Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.509
Revised: October 25, 2006
Accepted: December 7, 2006
Published online: January 28, 2007
AIM: To investigate the effects of 2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid (NM-3) alone and in combination with carboplatin on tumor growth and apoptosis in mouse models of human gastric cancer constructed by subcutaneous implantation of histologically intact tumor tissue.
METHODS: Human gastric cancer SGC-7901 tissues were implanted into the dorsal subcutis of nude mice. One week after tumors reached to a volume of 50-100 mm3 for around 1 wk, these mice were randomly divided into 8 groups (n = 10). NM-3 was injected peritoneally at the dose of 10 mg/kg, 20 mg/kg or 40 mg/kg every other day for 5 wk, combined with carboplatin (5 mg/kg) every third day for 4 wk. As controls of combined treatment, another 4 groups of mice were injected with either NM-3 at 10 mg/kg, 20 mg/kg or 40 mg/kg, or with carboplatin alone (5 mg/kg). The control mice received normal saline. Tumor weight, tumor growth inhibition (TGI), and intratumoral microvessel density (MVD) were evaluated. Apoptosis of human gastric cancer was detected by TUNEL method and flow cytometry analysis, respectively.
RESULTS: The mean tumor volume (692.40 ± 58.43 mm3, 548.30 ± 66.02 mm3, 382.13 ± 43.52 mm3) after treatment with carboplatin combined NM-3 at the dose of 10 mg/kg, 20 mg/kg or 40 mg/kg was lower than that after treatment with either NM-3 at the dose of 10 mg/kg, 20 mg/kg or 40 mg/kg or with carboplatin alone. Compared with the normal saline group, NM-3 administered at 10 mg/kg, 20 mg/kg or 40 mg/kg significantly reduced the tumor weight in these groups (P < 0.05). Carboplatin used alone at 5 mg/kg showed minimal effects. But NM-3 in combination with carboplatin had greater effects of tumor weight than either NM-3 or carboplatin alone. NM-3 alone at the dose 10 mg/kg or in combination with carboplatin had no obvious effects on body changes. Two mice died of diarrhea in each of the two groups treated with 40 mg/kg NM-3 or with 40 mg/kg NM-3 in combination with carboplatin. A significant increase in apoptosis was observed in the NM-3 treated groups, and the effect was more significant in the groups treated with carboplatin in combination with NM-3 at 10 mg/kg, 20 mg/kg and 40 mg/kg, than in the control group. The induction of apoptosis was positively associated with the dose of NM-3. NM-3 significantly reduced the neo-microvascular formation of gastric cancer. The MVD was lower in the groups treated with NM-3 or with NM-3 in combination with carboplatin than in the group treated with carboplatin or in the normal saline group (P < 0.05).
CONCLUSION: The results suggest that the inhibitory effect of NM-3 on gastric cancer growth is mediated through decreased angiogenesis and the increased induction of apoptosis. Furthermore, NM-3 alone at the dose of 10 mg/kg or in combination with carboplatin has no obvious effects on body changes, indicating that NM-3 in combination with carboplatin may be effective in the treatment of gastric cancer. The toxicity of NM-3 needs further studies.
-
Citation: Chen JL, Zhu JS, Hong J, Chen MX, Lu JL, Chen WX, Shen B, Zhu ZM, Chen NW. Effect of 2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid in combination with carboplatin on gastric carcinoma growth
in vivo . World J Gastroenterol 2007; 13(4): 509-514 - URL: https://www.wjgnet.com/1007-9327/full/v13/i4/509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.509
Gastric carcinoma is one of the most frequent malignancies and one of the major causes of cancer deaths in China[1]. Up to now, the prognosis of patients with gastric cancer is very poor because gastric cancer is usually diagnosed at its advanced stage throughout the world. Even after curative resection, it remains at a high risk of relapse. Chemotherapy is one of the most important treatment modalities for gastric cancer. However, its effect is limited due to its adverse reactions and resistance of tumor cells to chemotherapeutic agents[2].
Apoptosis plays an important role in the growth of malignant tumor cells. It has been shown that apoptosis can be induced in gastric cancer by some chemotherapeutic drugs, such as 5-fluorouracil, cisplatin and paclitaxel[3]. Recent studies have demonstrated that angiogenesis plays a crucial role in tumor growth and metastasis. Vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) are the main factors promoting angiogenesis[4-6]. It has been shown that radiation-induced tumor regression is enhanced by angiogenesis inhibitors. Angiostatin or antibody to VEGF in combination with chemotherapy produces greater antitumor effects than either treatment alone[7,8].
Recently, induction of apoptosis by antiangiogenic therapy has been suggested as a new anticancer strategy[9]. 2- (8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid (NM-3), is a synthetic derivative of cytogenin. In vitro studies have demonstrated that the inhibitory effects of NM-3 at lower concentrations are stronger on the growth of human umbilical vein endothelial cells than on the growth of normal fibroblasts or tumor cells[10]. NM-3 alone inhibits endothelial sprouting and tube formation in vitro. It has been shown that NM-3 can enhance 5-fluorouracil-induced tumor growth inhibition in breast carcinoma xenografts with no effects on body changes[11]. In this study, we investigated the effects of NM-3 alone or in combination with carboplatin on tumor growth and apoptosis in mouse models of human gastric cancer constructed by subcutaneous implantation of histologically intact tumor tissue.
Male BALB/c/nu/nu nude mice were obtained from Shanghai Experimental Animal Center of the Chinese Academy of Sciences. Animals used were 6-wk old and weighed 20-25 g. Human gastric cancer SGC-7901 was obtained from Shanghai Cancer Institute. NM-3 was provided by professor Robert (National Cancer Research Center of America), concentration of NM-3 was 20 mg/mL.
Animal models were made using subcutaneous implantation of histologically intact tissue of human gastric carcinoma. Tumors were resected aseptically. Necrotic tissue was removed and the remaining tumor tissues were minced into pieces about 2 mm in diameter and implanted into the dorsal subcutis of mice.
One week after tumors reached a volume of 50-100 mm3 for around 1 wk, these mice were randomly divided into 8 groups (n = 10). NM-3 was injected peritoneally at the dose of 10 mg/kg, 20 mg/kg or 40 mg/kg every other day for 5 wk, in combination with carboplatin (5 mg/kg) every third day for 4 wk. As controls of combined treatment, another four groups of mice were injected NM-3 or carboplatin alone. The control mice received normal saline as indicated. The mice were weighed twice weekly, and tumor measurements were taken by calipers twice weekly. Tumor volume was measured using the formula (V = ab2/2), where a is the largest diameter and b the smallest diameter. Tumor growth inhibition (TGI) in each group was (mean control tumor weight - mean treated tumor weight)/mean control tumor weight × 100%.
All animals were sacrificed 7 wk after the implantation. Tumors were biopsied, fixed in 10% formalin, and processed for routine paraffin embedding. Tumors were evaluated histologically under microscope.
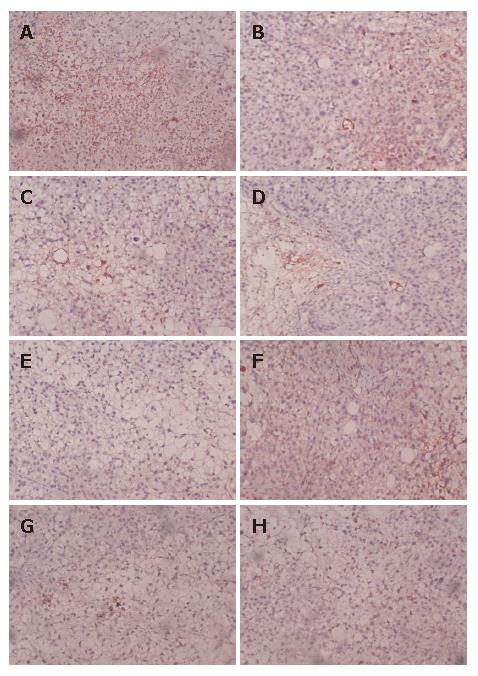
Four-micron-thick sections were deparaffined in xylene and rehydrated in graded alcohol. Immunostaining was performed using a labeled streptavidin biotin method. Immunohistochemical staining was carried out to detect CD34 expression following the manufacturer’s protocol (Santa Cruz Biotech Company). The modified Weidner’s method was used for the evaluation of MVD according to CD34 endothelial cell immunostaining. For microvessel counting, positive staining for MVD in five most highly vascularized areas in each section was counted in 200 × fields. MVD was expressed as the average of the microvessel count in the 5 areas. Any endothelial cell or endothelial cluster positive for CD34 (brown-yellow staining) was a single countable microvessel.
Apoptosis of human gastric cancer was detected by terminal deoxynuclotidyl transferase- mediated deoxy-uridine triphosphate-fluorescene nick end labeling (TUNEL) method and flow cytometry analysis, respectively. TUNEL method was performed as indicated. Flow cytometry analysis was conducted as follows. In brief, propidium iodide (PI) staining was used for flow cytometric detection of apoptosis, 1 × 106 cells from each of the samples were treated with RNase and stained with PI. DNA strand-labeled apoptotic cells were calculated with a flow cytometer (FACS Calibur, Becton Dickinson, USA.). Data were collected from 1 × 106 cells/sample, stored and analyzed using CELLQUES’T and MODFITLT for macV 1.01 software.
All data were expressed as mean ± SD. Student’s t test was used to determine changes in different groups. P < 0.05 was considered statistically significant.
The mean tumor volume (MTV) in the groups treated with 10 mg/kg, 20 mg/kg or 40 mg/kg NM-3 was 989.50 ± 102.17 mm3, 826.20 ± 76.52 mm3, and 709.75 ± 89.30 mm3, respectively, which was significantly smaller than that in the control group receiving normal saline (1609.60 ± 122.11 mm3, P < 0.05). Carboplatin at the dose of 5 mg/kg had no significant inhibitory action on gastric carcinoma with MTV being 1532.14 ± 110.12 mm3. However, the mean tumor volume in groups treated with carboplatin in combination with NM-3 was 692.40 ± 58.43 mm3 , 548.30 ± 66.02 mm3, and 382.13 ± 43.52 mm3, respectively, which was lower than that in the groups treated either with NM-3 or with carboplatin alone. Compared with the normal saline group, NM-3 administered at 10 mg/kg, 20 mg/kg or 40 mg/kg significantly reduced the tumor weight in these groups, and the effect was more significant when NM-3 was given at a dose of 40 mg/kg (P < 0.05). Carboplatin used alone at the dose of 5 mg/kg showed minimal effects. But NM-3 in combination with carboplatin, however, had a more significant effect on tumor weight than NM-3 or carboplatin alone (Table 1).The mean tumor volume did not differ among groups before treatment (Table 1).
| Groups | n | MTV (mm3) | Body weight (g) | Tumor weight (mg) | TGI (%) |
| Normal saline | 10 | 81.24 ± 12.63 | 25.8 ± 1.04 | 1754.0 ± 144.2 | |
| NM-3 (10 mg/kg) | 10 | 79.68 ± 13.72 | 24.4 ± 0.76 | 1351.0 ± 116.9 | 23.0a |
| NM-3 (20 mg/kg) | 10 | 81.08 ± 12.90 | 23.1 ± 0.82 | 1041.1 ± 143.5 | 40.6a |
| NM-3 (40 mg/kg) | 8 | 81.36 ± 11.20 | 22.9 ± 1.06 | 765.5 ± 140.1 | 56.2ac |
| NM-3 (10 mg/kg) + carplatin | 10 | 80.29 ± 14.26 | 24.2 ± 0.88 | 1002.0 ± 101.4 | 42.7ac |
| NM-3 (20 mg/kg) + carplatin | 10 | 82.30 ± 14.53 | 24.4 ± 0.78 | 919.0 ± 149.8 | 47.6ac |
| NM-3 (40 mg/kg) + carplatin | 8 | 81.97 ± 12.77 | 22.5 ± 1.13 | 645.7 ± 135.1 | 63.2ac |
| Carplatin | 10 | 80.01 ± 13.67 | 23.0 ± 1.03 | 1655.0 ± 157.4 | 5.6 |
During the experiment, diarrhea occurred in some mice when NM-3 was given at the dose of 20 mg/kg or 40 mg/kg. Two mice died of diarrhea in each of the two groups treated with 40 mg/kg NM-3 or with 40 mg/kg NM-3 in combination with carboplatin. The remaining mice recovered at the latter stage of the experiment. NM-3 alone at the dose of 10 mg/kg or in combination with carboplatin had no obvious effects on body changes.
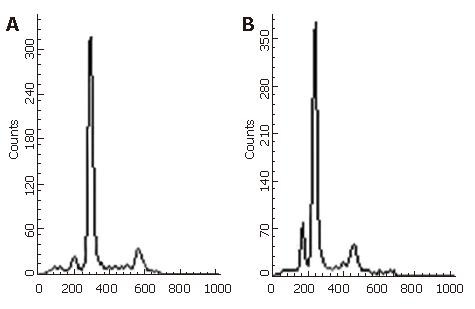
Apoptosis of gastric cancer cells was observed microscopically. Manifestations of apoptosis could be found more frequently in NM-3-treated groups, such as cell shrinkage, nuclear condensation, DNA fragmentation and formation of apoptotic bodies. Apoptosis was detected with flow cytometry (Figure 1).
The apoptosis index of tumors treated with carboplatin alone was not significantly different from that of the control group (P > 0.05). However, a significant increase in apoptosis was observed in the NM-3-treated groups, and the effect was more significant in the groups treated with carplatin in combination with NM-3 at 10 mg/kg, 20 mg/kg and 40 mg/kg than in the control group (Table 2). The induction of apoptosis was positively associated with the dose of NM-3.
| Groups | n | AI (TUNEL ) | AI (FAScan) |
| Normal saline | 10 | 2.12 ± 2.19 | 1.59 ± 0.24 |
| NM-3 (10 mg/kg) | 10 | 6.02 ± 1.63a | 3.84 ± 0.68a |
| NM-3 (20 mg/kg) | 10 | 10.43 ± 3.15ac | 8.21 ± 1.01ac |
| NM-3 (40 mg/kg) | 8 | 22.06 ± 5.68ac | 18.26 ± 4.46ac |
| NM-3 (10 mg/kg) + carplatin | 10 | 8.66 ± 2.35ac | 6.96 ± 0.65ac |
| NM-3 (20 mg/kg) + carplatin | 10 | 12.63 ± 3.75ac | 10.65 ± 1.43ac |
| NM-3 (40 mg/kg) + carplatin | 8 | 24.63 ± 3.67ac | 21.66 ± 2.96ac |
| Carplatin | 10 | 2.47 ± 0.31 | 1.85 ± 0.34 |
NM-3 significantly reduced the neo-microvascular formation of gastric cancer implanted into nude mice. The MVD was lower in groups treated with NM-3 or with NM-3 in combination with carboplatin than in carboplatin group or normal saline group (P < 0.05). More significant inhibitory effects on MVD of tumor were observed in NM-3-treated groups at the dose of 40 mg/kg or in combination with carboplatin than in control group (P < 0.05, Table 3, Figure 2A-H).
Recent studies have shown that angiogenesis plays a critical role in solid tumor growth and its development[8]. Antiangiogenic agents inhibit tumor growth by preventing proliferation, migration and sprouting of tumor endothelial cells and formation of new blood vessels. Antiangiogenic therapy plays an important role in improving prognosis of patients with gastric carcinoma[12-15]. NM-3, a small molecule isocoumarin, is a recently discovered angiogenesis inhibitor. It has been shown that NM-3 enhances the antitumor effects of some chemotherapeutic drugs in breast and prostate tumor models[11]. The increased antitumor effects of chemotherapy in combination with NM-3 can be achieved without any apparent increase in toxicity. To date, the effect of antitumor and induction of cell apoptosis of NM-3 on human gastric cancer have not been reported. It is worthwhile, therefore, to further research its antitumor mechanisms underlying gastric cancer.
In the present study, NM-3 significantly inhibited the growth of human gastric cancer in mice. Compared with the controls, growth of the tumor implanted subcutaneously was remarkably reduced in size and weight in the mice treated with NM-3 at the doses of 10 mg/kg, 20 mg/kg, 40 mg/kg. These doses of NM-3 in combination with carboplatin delayed the growth of SGC-7901 human gastric cancer in mice, compared with NM-3 or carboplatin alone. NM-3 alone at the dose of 10 mg/kg or in combination with carboplatin had no obvious effects on body changes. However, two mice died of diarrhea in each of the two groups treated with 40 mg/kg NM-3 or with 40 mg/kg NM-3 in combination with carboplatin, suggesting that the toxicity and doses of NM-3 used in patients need further studies. Although tumor growth was inhibited by NM-3 in combination with carboplatin as compared with normal saline group, tumor weight increased. This may be due to the lower dose of carboplatin used. In our study, carboplatin at the dose of 5 mg/kg showed minimal effects on tumor growth. Previous studies have demonstrated that chemotherapy in combination with an angiogenesis inhibitor can enhance tumor growth inhibition. Reimer et al[11] demonstrated that NM-3 can significantly enhance tumor growth inhibition in breast and prostate carcinoma xenografts at nontoxic doses in combination with 5-fluorouoacil, paclitaxel or cyclophosphamide given at subtherapeutic doses. These effects were particularly marked when NM-3 was combined with cyclophosphamide. Vinblastine in combination with VEGF receptor-2 antibody could cause sustained tumor regression. Salloum et al[10] studied the antitumor effects of NM-3 in combination with radiotherapy, and found that the tumor is significantly regressed after combined treatment compared with radiotherapy alone with no increase in systemic or local tissue toxicity, suggesting that NM-3 in combination with chemotherapy or radiotherapy can increase the efficacy of cancer treatment.
Cell apoptosis is an active death process of cells, its imbalance or changes are related to the occurrence of many diseases. Gastric cancer is not only a disease with abnormal cell proliferation and differentiation, but also a disease with abnormal apoptosis[16,17]. Increased apoptosis in human gastric cancer cells could be observed after treatment with 5-fluorouracil, cisplatin, etc. The results suggest that these drugs can be used in the treatment of patients with gastric cancer by inducing apoptosis of cancer cells. The results obtained by TUNEL method and cytometry analysis indicate that apoptosis is induced by NM-3. Matsuhashi et al[18] investigated the relationship between p53 expression and apoptosis induction of 5-fluorouracil and cisplatin on gastric cancer cells, and found that combined administration of 5-fluorouracil and cisplatin does not induce apoptosis of MKN-28 (mutant-type p53), while apoptotic cells can be observed in the case of MKN-45 (wild-type p53). Browder et al[19] demonstrated that TNP-470 at a low dose in combination with cyclophosphamide can eradicate drug-resistant Lewis lung carcinoma. Agata et al[20] revealed that NM-3 potentiates dexamethasone-induced apoptosis of human multiple myeloma cells. Moreover, NM-3 is effective against dexamethasone-resistant RPMI8226 and U266 multiple myeloma cells. NM-3 enhances dexamethasone-induced release of mitochondrial apoptogenic factors (cytochrome c and smac/DIABLO) and dexamethasone-induced activation of intrinsic caspase-9→caspase-3 apoptotic pathway. These results suggest that NM-3 inhibits the growth of gastric cancer by enhancing apoptosis of cancer cells.
Angiogenesis has been implicated in the growth and metastasis of gastric cancer. MaCarty et al[21] reported that ZD6474, a vascular endothelial growth factor receptor (tyrosine kinase inhibitor) inhibits orthotopic growth and angiogenesis of gastric cancer and increases tumor cell apoptosis. Stoeltzing et al[22] demonstrated that inhibition of hypoxia-inducible factor 1 activity can inhibit gastric cancer growth and angiogenesis. Kamiya et al[23] showed that the antitumor effect of VEGF Ab on gastric cancer is exerted by inducing mild hypoxia and apoptosis. Reimer et al[11] reported that the antitumor effects of NM-3 in combination with chemotherapeutic agents are mediated through decreased proliferation of endothelial cells. The present study indicated that NM-3 significantly inhibited angiogenesis in gastric cancer, suggesting that apoptosis of gastric cancer is mediated by NM-3 through decreased angiogenesis and that the inhibitory effect of NM-3 on gastric cancer growth is related to the induction of apoptosis.
In conclusion, NM-3 in combination with carboplatin is effective against gastric cancer. The toxicity and mechanism of NM-3 underlying apoptosis of gastric cancer need further studies.
Gastric carcinoma is one of the most frequent malignancies in China. Angiogenesis plays a crucial role in tumor growth and metastasis. Recently, induction of apoptosis by antiangiogenic therapy has been suggested as a new anticancer strategy.
2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid (NM-3) is a synthetic derivative of cytogenin. In vitro studies have demonstrated that the inhibitory effects of NM-3 at lower concentrations are stronger on human umbilical vein endothelial cells than on normal fibroblasts or tumor cells. NM-3 alone inhibits endothelial sprouting and tube formation in vitro.
There is some experience of NM-3 as an apoptotic and antiangiogenic inducer in other types of tumors such as lung and prostatic cancers, but not in gastric cancer which is associated with high chemotherapy resistance. Inhibitory effects of NM-3 on gastric cancer growth are mediated through decreased angiogenesis and the increased induction of apoptosis. NM-3 at the dose of 10 mg/kg alone or in combination with carboplatin has no obvious effects on body changes.
NM-3 in combination with carboplatin may be effective against gastric cancer.
This is a well designed experimental study of apoptosis and effects of NM-3 and carboplatin on gastric cancer model (SGC-7901). There is some experience in NM-3 as an apoptotic and antiangiogenic inducer in other types of tumors such as lung and prostatic cancers, but not in gastric cancer which is associated with high chemotherapy resistance. The paper is interesting.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Chen JL, Chen WX, Zhu JS, Chen NW, Zhou T, Yao M, Zhang DQ, Wu YL. Effect of P-selectin monoclonal antibody on metastasis of gastric cancer and immune function. World J Gastroenterol. 2003;9:1607-1610. [PubMed] |
| 2. | Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23:6220-6232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. Apoptosis induced by 5-fluorouracil, cisplatin and paclitaxel are associated with p53 gene status in gastric cancer cell lines. Int J Oncol. 2005;26:1563-1567. [PubMed] |
| 4. | Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Sun J, Blaskovich MA, Jain RK, Delarue F, Paris D, Brem S, Wotoczek-Obadia M, Lin Q, Coppola D, Choi K. Blocking angiogenesis and tumorigenesis with GFA-116, a synthetic molecule that inhibits binding of vascular endothelial growth factor to its receptor. Cancer Res. 2004;64:3586-3592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Levin EG, Sikora L, Ding L, Rao SP, Sriramarao P. Suppression of tumor growth and angiogenesis in vivo by a truncated form of 24-kd fibroblast growth factor (FGF)-2. Am J Pathol. 2004;164:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Atiqur Rahman M, Toi M. Anti-angiogenic therapy in breast cancer. Biomed Pharmacother. 2003;57:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Wahl ML, Moser TL, Pizzo SV. Angiostatin and anti-angiogenic therapy in human disease. Recent Prog Horm Res. 2004;59:73-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hinoda Y, Sasaki S, Ishida T, Imai K. Monoclonal antibodies as effective therapeutic agents for solid tumors. Cancer Sci. 2004;95:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Salloum RM, Jaskowiak NT, Mauceri HJ, Seetharam S, Beckett MA, Koons AM, Hari DM, Gupta VK, Reimer C, Kalluri R. NM-3, an isocoumarin, increases the antitumor effects of radiotherapy without toxicity. Cancer Res. 2000;60:6958-6963. [PubMed] |
| 11. | Reimer CL, Agata N, Tammam JG, Bamberg M, Dickerson WM, Kamphaus GD, Rook SL, Milhollen M, Fram R, Kalluri R. Antineoplastic effects of chemotherapeutic agents are potentiated by NM-3, an inhibitor of angiogenesis. Cancer Res. 2002;62:789-795. [PubMed] |
| 12. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 470] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 13. | Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F, Takeda A, Akagi M, Ellis LM. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech. 2003;60:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Saito H, Tsujitani S. Angiogenesis, angiogenic factor expression and prognosis of gastric carcinoma. Anticancer Res. 2001;21:4365-4372. [PubMed] |
| 15. | Tenderenda M, Rutkowski P, Jesionek-Kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001;7:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Forones NM, Carvalho AP, Giannotti-Filho O, Lourenço LG, Oshima CT. Cell proliferation and apoptosis in gastric cancer and intestinal metaplasia. Arq Gastroenterol. 2005;42:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep. 2005;14:609-615. [PubMed] |
| 19. | Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878-1886. [PubMed] |
| 20. | Agata N, Nogi H, Milhollen M, Kharbanda S, Kufe D. 2-(8-Hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl)propionic acid, a small molecule isocoumarin, potentiates dexamethasone-induced apoptosis of human multiple myeloma cells. Cancer Res. 2004;64:8512-8516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | McCarty MF, Wey J, Stoeltzing O, Liu W, Fan F, Bucana C, Mansfield PF, Ryan AJ, Ellis LM. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther. 2004;3:1041-1048. [PubMed] |
| 22. | Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Kamiya K, Konno H, Tanaka T, Baba M, Matsumoto K, Sakaguchi T, Yukita A, Asano M, Suzuki H, Arai T. Antitumor effect on human gastric cancer and induction of apoptosis by vascular endothelial growth factor neutralizing antibody. Jpn J Cancer Res. 1999;90:794-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |