Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.600
Revised: September 21, 2006
Accepted: November 21, 2006
Published online: January 28, 2007
AIM: To investigate the immunogenicity of H pylori proteins, to evaluate the production rate of anti H pylori IgG antibodies in relation to time and to demonstrate the fidelity of newly optimized in-house enzyme-linked immunosorbent assay (ELISA) technique as an alternative for H pylori infection assay.
METHODS: In the present study, 100 μg of formalin-fixed H pylori whole cell antigens was injected into an experimental animal (New Zealand white female rabbit) intramuscularly on d 0, 16, 27 and 36. The first two doses were injected with adjuvants. On d 0, a serum sample was collected from the rabbit before immunization and this pre-immunized serum was used as a negative control for the whole study. To evaluate the immunogenic responses of the injected antigen, serum samples were collected from the rabbit at regular intervals up to d 42. The sera were analyzed using in-house ELISA and Western blot techniques.
RESULTS: The production of anti H pylori IgG antibodies in the rabbit in response to the injected antigen increased almost exponentially up to d 14 and after that it was maintained at the same level until the last day (d 42). By analyzing the immune profiles of immunized sera, 11 proteins were identified to be immunogenic, among them 2 (approximately 100 kDa and 85 kDa) were most prominent.
CONCLUSION: Analysis of the immune responses against pathogenic microorganisms like H pylori is necessary for the development of various diagnostic and preventive approaches. The results of this experiment reveal that the formalin-fixed H pylori whole cell antigens injected into the rabbit are highly immunogenic. These prominent proteins (approximately 100 kDa and 85 kDa) might have higher immunogenic effects among humans infected with H pylori and some of these immunogenic proteins can be included in diagnostic approaches based on serology and also for vaccine formulation. The in-house ELISA is a promising alternative compared to invasive techniques.
-
Citation: Islam K, Khalil I, Ahsan CR, Yasmin M, Nessa J. Analysis of immune responses against
H pylori in rabbits. World J Gastroenterol 2007; 13(4): 600-606 - URL: https://www.wjgnet.com/1007-9327/full/v13/i4/600.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.600
In the early 1980’s, Barry Marshall and Robin Warren of Australia discovered the bacterium H pylori in the stomach lining of patients with chronic gastritis and peptic ulcers[1]. The discovery of the infective organism H pylori and its involvement in these diseases has changed our views on how to diagnose and treat these diseases. Strains carrying the genes encoding the cytotoxin-associated protein (Cag-A) cause chronic active gastritis[2]. Gastric infection with H pylori is one of the common chronic infections in humans, causing substantial morbidity and some mortality[3]. Before an active protective response occurs, the gut must first be exposed to H pylori, which is a slowly growing microaerophilic, highly motile, Gram-negative spiral organism whose most striking biochemical characteristic is the abundant production of urease[4]. Colonization of H pylori in the gastric epithelium leads to a chronic inflammatory reaction[5-7]. Such a reaction may involve specific IgG and/or IgA antibody responses against the bacterium both in the peripheral blood and in the gastric mucosa. However, despite the production of such antibodies, the microorganism usually persists and gastritis progresses chronically through unknown mechanisms[8].
H pylori infection and peptic ulcer disease are more common in developing countries than in developed countries. Until the mid 1980s, it was felt that one or more of these factors working together could lead to the development of gastritis and ulcers. Since then, evidence has been mounting that H pylori has a major role in causing these diseases. Today the standard triple antibiotic therapy is amoxicillin, clarithromycin and proton pump inhibitors such as omeprazole. Unfortunately, an increasing number of infected individuals are found to harbour bacteria resistant to first-line antibiotics. This results in initial treatment failure and requires additional rounds of antibiotic therapy[9]. One of the promising recent developments in medicine is the concept that chronic afflictions, such as peptic ulcer disease and cancer, can be controlled through immunization like classic infectious diseases. One approach has been the oral administration of purified recombinant subunit proteins of H pylori and a mucosal adjuvant, the labile toxin (LT) of Escherichia coli[10,11]. As a single-component vaccine, urease protein has shown some prophylactic and therapeutic activity in animal models and partial therapeutic activity in humans[12]. Another research was directed at the comparison of adjuvants and vaccine delivery systems and toward the immunologic mechanisms mediating protection[13].
Serological methods for detection of H pylori infection have reached sufficient accuracy and can be used as screening tests before endoscopy or for seroepidemiological surveys[4]. A number of different serological techniques have been used to detect antibodies, including haemagglutination, complement fixation, coagglutination, indirect immunofluorescence and latex agglutination[14]. Antibodies developed in rabbits against H pylori antigen can easily be detected by slide agglutination test. However, immunoblotting and enzyme-linked immunosorbent assay (ELISA) have emerged as the most frequently used techniques. A combination of immunoblotting and ELISA is the most efficient means of detecting serum antibodies to H pylori antigens and can be applied to the screening of rabbit sera for H pylori-specific antibodies[15]. These two techniques can be used in analysis of immune responses against H pylori in rabbits.
It is difficult to eradicate H pylori by antibiotic therapy and to date no vaccine is available for use in humans[16]. An effective vaccine would be a desirable way to control H pylori-induced gastric disease. Initial studies in animal models have demonstrated the feasibility of immunization, thus leading to high hopes for a human vaccine. In the mouse model, immunological approaches have to date not brought a satisfactory explanation for the mechanisms of protection against this largely luminal pathogen. In the present study, we used a rabbit model with whole cell extract from H pylori as antigen to analyze the immune responses.
In this study, two healthy New Zealand white female rabbits aged 2 mo (weighing 2 kg) were used for serum antibody response. Although other strains of rabbits (e.g., Californian, Giant blank, Beveren etc) were available, this strain could easily adapt to the tropical area like Bangladesh (studied area). A great care was taken during the study with proper feeding and supplying adequate amount of fresh water daily. Rabbit house was cleaned daily. During the study, the climate was fine. Hygiene condition was maintained properly.
Bacterial cells were grown and harvested from agar plates. Bacterial cells were suspended in phosphate buffered saline (PBS) containing 1% (w/v) formalin and kept at 4°C for 1 h. The cells were then centrifuged at 12 500 ×g for 5 min and the pellet was resuspended in 1 mL of PBS. The cells were washed 4 times in PBS to remove the formalin. Finally, a suspension of 1 mg/mL cells in PBS was made.
H pylori antigens were administered in combination with incomplete Freund’s adjuvant (which does not contain killed mycobacteria) to enhance the response to the first two doses. Freund’s adjuvant is a water-in-oil emulsion consisting primarily of mineral oil. The oil acts as a repository, which releases the immunogen. The mixture was prepared by taking 250 μL of H pylori whole cell antigen (formalin fixed, 1 mg/mL, stored at -20°C) with 250 μL of incomplete Freund’s adjuvant and then 200 μL of antigen-adjuvant mixture was injected intramuscularly.
Using a 22G needle, rabbits were immunized intramuscularly with whole cell antigen adjuvant mixture on d 0 and 16. Subsequent doses without adjuvant were administered on d 27 and 36. However, before immunization 1.5 mL blood was collected from the marginal vein of the ear for collecting pre-immunization sera. This was used as a negative control.
After immunization, rabbit blood were collected from marginal ear vein on d 0, 7, 17, 21, 27 and 35. Blood was also collected by cardiac puncture on d 42. Serum was separated from these blood samples and analyzed for antibody response by in-house ELISA and immunoblot techniques.
An aliquot of formalin-fixed bacteria was diluted in coating buffer to a final concentration of 1 μg/100 μL. One hundred μL of antigen preparation was added to all the wells except wells A1 and B1, which were used to calibrate the ELISA reader. The plates were covered with plate sealer and incubated at 4°C overnight. On the following day, the plates were washed 3 times with PBS containing 0.05% Tween-20 (PBS-Tween 20). The wells were blocked with 200 μL of 1% (w/v) bovine serum albumin (BSA) in PBS, and then plates were incubated at 37°C for 30 min. The PBS-BSA was discarded and the plates were washed 3 times with PBS-Tween 20. Then, 100 μL of diluted serum samples (neat, 1:50, 1:100, 1:200, 1:400 and 1:800 dilution in PBS) collected on d 7, 14, 21, 27, 35 and 42 was added into each of two consecutive wells, i.e. duplicate wells were used for each sample and each dilution. To the wells A1 and B1, 100 μL of PBS was added. The plate was covered with the plate sealer and incubated at room temperature for 2 h. The plate was then washed three times as described above and 100 μL of diluted secondary antibody conjugate (1:3000 in PBS) was added (goat anti-rabbit polyvalent antibody conjugated with alkaline phosphatase; A-3937, Sigma Chemical Co, Ltd. UK), and incubated at room temperature for 2 h. The plate was again washed three times with PBS Tween-20 and 200 μL of 1 mg/mL substrate (p-nitrophenyl phosphate in diethanolamine buffer) was added to each well. The plate was placed in dark and an optical density (A405) was measured after exactly 25 min. In this study, a sample was considered positive for antibodies to H pylori if the absorbance of the reaction was ≥ 1. Any value < 1 was considered seronegative[17]. For the validation of the experiment, a positive serum sample from humans was tested by in-house ELISA using similar dilution (Neat, 1:50, 1:100, 1:200, 1:400, and 1:800). For ELISA, all the tests were done in duplicate well to minimize the handling error. The average of the two values from duplicate well was used for further data analysis.
The 7 serum samples collected from rabbits on d 7, 14, 21, 35 and 42 were examined by immunoblot assay to check the immunological response of the pre-immunized polyclonal rabbit serum to the antigens from whole cell extract of H pylori.
After confluent growth, bacterial cells were harvested and taken into preweighed, screw-capped Eppendorf tubes. Bacteria were sedimented by centrifugation at 12 500 ×g for 2 min and suspended in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) solubilization buffer[18] to a cell concentration of 500 μg cells per 5 μL. The suspension was incubated at 100°C for 5 min to denature bacterial proteins and DNA was disrupted by brief sonication (30 s). The suspension was reheated at 100°C for 2 min and then diluted in SDS-PAGE solubilization buffer to produce a final concentration of 70 μg whole cell extract per 5 μL. The aliquots were stored at -20°C for SDS-PAGE.
The SDS-PAGE profile of whole cell extract was prepared as described previously[19]. Gels comprised a 4.5% (w/w) acrylamide stacking gel and a 12.5% (w/w) acrylamide separation gel. Samples were applied to gels alongside protein molecular weight standards (1 610 305; Bio-Rad, UK). Electrophoresis was performed using a minigel system (Consort, UK) with a constant current of 40 mA for 40 min. Gels were either stained with Coomassie blue[19] or used for immunoblotting.
H pylori serostatus in all serum samples was determined by immunoblot technique as described previously[20]. The SDS-PAGE protein profiles were transferred onto nitro-cellulose sheets[21] using a semidry electrotransfer apparatus (Bio-Rad). Individual protein profiles were prepared by cutting nitrocellulose sheets into strips. Strips were incubated separately with different rabbit serum (1:200 dilution in 3% skimmed milk) samples (primary antibody). Antibody-antigen complexes were detected with a goat anti-rabbit polyvalent antibody conjugated with alkaline phosphatase, diluted 1:5000 in 3% skimmed milk. Color development was carried out in a polythene bag at 37°C in the dark for 10 min. Individual serum antibodies binding to five or more protein bands were considered positive results and those binding to less than five protein bands were considered negative results[20].
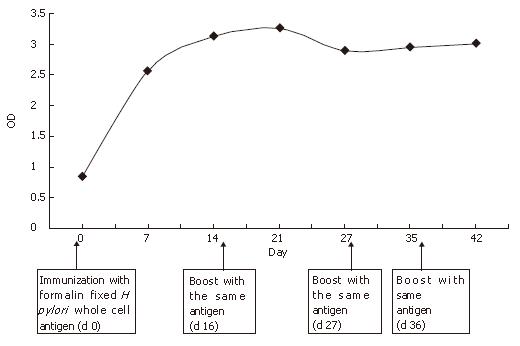
The serum collected from two rabbits for six weeks was examined for antibodies specific for antigen using in-house ELISA. Absorbance values ≥ 1 at 405 nm were considered seropositive (Table 1). The collected immunized sera from rabbits were evaluated and the result supported that the experimental animal (rabbit) was effectively immunized with H pylori whole cell antigen. For the validation of the experiment, a positive serum sample from humans was tested by using similar dilution (Neat, 1:50, 1:100, 1:200, 1:400, 1:800). The ELISA values (OD405) from all dilutions showed presence of adequate amount of anti H pylori antibody. There was also a negative control serum collected from the rabbit on d 0 (before immunization with formalin-fixed H pylori whole cell antigen). The ELISA values from different dilutions showed that the negative control serum had no antibodies against H pylori antigen. Using ELISA values for 1:50 dilution on different days (0, 7, 14, 21, 27, 35, and 42) a plot of antibody titer versus time was drawn (Figure 1). Similar results were obtained from other dilutions (neat, 1:50, 1:100, 1:200, 1:400 and 1:800) (data not shown). The graph showed a significant increase in anti H pylori antibody production with time. After immunization, the production of anti H pylori antibody increased almost exponentially up to d 14 and after that, it maintained at the same level until the last day.
| Dilution of sera | ||||||
| Serum collection | Neat | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 |
| d 0 | 0.998 | 0.847 | 0.620 | 0.063 | 0.024 | Not done |
| d 7 | 2.777 | 2.562 | 2.029 | 1.835 | 1.245 | 1.028 |
| d 14 | 2.003 | 3.136 | 2.792 | 2.241 | 0.408 | 1.404 |
| d 21 | 3.004 | 3.267 | 2.857 | 3.303 | 2.973 | 1.974 |
| d 27 | 2.805 | 2.894 | 2.021 | 2.074 | 2.323 | 1.004 |
| d 35 | 2.62 | 2.958 | 3.128 | 2.947 | 3.112 | 2.857 |
| d 42 | 3.002 | 2.945 | 2.763 | 2.719 | 2.999 | 3.303 |
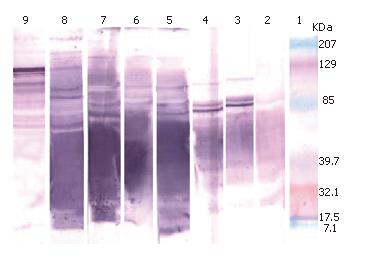
The serum samples collected from the rabbit were examined by immunoblot assay. A variable number of protein bands were observed among the immune profiles (Figure 2). Serum sample collected on d 0 represented pre-immunized serum and no visible protein band was observed, indicating absence of anti H pylori antibody. Several protein bands in other samples (collected on d 7, 14, 21, 27, 35 and 42) suggested that tested sera had antibodies against H pylori. Immunoreactivity of the protein bands increased gradually from d 7 to d 42, suggesting that the production of antibody against H pylori increased with time in the rabbit. A positive serum sample from humans was also tested and found to have antibodies against that antigen.
The presence or absence of anti H pylori antibodies in pre-immunized and immunized rabbits was examined by in-house ELISA and immunoblotting. The findings from the two immunoassays showed a significant correlation (P = 0.001) (Table 2).
Current antibiotic regimens against H pylori infection may be effective, but complex dosing and development of resistance are always concerns. Animal studies and limited clinical trials of H pylori antigens have been conducted, with no final conclusive findings. A number of data now exist, supporting the potential for protection against H pylori. However, we are still at a preliminary stage in clinical development. The best immunogens, the best mode of presentation, the number of doses needed, optimal age at immunization, expected benefit, cost-effectiveness, and other factors involved in vaccine development require further study[13]. The present study employed rabbits to clarify the immunogenicity of H pylori proteins and to evaluate the production rate of anti H pylori IgG antibodies in relation to time.
For serological diagnosis of H pylori infection, immune responses against the relevant microorganism can be analyzed in experimental animal models. When endoscopy is not performed, the most commonly used diagnostic approach is the laboratory-based serological test. Enzyme-linked immunosorbent assay (ELISA) detects various classes of antibodies to H pylori, indicating current or past infection. Because H pylori infection is not known to spontaneously resolve, a positive serologic test suggests active infection in patients who have not undergone eradication therapy[22]. Although H pylori is not an invasive bacterium, it actively stimulates the immune system in its host by releasing lipopolysaccharides and immunogenic proteins. An immune response accompanies the presence of the bacterium in 98% of the cases[23]. The sensitivity and specificity of ELISA to identify infectious microorganisms are quite high. In the present study, since the antibody response occurred a few days after a new infection or after a booster dose of antigen with or without adjuvant, the subject had to mount a strong immune response.
IgG usually appears several days after H pylori infection in rabbits. But after eradication of H pylori, the drop in antibody titer is not significant until the 6th mo in humans[24]. In the present study, specific IgG antibodies to H pylori were detected in rabbit sera with the help of enzyme immunoassay. Analysis of serum samples by in-house ELISA technique is an alternative for H pylori infection assay. The present study evaluated the noninvasive methods to screen for H pylori infection in rabbits. The relationship between time course and magnitude of antibody response showed the kinetics of the development of specific antibody responses in the studied animals (Figure 1). A clear kinetics of the development of anti H pylori antibody response found in the rabbit showed a significant increase in anti H pylori IgG antibody production with time. After immunization, the production of anti H pylori antibody increased almost exponentially up to d 14 and after that, it maintained at the same level till the last day of serum collection. The ELISA values from different dilutions showed that the negative control serum had no antibodies against H pylori antigen. However, 1:400 dilution of serum (collected at d 14) showed a negative ELISA value, which could be due to the handling error.
A large number of different proteins present in single cells and may play a major antigenic role in infection. The complex pathogenesis of this infection[25,26], including the presence of antigens on H pylori in the host[27], demands better approaches to the identification of novel immunogens that would give substantial protection. The selection of defined and well-characterized antigens appears to be the most viable approach. The present in-house ELISA with formalin-fixed whole cell antigens had a high diagnostic value for studied animals. Antibody was raised against whole cell antigens which were at first confirmed by the slide agglutination test. The collected sera subjected to immunoblot analysis showed a number of antibody bands. Some bands were more prominent than others in relation to color intensity. Previous studies revealed that H pylori whole cell lysate contains protein bands like CagA (140-121 kDa), VacA (87 kDa), heat-shock protein (60 kDa), two urease subunits of 62 and 26 kDa and thiol peroxidase (18 kDa)[28], suggesting that the concentration of some proteins from the whole cells is high. These proteins might have immunogenic effects in the rabbit. By analyzing the immune profiles of immunized serum samples collected at regular intervals from the rabbit, 11 proteins were identified to be immunogenic, of which 2 were more prominent than others (approximately 100 kDa and 85 kDa). Haque et al[29] showed that approximately 61 kDa, 58 kDa and 24 kDa proteins from whole cell extract of H pylori are immunogenic. However, they could not prove it conclusively. The present study identified two such potential antigens which were proved most potent in eliciting protective immunity.
It is reasonable to assume that more than one protective component is needed in a vaccine[13]. Therefore the previously identified urease[8] or catalase[30] alone could not be used for final vaccine formulation. There is also concern about inducing an immune response to heat shock protein B (HspB) because this protein has homologies to the GroEL family of heat shock proteins[31]. In the present study, we demonstrated some potential immunogens which could give better protection and could be used for diagnosis purpose.
Adjuvant is an important component of any vaccine. It is responsible for stimulating immune system. In some previous trials[10,30], cholera toxin was used as adjuvant for immunization of mice. However this effort might raise multiple problems including safety. E. coli heat-labile toxin (LT) that was used as an oral adjuvant in humans does not show a significant decrease in gastric H pylori density but is associated with cramping and diarrhea[32]. Due to all these adverse effects, no suitable and safe adjuvants are currently available for use in humans[33]. However, in the present study an incomplete form of Freund’s adjuvant not containing such a cytotoxic agent was used to enhance the immune response. Therefore, this adjuvant could be considered a substitute to cholera toxin or other toxic adjuvants.
The identified proteins might have higher immunogenic effects among humans infected with H pylori and some of these immunogenic proteins could be included in diagnostic approaches based on serology and also in vaccine preparation. However, further characterization of these antigens is required. Finally, in-house ELISA and immunoblot can be better applied in analysis of antigenic response in experimental animal model (rabbits).
Gastric infection with H pylori is one of the common chronic infections in humans, causing substantial morbidity and some mortality. Still there is still no effective vaccine against H pylori, a causative agent of gastric and peptic ulcer. We are still at a preliminary stage in clinical development. The best immunogens, the best mode of presentation, the number of doses needed, optimal age at immunization, expected benefit, cost-effectiveness, and other factors involved in vaccine development require further study. Initial studies in animal models have demonstrated the feasibility of immunization, thus leading to high hopes for a human vaccine. In the mouse model, immunological approaches have to date not brought a satisfactory explanation for the mechanisms of protection against this largely luminal pathogen. In the present study, we used a rabbit model with the whole cell extract antigens from H pylori.
H pylori infection is a newly discovered stomach infection which was first reported by Barry Marshall and Robin Warren of Perth, Western Australia, in 1983, who were awarded the Nobel Prize in Medicine in 2005 for their work on H pylori. The Sydney gastroenterolgist Thomas Borody invented the first triple therapy in 1987. Such a therapy has revolutionized the treatment of gastric ulcer. Mode of infection, mechanism of pathogenesis, host immune response, chemotherapy and vaccine development are important areas of research.
Initial studies in animal models have demonstrated the feasibility of immunization, thus leading to high hopes for a human vaccine against H pylori. In the mouse model, immunological approaches have to date not brought a satisfactory explanation for the mechanisms of protection against this luminal pathogen. In the present study, we used a rabbit model with the whole cell extract from H pylori as antigen. The experiment identified two prominent proteins (approximately 100 kDa and 85 kDa) which have higher immunogenic effects among humans infected with H pylori and some of these immunogenic proteins could be included in diagnostic approaches based on serology and also for vaccine formulation. Most of the previous studies used cholera toxin as adjuvants which have some side effects. In the present study, we used an incomplete form of Freund’s adjuvant which does not contain cytotoxic agent. We established and optimized the low cost, non-invasive in-house ELISA technique for the detection of H pylori infection in Bangladeshi people.
Analysis of the immune responses against pathogenic microorganisms like H pylori is necessary for the development of various diagnostic and preventive approaches. The results of our experiment reveal that formalin-fixed H pylori whole cell antigens injected into the rabbit are highly immunogenic. These prominent proteins (approximately 100 kDa and 85 kDa) might have higher immunogenic effects among humans infected with H pylori and some of these immunogenic proteins could be included in diagnostic approaches based on serology. The in-house ELISA is a promising alternative in the developing countries compared to high cost invasive techniques.
Freund’s adjuvant is an antigen solution emulsified in mineral oil, and can be used as an immunopotentiator (booster of the immune system). The so-called complete form (FCA) is composed of inactivated and dried mycobacteria, usually Mycobacterium tuberculosis (the pathogenic agent of tuberculosis). The so-called incomplete form (FIA) is the same adjuvant without the mycobacterial components and is named after Jules T. Freund (1890-1960), a Hungarian-born American immunologist.
It is an interesting study. The science seems good and the study is well performed. The results are also of interest.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Van den Bulck K, Decostere A, Baele M, Marechal M, Ducatelle R, Haesebrouck F. Low frequency of Helicobacter species in the stomachs of experimental rabbits. Lab Anim. 2006;40:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Aucher P, Petit ML, Mannant PR, Pezennec L, Babin P, Fauchere JL. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J Clin Microbiol. 1998;36:931-936. [PubMed] |
| 3. | Lepper PM, Möricke A, Vogt K, Bode G, Trautmann M. Comparison of different criteria for interpretation of immunoglobulin G immunoblotting results for diagnosis of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2004;11:569-576. [PubMed] |
| 4. | Barrow GI, Felthem RKA. Cown and Steel's Manual for the Identification of Medical Bacteria. 3rd editors. London: Cambrigde University Press 2004; 121-130. |
| 5. | Berstad AE, Brandtzaeg P, Stave R, Halstensen TS. Epithelium related deposition of activated complement in Helicobacter pylori associated gastritis. Gut. 1997;40:196-203. [PubMed] |
| 6. | Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Mai UE, Perez-Perez GI, Wahl LM, Wahl SM, Blaser MJ, Smith PD. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991;87:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Futagami S, Takahashi H, Norose Y, Kobayashi M. Systemic and local immune responses against Helicobacter pylori urease in patients with chronic gastritis: distinct IgA and IgG productive sites. Gut. 1998;43:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Mirbagheri SA, Hasibi M, Abouzari M, Rashidi A. Triple, standard quadruple and ampicillin-sulbactam-based quadruple therapies for H. pylori eradication: a comparative three-armed randomized clinical trial. World J Gastroenterol. 2006;12:4888-4891. [PubMed] |
| 10. | Weltzin R, Guy B, Thomas WD, Giannasca PJ, Monath TP. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect Immun. 2000;68:2775-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Nyström J, Raghavan S, Svennerholm AM. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect. 2006;8:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Ermak TH, Giannasca PJ, Nichols R, Myers GA, Nedrud J, Weltzin R, Lee CK, Kleanthous H, Monath TP. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 321] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Monath TP, Lee CK, Ermak TH, Myers GA, Weltzin RA, Giannasca PJ, Thomas WD, Soman G, Bhagat H, Ackerman SA. The Search for Vaccines Against Helicobacter pylori. Infect in Med. 1998;15:534-546. |
| 14. | Mayo K, Pretolani S, Gasbarrini G, Ghironzi G, Megraud F. Heterogeneity of immunoglobulin G response to Helicobacter pylori measured by the unweighted pair group method with averages. Clin Diagn Lab Immunol. 1998;5:70-73. [PubMed] |
| 15. | Nessa J, Chart H, Owen RJ, Drasar B. Human serum antibody response to Helicobacter pylori whole cell antigen in an institutionalized Bangladeshi population. J Appl Microbiol. 2001;90:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Axon AT. Treatment of Helicobacter pylori: future therapeutic and prophylactic perspectives. Gut. 1998;43 Suppl 1:S70-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Islam ABMMK, Yasmin M, Ahasan R, Nessa J. Helicobacter pylori Infection in Bangladeshi Population: Serodiagnosis, Seroprevalence and Risk Factors. Dhaka Univ J Biol Sci. 2005;14:1-8. |
| 18. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188829] [Article Influence: 3433.3] [Reference Citation Analysis (0)] |
| 19. | Chart H, Jenkins C, Smith HR, Rowe B. Serum antibodies to secreted proteins in patients infected with Escherichia coli O157 and other VTEC. Epidemiol Infect. 1998;120:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Rahman F, Islam ABMMK, Nessa J, Ahsan CR Yasmin M. Correlation in prevalence, risk factors and serological detection of Helicobacter pylori by different techniques. Bangladesh J Microbiol. 2005;22:152-157. |
| 21. | Towbin H, Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984;72:313-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 692] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 22. | Feldman RA, Eccersley AJ, Hardie JM. Epidemiology of Helicobacter pylori: acquisition, transmission, population prevalence and disease-to-infection ratio. Br Med Bull. 1998;54:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Tam YH, Yeung CK, Lee KH. Seven-day is more effective than 4-day ranitidine bismuth citrate-based triple therapy in eradication of Helicobacter pylori in children: a prospective randomized study. Aliment Pharmacol Ther. 2006;24:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Kivi M, Tindberg Y. Helicobacter pylori occurrence and transmission: a family affair? Scand J Infect Dis. 2006;38:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191-202. [PubMed] |
| 27. | Appelmelk BJ, Simoons-Smit I, Negrini R, Moran AP, Aspinall GO, Forte JG, De Vries T, Quan H, Verboom T, Maaskant JJ. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031-2040. [PubMed] |
| 28. | Ji KY, Hu FL. Interaction or relationship between Helicobacter pylori and non-steroidal anti-inflammatory drugs in upper gastrointestinal diseases. World J Gastroenterol. 2006;12:3789-3792. [PubMed] |
| 29. | Haque M, Rahman KM, Khan AK, Hassan M, Miah MR, Qadri F, Akhter Q. Antigen profile of Helicobacter pylori strains isolated from peptic ulcer patients in Dhaka, Bangladesh. Bangladesh Med Res Counc Bull. 1993;19:71-78. [PubMed] |
| 30. | Radcliff FJ, Hazell SL, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668-4674. [PubMed] |
| 31. | Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499-6503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 207] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Arora S, Czinn SJ. Vaccination as a method of preventing Helicobacter pylori-associated gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1890-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |