: CNR = [SI (colonic wall/colonic lesion)-SI (lumen)]/noise.
Localization and size of all detected colonic neoplasm was recorded. Colorectal neoplasm was classified based on size: (a) < 5 mm; (b) 5-10 mm; (c) > 10 mm in diameter. Extracolonic lesions, including hepatic metastasis, were also recorded as additional findings. Colonoscopic findings were considered as the standard of reference.
All patients completed the MRC without any complication, but 4 patients did not complete colonoscopy for obvious stenosis of colon. Of the 132 colonic segments examined, distensions were documented as follows: 120 (90.9%) had a score of 1, 11 (8.3%) had a score of 2, and 1 (0.8%) had a score of 3. Distinct motion artefacts affecting diagnosis were not found.
Mean CNR values were documented as follows: between the normal colonic wall and lumen, pre contrast enhancement scan 8.4 ± 2.1, post contrast enhancement scan 18.5 ± 2.9; between colorectal neoplasm and lumen, post contrast enhancement scan 20.2 ± 3.1.
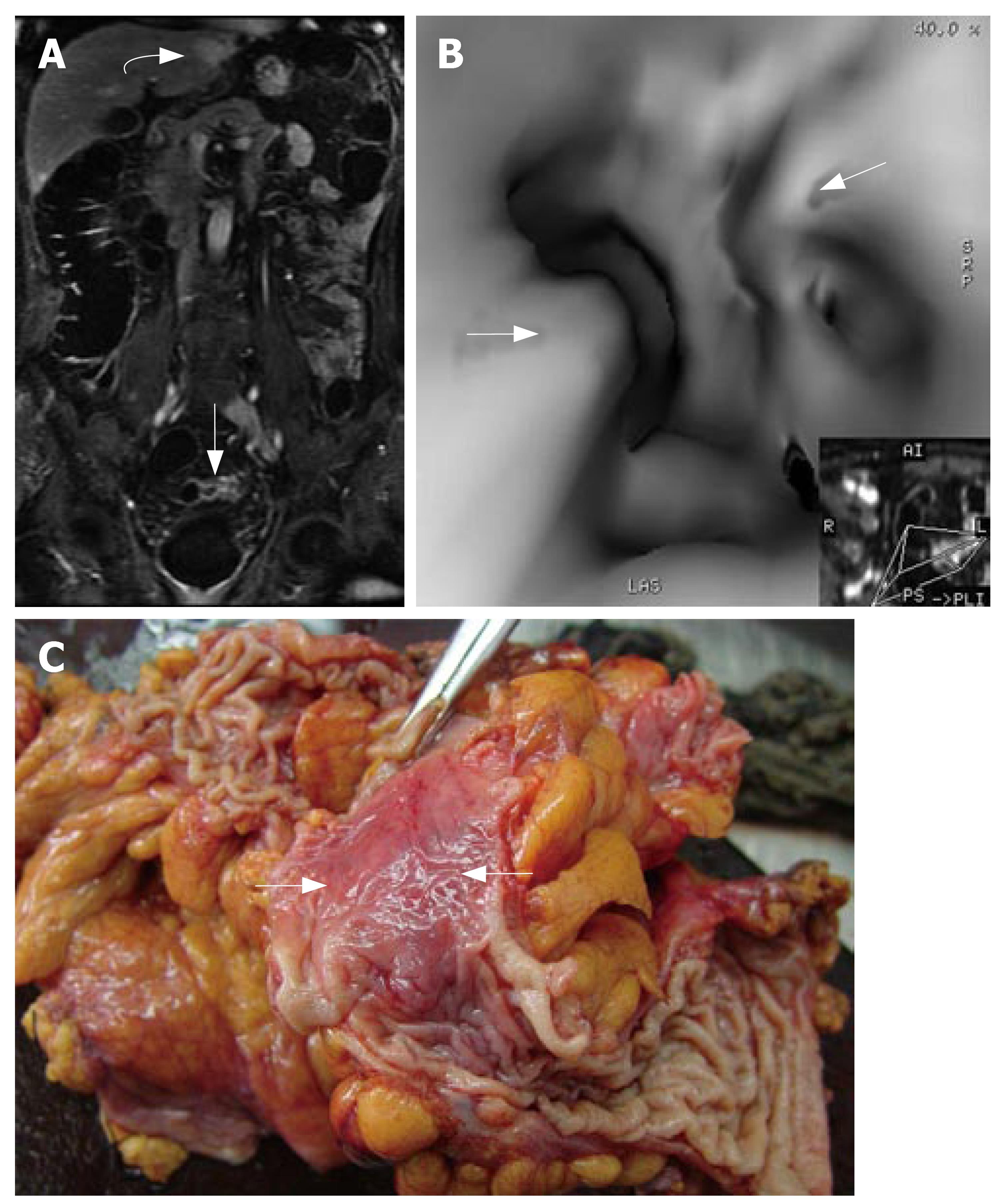
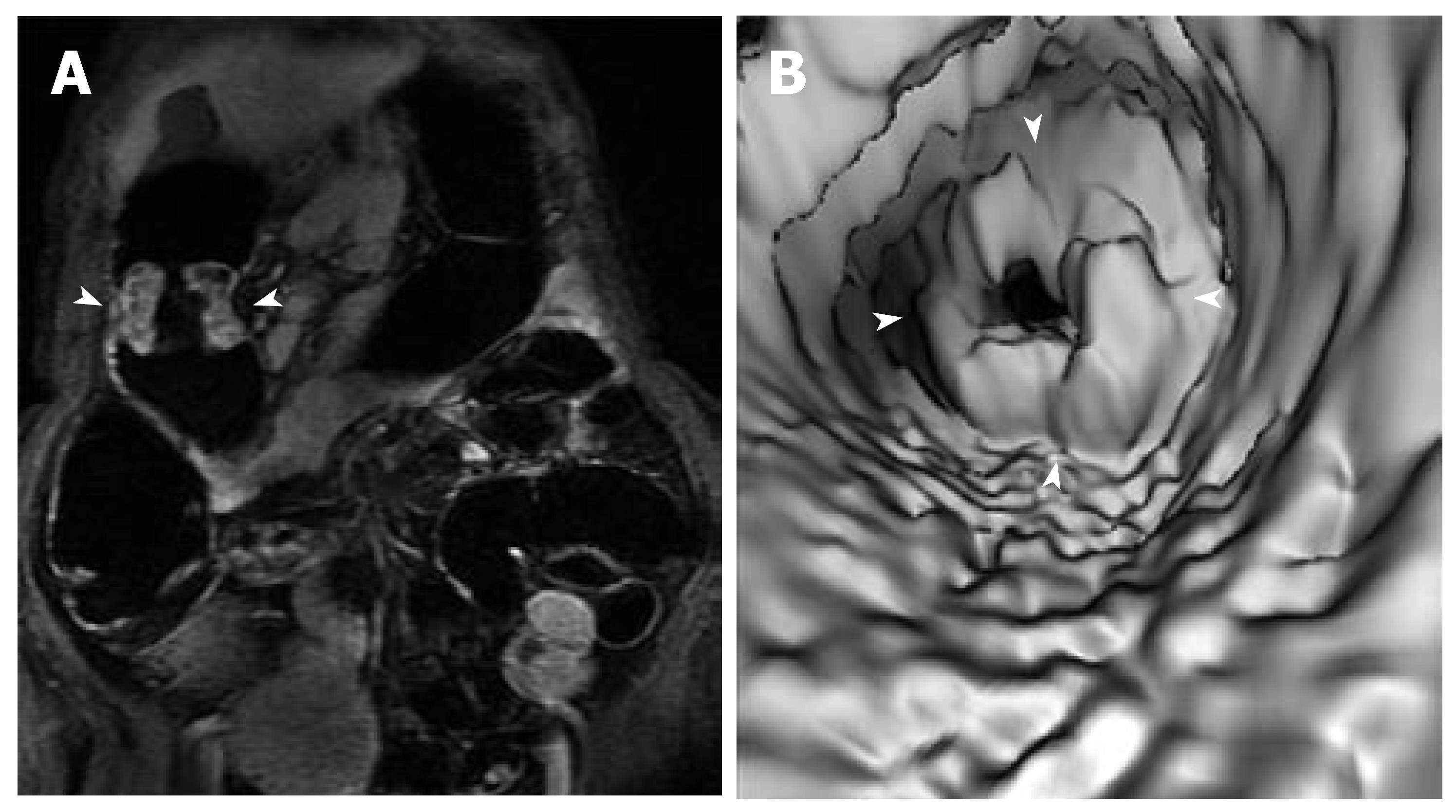
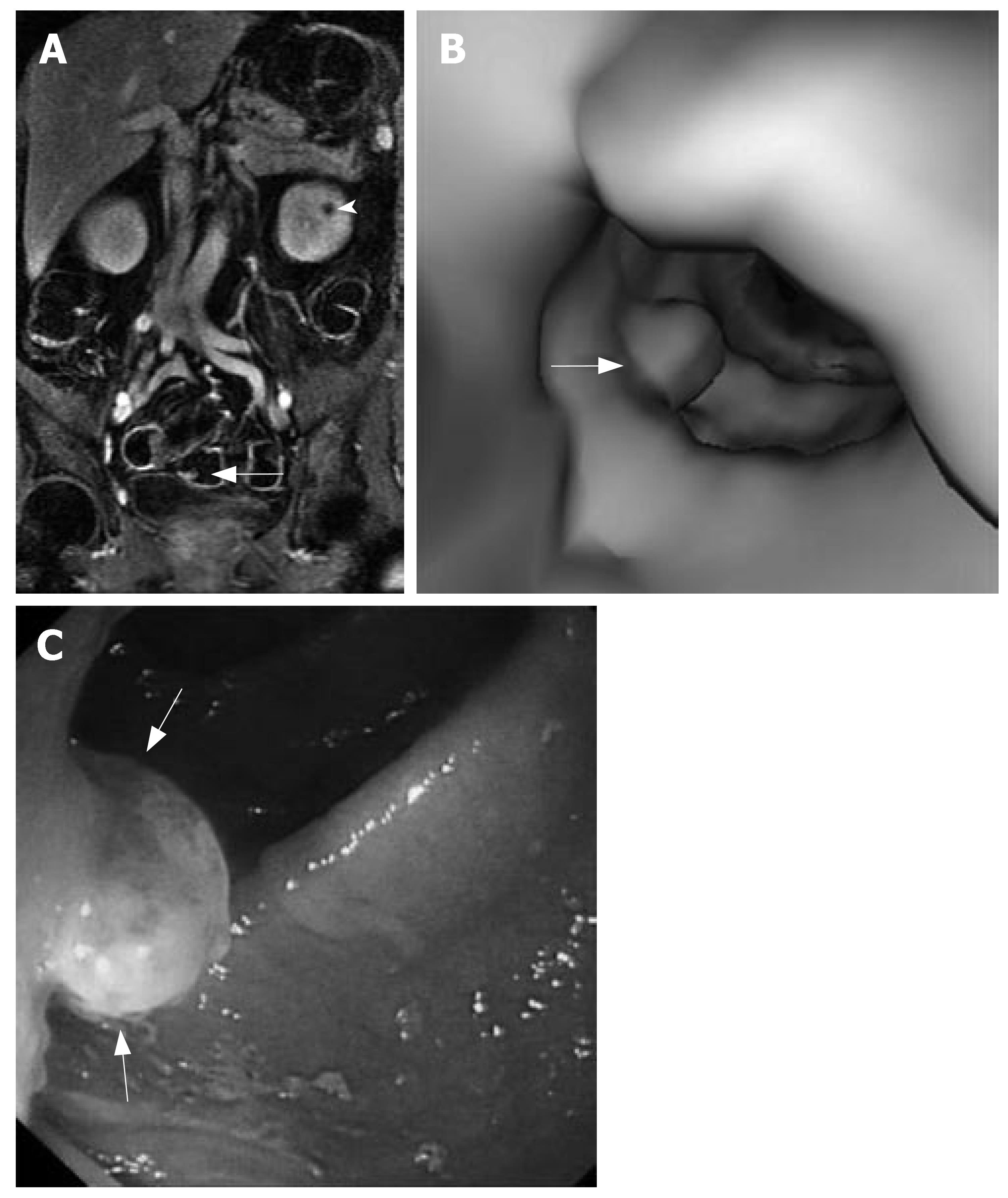
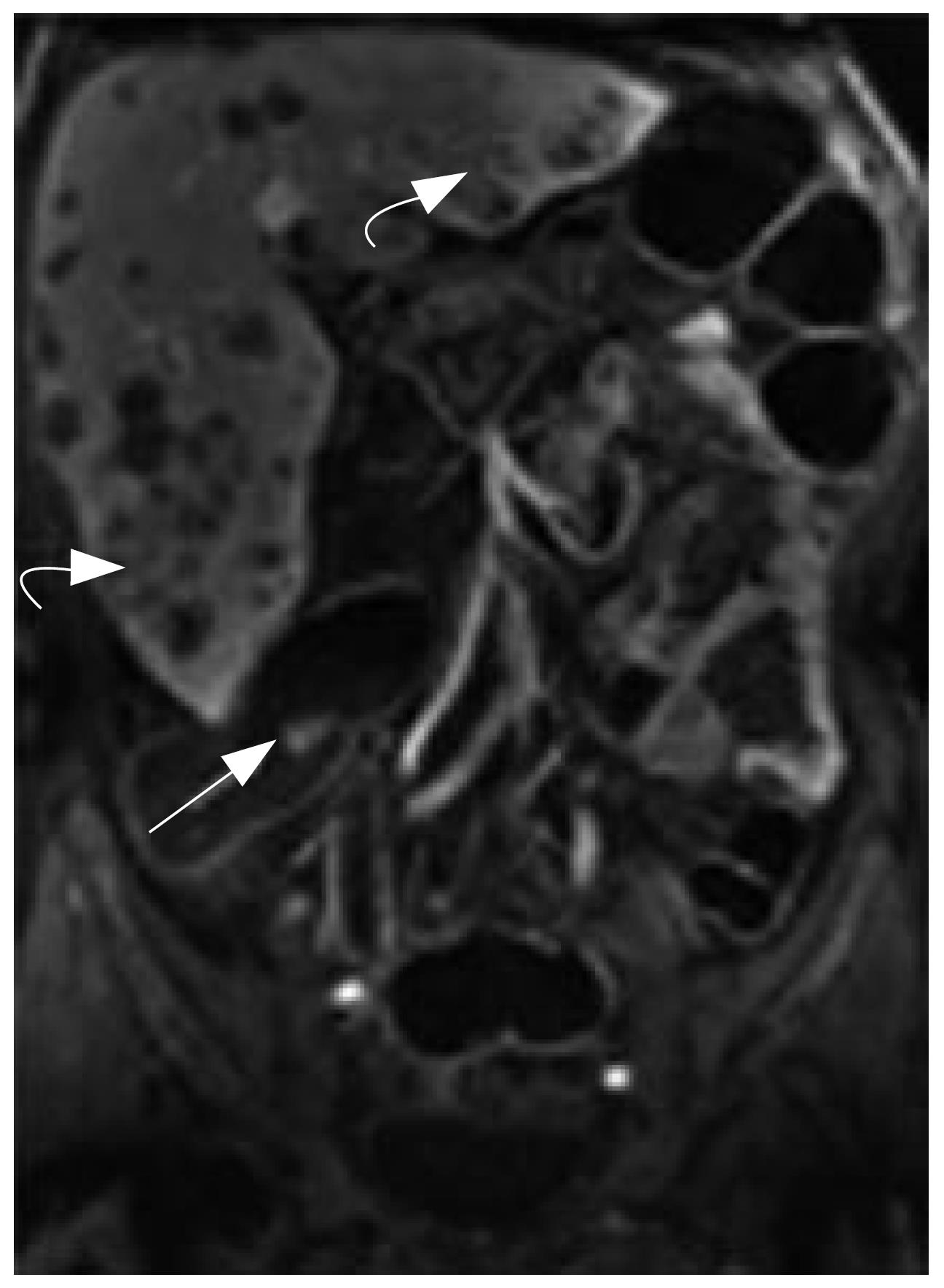
MRC revealed 26 neoplasms including an inflammatory neoplasm (Figure 1), 16 carcinomas (Figure 2) and 9 polyps in 22 patients. The size of five polyps (Figure 3) was between 5 mm and 10 mm and the size of the other 21 neoplasm (Figure 4) was larger than 10 mm in diameter. In patients, there were no false positive MR findings. However, four polyps less than 5 mm, four 5-10 mm polyps and one 20 mm polyp were not found by MRC but by colonoscopy. The 20 mm polyp was a flat polyp. On MRC, neoplasm larger than 10 mm had a higher sensitivity and neoplasm less than 10 mm had a lower sensitivity. The sensitivity for neoplasm of a diameter of < 5 mm, 5-10 mm, and > 10 mm was 0% (0/4), 55.5% (5/9), and 95.5% (21/22). The overall sensitivity was 74.3% (26/35).
MRC permitted assessment of extracolonic organs. Hepatic metastases were discovered in two patients. Hepatic and/or renal cysts were seen in three patients.
MRC is a promising, less intrusive imaging technique that can image the entire colon. The potential advantages of MRC over CT colonography include powerful multi-planar data acquisition, superior contrast resolution and absence of ionizing radiation[8].
To date, most MRC techniques require a water enema containing paramagnetic contrast material gadolinium at 10 mmol/L concentration[9,11,12]. Despite Luboldt et al[9] reporting promising results, the gadolinium enema may limit extensive use of MRC. Given 10 mmol/L as optimal concentration of gadolinium, 1.5 L enema would need 30 mL of 0.5 mol/L gadopentetate dimeglumine. The cost of dilute gadolinium enema would be considered as big setback to MRC. In addition, a 3D gradient echo sequence method that is designed to generate maximal signal from interior of the lumen and depress all the other tissue including the luminal wall was performed. Colorectal neoplasms were identified solely on filling defects in the bright colonic lumen. Hence the differentiation between polyps or carcinomas and residual fecal materials or air bubbles proved to be difficult and sometimes even impossible. So the MRC technique needs to be further amplified by the acquisition of 2D gradient echo sequence datasets. And to compensate for the residual air in the lumen, the MRC requires data acquisition both in the supine and the prone patient positions, which results in the complicated examination and longer examination time.
Recently, MRC has been used with air or carbon dioxide insufflation and Susceptibility artefacts which thought to be a big obstacle had been minimized by using a half-Fourier single-shot fast spin-echo (SE) sequence or a short echo time (TE) multi-slice half-Fourier acquisition single-shot turbo spin-echo (HASTE ) sequence[14,17,18]. Although air is cheap and abundant, HASTE sequence acquires two or more breath hold acquisitions to cover the entire colon, and can not afford virtual endoscopic observation. In addition, the MRC with air or carbon dioxide insufflation analysis had shortage of clinical cases.
The fat contrast medium that we used in MRC mainly contains salad oil, acacia, menthol and distilled water. It is not absorbed by gastrointestinal tract and has no obvious side-effects. The estimated price of 500 mL of fat contrast medium is $1.25. It is cheaper than paramagnetic contrasts such as gadolinium and less sensitive to susceptibility artefacts than air. It could provide negative contrast within the bowel lumen with a fat-depression sequence. On T1WI, the intensity of fat contrast medium was slightly lower compared to pericolonic fat; however it was too low to visualize air-fat interface when the fat-depression sequence was used. But the intensity of residual water in colon on T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence was higher than that of fat, which led to distinction of fat-water interface. At the same time, residual water blurred colonic wall and neoplasm, even resulting in neoplasm being missed in diagnosis. But after gadolinium administration, the intensity of colonic wall and neoplasm increased considerably, and the intensity difference between colonic wall, neoplasm and water became distinct. Thus the diagnosis of neoplasm obscured by residual water could be done without a need to change patients' position.
Residual fecal materials can not take up paramagnetic contrast, so it is easy to differentiate between residual fecal materials and neoplasm. This form of direct visualization of the colonic wall and colorectal pathologies stemming from it can reduce the possibility of false positive findings that might mimic neoplasm in MRC with gadolinium solution.
T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence is a 3D SPGR acquisition that automatically uses a Partial K spatial filling technique and a segmented special technique. Thus T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence allows shortening of acquisition time, enlargement of spectrum of scan, optimization of fat saturation, and boosting spatial resolution. However, it is essential that the patients maintain the breath-hold position throughout the data acquisition. Minor movements can lead to significant motion artefacts. After being trained, all patients can hold their breath for approximately 25 s. The mean acquisition time of 20 s for covering entire colon can be tolerated by the patients. And thus T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence is able to accomplish MRC.
In terms of lesion detection, the sensitivity of barium enema in detecting polyps of 10 mm or larger has been reported to be varied between 39% and 56%[19]. For CT colonography, the sensitivities of detecting > 9 mm, 6-9 mm and < 6 mm polyps were 85%, 70% and 48% and specificities of detecting > 9 mm, 6-9 mm and < 6 mm polyps were 97%, 93% and 92%, respectively[20]. Using a water enema containing paramagnetic contrast material as a contrast medium, MRC of colorectal neoplasm larger than 10 mm has been reported to have a sensitivity of 93%, specificity of 99%, positive predictive value of 92%, and negative predictive value of 98%[9]. While using water as a contrast medium, sensitivity was 93% and specificity was 100% for colonic neoplasm exceeding 5 mm[15]. However, colorectal neoplasm less than 5 mm are often missed in diagnosis[9,15,16,21]. In contrast, MRC using air has shortcomings of low sensitivity, limited coverage and signal drop-off at the borders of the field of view as well as needs further large-scale studies[14,22]. Although the number of patients in our study was small, our results suggest that MRC with fat enema could correctly detect neoplasm lager than 10 mm and neoplasm less than 5 mm would most likely were missed.
This technique can be helpful in patients with colonic carcinoma. It can demonstrate the entire colon and exclude synchronous colorectal neoplasm, as well as detect extra-colonic involvement, such as hepatic metastases.
But there are three limitations of this study. The first is the relatively large slice thickness, 5 mm slice thickness. It may be a reason for missing colorectal neoplasm less than 5 mm in diameter. With technical advancements, it is more likely that colorectal neoplasm less than 5 mm would be identified on MRC with fat enema. The second is that patients required a standard bowel preparation, which results in the limitation of patient acceptance for the technique. Recent studies have shown that fecal tagging technique which does not need a cleansed colon can improve patient acceptance, evaluate the majority of colonic segments inaccessible with conventional colonoscopy and depict polyps exceeding 5 mm in size[23-25]. The third is the small number of the patients and almost all patients with colorectal carcinoma that have large neoplasm. It could be a reason for high sensitivity.
In summary, the present data indicates that MRC with fat enema is a promising alternative to colonoscopy for the detection of colorectal neoplasm larger than 10 mm. Fat contrast medium distention seems to be well tolerated by patients. In the future, advancements in technique together with large-scale clinical cases analysis might improve the sensitivity of the method and hence reliability of its results.
MR colonograhpy (MRC) is an effective diagnostic tool for colorectal lesions. Currently two techniques were introduced for MRC based on the signal in the colorectal lumen: "bright lumen" and "dark lumen". Usually, bowel cleansing is essential for two techniques. Bright lumen MRC can be performed by fast T1 weighted 3D gradient-echo acquisitions within the conforms of a single breath hold and requires a rectal enema containing paramagnetic contrast. Dark lumen MRC is based on contrast generated between an enhancing colorectal wall and a homogeneously dark colonic lumen. The technique requires intravenous administration of paramagnetic contrast and administration of a water or air enema.
Recently, several studies laid emphasis on dark lumen MRC. Compared with bright lumen MRC, dark lumen MRC has considerable advantages including reduced examination and post-processing time, good differentiation between polyps and stool, direct analysis of the bowel wall and comprehensive assessment of parenchymal abdominal organs within the field of view. However, bowel cleansing is not well accepted by more than half of patients. To assure high patient acceptance of MRC, fecal tagging has been introduced. It is based on the principle of altering the signal intensity of fecal material by adding contrast compounds to regular meals. For fecal tagging, a paramagnetic MR contrast medium and a highly concentrated barium sulphate containing contrast are administrated for bright lumen MRC and dark lumen MRC, respectively.
MRC with fat enema is a technique that could generate a dark colonic lumen with a fat-depression sequence. So it has almost all of the advantages of dark lumen MRC. The data sets required could render an endoluminal virtual view through post-processing. In addition, fat contrast medium is cheaper than paramagnetic contrasts such as gadolinium and less sensitive to susceptibility artefacts than air.
MRC with fat enema is a promising alternative to colonoscopy for the detection of colorectal neoplasms larger than 10 mm. Moreover, it could depict the infiltration of extra-colonic fat and evaluate other abdominal organs.
MRC is based on 3D datasets required from cross sectional images. Compared with a conventional colonography, MRC is not limited to endoscopic viewing. Multiplanar reformation analysis can not only depict the colonic lumen and colonic wall, but also assess parenchymal abdominal organs.
The authors assessed Magnetic resonance colonography with fat enema as a method for detection of colorectal neoplasms. Colorectal neoplasms identified by MR colonography were compared with those identified on colonoscopy and sensitivity of detecting the lesions was calculated accordingly. They concluded that MR colonography with fat enema and T1-weighted three-dimensional fast spoiled gradient-echo with inversion recovery sequence is feasible in detecting colorectal neoplasms larger than 10 mm.
S- Editor Zhu LH L- Editor Li M E- Editor Yin DH
| 1. | Dachman AH, Yoshida H. Virtual colonoscopy: past, present, and future. Radiol Clin North Am. 2003;41:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Angtuaco TL, Banaad-Omiotek GD, Howden CW. Differing attitudes toward virtual and conventional colonoscopy for colorectal cancer screening: surveys among primary care physicians and potential patients. Am J Gastroenterol. 2001;96:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Chung DJ, Huh KC, Choi WJ, Kim JK. CT colonography using 16-MDCT in the evaluation of colorectal cancer. AJR Am J Roentgenol. 2005;184:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Johnson KT, Carston MJ, Wentz RJ, Manduca A, Anderson SM, Johnson CD. Development of a cathartic-free colorectal cancer screening test using virtual colonoscopy: a feasibility study. AJR Am J Roentgenol. 2007;188:W29-W36. [PubMed] |
| 5. | Pickhardt PJ, Taylor AJ, Kim DH, Reichelderfer M, Gopal DV, Pfau PR. Screening for colorectal neoplasia with CT colonography: initial experience from the 1st year of coverage by third-party payers. Radiology. 2006;241:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Doshi T, Rusinak D, Halvorsen RA, Rockey DC, Suzuki K, Dachman AH. CT colonography: false-negative interpretations. Radiology. 2007;244:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Martin DR, Yang M, Thomasson D, Acheson C. MR colonography: development of optimized method with ex vivo and in vivo systems. Radiology. 2002;225:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Saar B, Rösch T, Rummeny EJ. Colorectal cancer screening: a challenge for magnetic resonance colonography. Top Magn Reson Imaging. 2002;13:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Luboldt W, Bauerfeind P, Wildermuth S, Marincek B, Fried M, Debatin JF. Colonic masses: detection with MR colonography. Radiology. 2000;216:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Pappalardo G, Polettini E, Frattaroli FM, Casciani E, D'Orta C, D'Amato M, Gualdi GF. Magnetic resonance colonography versus conventional colonoscopy for the detection of colonic endoluminal lesions. Gastroenterology. 2000;119:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Haykir R, Karakose S, Karabacakoglu A, Sahin M, Kayacetin E. Three-dimensional MR and axial CT colonography versus conventional colonoscopy for detection of colon pathologies. World J Gastroenterol. 2006;12:2345-2350. [PubMed] |
| 12. | Saar B, Meining A, Beer A, Settles M, Helmberger H, Frimberger E, Rummeny EJ, Rösch T. Prospective study on bright lumen magnetic resonance colonography in comparison with conventional colonoscopy. Br J Radiol. 2007;80:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lam WW, Leung WK, Wu JK, So NM, Sung JJ. Screening of colonic tumors by air-inflated magnetic resonance (MR) colonography. J Magn Reson Imaging. 2004;19:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Lomas DJ, Sood RR, Graves MJ, Miller R, Hall NR, Dixon AK. Colon carcinoma: MR imaging with CO2 enema--pilot study. Radiology. 2001;219:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Ajaj W, Pelster G, Treichel U, Vogt FM, Debatin JF, Ruehm SG, Lauenstein TC. Dark lumen magnetic resonance colonography: comparison with conventional colonoscopy for the detection of colorectal pathology. Gut. 2003;52:1738-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hartmann D, Bassler B, Schilling D, Adamek HE, Jakobs R, Pfeifer B, Eickhoff A, Zindel C, Riemann JF, Layer G. Colorectal polyps: detection with dark-lumen MR colonography versus conventional colonoscopy. Radiology. 2006;238:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Semelka RC, Kelekis NL, Thomasson D, Brown MA, Laub GA. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996;6:698-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Morrin MM, Hochman MG, Farrell RJ, Marquesuzaa H, Rosenberg S, Edelman RR. MR colonography using colonic distention with air as the contrast material: work in progress. AJR Am J Roentgenol. 2001;176:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Johnson CD, MacCarty RL, Welch TJ, Wilson LA, Harmsen WS, Ilstrup DM, Ahlquist DA. Comparison of the relative sensitivity of CT colonography and double-contrast barium enema for screen detection of colorectal polyps. Clin Gastroenterol Hepatol. 2004;2:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Ajaj W, Ruehm SG, Gerken G, Goyen M. Strengths and weaknesses of dark-lumen MR colonography: clinical relevance of polyps smaller than 5 mm in diameter at the moment of their detection. J Magn Reson Imaging. 2006;24:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Bielen DJ, Bosmans HT, De Wever LL, Maes F, Tejpar S, Vanbeckevoort D, Marchal GJ. Clinical validation of high-resolution fast spin-echo MR colonography after colon distention with air. J Magn Reson Imaging. 2005;22:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Lauenstein TC, Goehde SC, Ruehm SG, Holtmann G, Debatin JF. MR colonography with barium-based fecal tagging: initial clinical experience. Radiology. 2002;223:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Ajaj W, Lauenstein TC, Pelster G, Holtmann G, Ruehm SG, Debatin JF, Goehde SC. MR colonography in patients with incomplete conventional colonoscopy. Radiology. 2005;234:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Kuehle CA, Langhorst J, Ladd SC, Zoepf T, Nuefer M, Grabellus F, Barkhausen J, Gerken G, Lauenstein TC. Magnetic resonance colonography without bowel cleansing: a prospective cross sectional study in a screening population. Gut. 2007;56:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |