Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5245
Revised: June 26, 2007
Accepted: August 17, 2007
Published online: October 21, 2007
AIM: To determine the gastroesophageal refluxate in the cervical esophagus (CE) and measure transcutaneous cervical esophageal ultrasound (TCEUS) findings [anterior wall thickness (WT) of CE, esophageal luminal diameter (ELD), esophageal diameter (ED)]; to compare TCEUS findings in the patient subgroups divided according to 24-h esophageal pH monitoring and manometry; and to investigate possible cut-off values according to the TCEUS findings as a predictor of gastroesophageal reflux (GER).
METHODS: In 45/500 patients, refluxate was visualized in TCEUS. 38/45 patients underwent esophagogastroduodenoscopy (EGD), 24-h pH monitoring and manometry.
RESULTS: The 38 patients were grouped according to 24-h pH monitoring as follows: Group A: GER-positive (n = 20) [Includes Group B: isolated proximal reflux (PR) (n = 6), Group C: isolated distal reflux (DR) (n = 6), and Group D: both PR/DR (n = 8)]; Group E: no reflux (n = 13); and Group F: hypersensitive esophagus (HSE) (n = 5). Groups B + D indicated total PR patients (n = 14), Groups E + F reflux-negatives with HSE (n = 18), and Groups A + F reflux-positives with HSE (n = 25). When the 38 patients were grouped according to manometry findings, 24 had normal esophageal manometry; 7 had hypotensive and 2 had hypertensive lower esophageal sphincter (LES); and 5 had ineffective esophageal motility disorder (IEM). The ELD measurement was greater in group A + F than group E (P = 0.023, 5.0 ± 1.3 vs 3.9 ± 1.4 mm). In 27/38 patients, there was at least one pathologic acid reflux and/or pathologic manometry finding. The cut-off value for ELD of 4.83 mm had 79% sensitivity and 61% specificity in predicting the PR between Groups B + D and E (AUC = 0.775, P = 0.015).
CONCLUSION: Visualizing refluxate in TCEUS was useful as a pre-diagnostic tool for estimating GER or manometric pathology in 71.1% of adults in our study, but it was not diagnostic for CE WT.
- Citation: Kacar S, Uysal S, Kuran S, Dagli U, Ozin Y, Karabulut E, Sasmaz N. Transcutaneous cervical esophagus ultrasound in adults: Relation with ambulatory 24-h pH-monitoring and esophageal manometry. World J Gastroenterol 2007; 13(39): 5245-5252
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5245.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5245
Esophageal ultrasonography (US) is a non-invasive, readily available, repeatable, cheap, fast and highly sensitive technique[1-4] in the diagnosis of gastroesophageal reflux (GER) in infants and children[5-8]. The esophageal US studies in GER have mainly focused on the evaluation of the gastroesophageal junction (GEJ)[9-11] and esophageal motility[12-14]. These studies were performed by transabdominal or endoluminal routes. Although cervical US is a part of neck US, it has not been routinely used in infants and adults for diagnosis of GER[9]. There are only a few studies about the transcutaneous cervical esophagus ultrasonography (TCEUS), but these were in normal[15,16] and pathologic conditions[17,18].
Intraluminal refluxate can be recognized by US images. Sonographic GER diagnosis was made by backward movement of gastric content into the esophagus and the visualization of the clearance of refluxate material[15,19,20]. The visualization of GER episodes or gastroesophageal reflux disease (GERD) estimation in the GEJ region in US provided the background for our study. The aims of this study were to evaluate the possible pathologies in 24-h (h) pH monitoring and esophageal manometry in patients with refluxate in the lumen of the cervical esophagus (CE) during TCEUS; to compare TCEUS findings in the patient subgroups divided according to 24-h esophageal monitoring and manometry; and to investigate possible cut-off values according to the TCEUS findings as a predictor of GER.
Patient features: Five hundred patients (45.82 ± 14.15 years, 163 M/337 F) who were admitted to the outpatient clinic between the years from January 2006 to January 2007 with complaints other than of the gastrointestinal system underwent TCEUS. Refluxate material was found in the esophageal lumen in 45 (9%) of the 500 patients during TCEUS. Forty-five patients were questioned regarding GERD symptoms, and all had reflux symptoms.
Thirty-eight of the 45 patients underwent esophagogastroduodenoscopy (EGD), 24-h pH monitoring and esophageal manometry [7 patients were excluded as follows: pH monitoring not accepted (n = 5), nasal cannulation could not be performed due to nasal operation history (n = 1), inability to continue the 24-h pH monitoring/pH catheter extracted (n = 1)]. The period between the TCEUS and the pH monitoring was 1-3 d.
Patients had no history of weight loss, gastrointestinal bleeding, gastrointestinal motility disorder, pneumonic dilatation, collagen vascular disease, any operation around the cervical region, or gastrointestinal operation. None of the patients was taking medications known to affect esophageal motor function, including promotility agents, antacids, H2 receptor antagonist, or proton-pump inhibitors (PPI); 3 patients had been taking PPI but they had been discontinued for two weeks before manometric investigation and 24-h pH monitoring.
Questionnaire for GERD: All patients were evaluated for typical (acid regurgitation and heartburn) and extraesophageal (hoarseness, asthma-like clinical presentation, nocturnal cough, and nocturnal wake-up) GERD symptoms.
Esophagogastroduodenoscopy (EGD): The procedure was performed by Pentax EG 2940 with 2% xylocaine topical anesthesia after a 12-h overnight fast. Reflux esophagitis was evaluated according to Los Angeles classification[21]. The presence of hiatal hernia and the distance between GEJ and diaphragmatic impression were recorded.
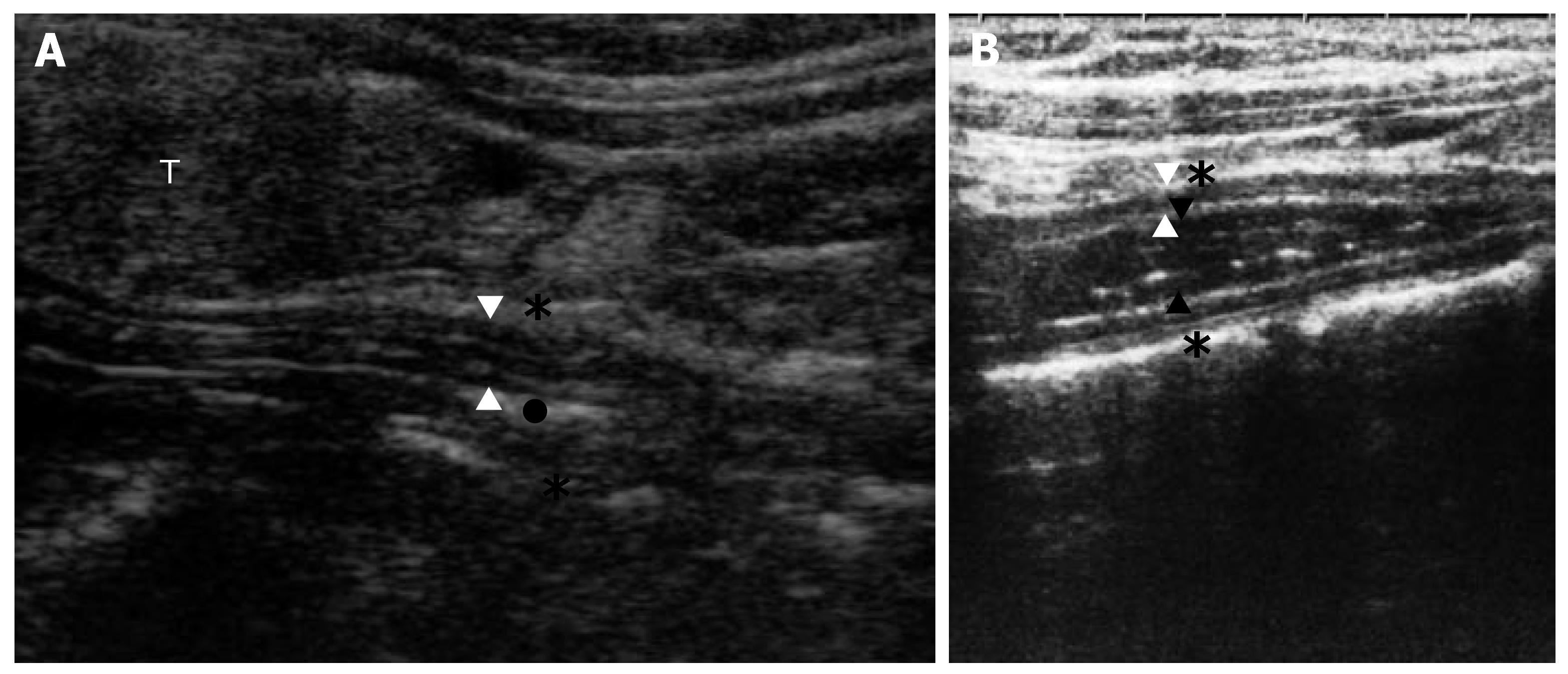
TCEUS: Each patient was given an 800 kcal standard meal (15% protein, 50% carbohydrate, and 35% fat) and TCEUS was performed at postprandial 1-2 h with patient in supine position (Hitachi EUB, 6-13 MHz linear probe). TCEUS was performed as defined by Zhu and Mateen[15,16]. The esophagus was demonstrated at thyroid cartilage level with the guidance of thyroid gland acoustic window up to the supraclavicular level to thoracal inlet (manubrium sterni) by linear probe in transverse and longitudinal sections without a pillow under the neck. CE was evaluated by using a slightly flexed neck position with head turned 45° to the opposite side by left and right lateral approaches over 15 min to determine the presence of refluxate (the luminal anechoic fluid and/or linear bright stratifying small lines indicating gas in refluxate) and its to-and-fro movement, with the patients not swallowing[15,20,22]. Then all patients were required to swallow and the clearance of refluxate was observed. The presence of comet-tail artifact (during swallowing, the presence of saliva mixed with air and downward movement of refluxate generated a strong echogenic appearance[15,20,22]) was observed in patients. After the clearance of refluxate was observed, a few patients had backward flow of refluxate into the esophagus, which can perhaps be considered by the terminology “re-reflux”[23].
Anterior wall thickness (WT) of the esophagus (distance between adventitia and the mucosa, with 5-7 esophageal wall layers), esophageal diameter (ED) [distance between the adventitia (outer to outer)], and esophageal luminal diameter (ELD) with or without refluxed material [distance between the mucosa (inner to inner)] were measured in longitudinal section at left lateral cervical approach. The GEJ was not evaluated during US in this study. The TCEUS appearance with or without refluxate is given in Figure 1.
Ambulatory 24-h pH monitoring: pH monitoring was performed using Synetics Digitrapper MHIII, and double-channel, 15 cm antimony catheter. The esophageal pH catheter was placed 5 cm above the upper border of the lower esophageal sphincter (LES). Findings were evaluated by Microsoft esophagram version 2.04. The pathologic measurements were evaluated as follows: Proximal reflux (PR): The upper esophageal sphincter (UES) localization was determined by manometry and PR was determined by the proximal probe localization and UES. If proximal probe was localized in the UES or above it, a single acid reflux synchronously occurring with distal probe was accepted as pathologic acid reflux; if the probe was localized under the UES, acid contact time above 1% of total time was accepted as pathologic in PR. De Meester score > 14.72 and acid contact greater than 4.0% of total time below pH 4 were accepted as pathologic in distal reflux (DR). Hypersensitive esophagus (HSE) was defined if symptom index (SI) for distal measurements (SI = number of symptoms in pH < 4/total number of symptoms) was ≥ 50% while there was no measurable DR or PR[23-25].
Esophageal manometry: Esophageal manometry was performed using MMS (ver. 8.4i Beta) and eight-channel Dent-sleeve catheter. After calibration, catheter was sent through the nose to the stomach and advanced 65 cm by swallowing. When all channels were in stomach, with patient in supine position, UES and LES were determined as the catheter was slowly withdrawn back into the esophagus. LES pressure (LESP), relaxation, esophageal body pressure, body contractions, contraction amplitudes and duration, peristalsis and upper esophageal contractions were recorded. Manometric findings were grouped as: normal, spastic (hypertensive LES, if LESP > 45 mmHg), non-spastic [hypotensive LES, if LESP < 10 mmHg) or ineffective esophageal motor contractions (IEM), if contraction amplitude was < 25 mmHg in > 30% of wet swallows][26].
Descriptive and comparative statistical analyses were performed using statistical software system (SPSS v11.0). Where appropriate, average data were presented as mean ± SD. Comparison between groups was performed by Kruskal Wallis analysis. All possible pair-wise comparisons were done by Mann-Whitney U test with Bonferroni correction. Fisher-Freeman-Halton test generalized at Fisher’s exact test to mxn tables was used for categorical variables. The cut-off values were determined using receiver operating characteristic (ROC) curve for TCEUS parameters between all possible patient group pairs according to pH-metry and manometry to determine reflux or any pathologic manometry finding. The sensitivity and the specificity were determined according to the measured cut-off values. The significance of the area under curve (AUC) was tested (P < 0.05).
Investigators interpreting sonography, 24-h pH monitoring and esophageal manometry were blinded to the patients’ features. None of the patients was sedated during EGD. All patients provided written informed consent and the study conformed to the guidelines of the Helsinki Declaration.
Forty-five (9%) of 500 patients who underwent TCEUS were found to have anechoic fluid and/or air echogenicities of refluxate in the cervical esophageal lumen. CE was not visualized clearly in 1 (0.2%) of the 500 patients due to neck anatomy.
Esophagitis (all grade A), hiatal hernia, antral gastritis, and grade 1 bulbitis were diagnosed in 10.7%, 10%, 14%, and 3%, respectively, in EGD. None of the patients had malignancy, or gastric or duodenal ulcer disease.
Thirty-eight patients were grouped according to 24-h pH monitoring as follows: Group A: Acid reflux-positive (n = 20, 52.7%) [includes Group B + Group C + Group D] [Group B: Isolated PR but no DR (n = 6, 15.8%); Group C: Isolated DR but no PR (n = 6, 15.8%); Group D: Both PR and DR (n = 8, 21.1%)]; Group E: No acid reflux (n = 13, 34.2%); and Group F: patients with hypersensitive esophagus (HSE) (n = 5, 13.1%). Group B + D indicated total patients with PR (n = 14, 36.9%) and Group E + F: acid reflux-negatives with HSE (n = 18, 47.3%) and Group A + F: Acid reflux-positives with HSE (n = 25, 65.8%). The demographic and TCEUS findings of subjects grouped according to 24-h pH monitoring are given in Table 1.
| Group A (n = 20) | Group B (n = 6) | Group C (n = 6) | Group D (n = 8) | Group E (n = 13) | Group F (n = 5) | Group B + D (n = 14) | Group E + F (n = 18) | Group A + F (n = 25) | P | |
| Age (yr) | 43.4 ± 12.0 | 40.0 ± 11.4 | 47.8 ± 12.7 | 42.6 ± 12.5 | 43.17 ± 9.6 | 37.2 ± 18.8 | 41.5 ± 11.7 | 40.5 ± 10.7 | 42.3 ± 13.7 | NS |
| (24-72) | (24-58) | (38-72) | (25-62) | (25-65) | (15-64) | (24-629 | (16-65) | (15-72) | ||
| Sex (F/M) | 8/12 | 3/3 | 1/5 | 4/4 | 11/2 | 5/0 | 7/7 | 2/16 | 12/13 | NS |
| n (%) | (40/60) | (50/50) | (16.7/83.3) | (50/50) | (84.6/5.4) | (100/0) | (50/50) | (88.9/11.1) | (52/48) | |
| BMI (kg/m2) | 27.4 ± 4.5 | 27.4 ± 4.4 | 27.4 ± 4.4 | 27.9 ± 5.6 | 27.7 ± 4.6 | 24.9 ± 2.1 | 27.7 ± 4.9 | 26.9 ± 4.24 | 26.9 ± 4.3 | NS |
| (19.6-37.4) | (19.6-32) | (19.6-32) | (20.6-37.5) | (17.6 ± 35.0) | (22.7-27.4) | (19.6-37.4) | (17.6-35.0) | (19.6-37.5) | ||
| ED (mm) | 9.4 ± 1.4 | 9.7 ± 0.9 | 9.1 ± 1.6 | 9.6 ± 1.71 | 8.54 ± 1.82 | 10.6 ± 1.3 | 9.6 ± 1.4 | 9.1 ± 1.9 | 9.7 ± 1.5 | NS |
| (6.1-12.1) | (8.2-10.8) | (8.0-12.0) | (6.1-11.4) | (5.5-11.1) | (8.8-12.4) | (6.2-11.4) | (5.5-12.4) | (6.1-12.4) | ||
| ELD (mm) | 5.0 ± 1.2 | 5.1 ± 1.1 | 4.6 ± 1.6 | 5.2 ± 1.18 | 3.9 ± 1.4 | 5.3 ± 1.7 | 5.16 ± 1.10 | 4.3 ± 1.6 | 5.0 ± 1.3 | 0.023a |
| (3.0-7.7) | (3.00-6.00) | (3.4-7.7) | (3.0-6.4) | (1.5-6.2) | (3.4-7.9) | (3.0-6.4) | (1.5-7.9) | (3.0-7.9) | ||
| Esophageal | 2.2 ± 0.2 | 2.3 ± 1.2 | 2.2 ± 0.1 | 2.1 ± 0.3 | 2.3 ± 0.3 | 2.6 ± 0.7 | 2.2 ± 0.3 | 2.4 ± 0.50 | 2.3 ± 0.4 | NS |
| WT (mm) | (1.5-2.6) | (2.2-2.6) | (2.0-2.3) | (1.5-2.5) | (2.0-3.3) | (2.2-4.0) | (1.6-2.6) | (2.0-4.0) | (1.6-4.0) | |
| DeMeester | 19.5 ± 12.1 | 7.9 ± 3.1 | 24.3 ± 14.4 | 24.5 ± 9.1 | 6.4 ± 4.7 | 7.5 ± 4.9 | 17.4 ± 10.9 | 6.7 ± 4.7 | 17.7 ± 11.8 | NS |
| score | (1.7-46.2) | (4.6-13.2) | (1.7-46.2) | (14.4-38.9) | (0.8-14.7) | (1.4-17.3) | (4.6-38.9) | (0.8-14.7) | (1.4-46.2) |
When the 38 patients were grouped according to manometry findings, 24 (63.2%) patients had normal esophageal manometry; 7 (18.4%) had hypotensive and 2 (5.3%) had hypertensive LES; 5 (13.1%) had ineffective esophageal motility disorder (IEM). Demographic and TCEUS findings of subjects grouped according to manometric results are given in Table 2.
| Normal (n = 24) | IEM (n = 5) | Hypo LES (n = 7) | Hyper LES (n = 2) | Total patients (n = 38) | P | |
| Age (yr) | 40.1 ± 11.4 (15-64) | 50.6 ± 11.9 (39-65) | 48.9 ± 11.3 (38-72) | 29.0 ± 4.3 (26-32) | 42.5 ± 12.2 (15.0-72.0) | NS |
| Sex (F/M), n (%) | 8/16 (66.7%/33.3%) | 4/1 (80%/20%) | 2/5 (28.6%/71.4%) | 2/0 (100%/0%) | 24/14 (63.2%/36.8%) | NS |
| BMI (kg/m2) | 26.7 ± 4.6 (17.6-375) | 26.7 ± 4.1 (20.6-31.1) | 29.58 ± 3.93 (23.1-35.0) | 25.4 ± 2.7 (23.4-27.3) | 27.2 ± 4.3 (17.6-37.4) | NS |
| ED (mm) | 9.1 ± 1.59 (6.0-12.4) | 9.5 ± 2.3 (5.5-11.4) | 9.8 ± 1.7 (7.5-12.1) | 9.6 ± 1.1 (8.9-10.4) | 9.3 ± 1.7 (5.5-12.4) | NS |
| ELD (mm) | 4.5 ± 1.3 (2.0-7.9) | 4.7 ± 1.9 (1.5-6.4) | 5.1 ± 1.7 (3.1-7.7) | 4.7 ± 0.9 (4.1-5.4) | 4.68 ± 1.43 (1.5-7.9) | NS |
| Esophageal WT (mm) | 2.2 ± 0.4 (1.6-4.0) | 2.4 ± 2.2 (2.0-2.6) | 2.3 ± 0.4 (2.0-3.3) | 2.35 ± 0.2 (2.2-2.5) | 2.3 ± 0.4 (1.6-4.0) | NS |
Patient symptoms are given in Table 3. None of the patients had complaints of dysphagia or asthma-like dyspnea.
| Group A (n = 20) | Group E (n = 13) | Group B + D (n = 14) | Group E + F (n = 18) | |
| Extra-esophageal symptom | 12 (60.0) | 7 (53.8) | 9 (64.3) | 12 (66.6) |
| Cough | 6 (30.0) | 6 (46.2) | 4 (28.5) | 8 (44.4) |
| Hoarseness | 5 (25) | 3 (23.1) | 4 (28.6) | 4 (22.2) |
| Nocturnal wake-up with reflux | 10 (50) | 5 (38.5) | 8 (57.1) | 9 (50.0) |
| Typical symptom | 20 (100) | 12 (92.3) | 14 (100.0) | 17 (94.4) |
| Heartburn | 18 (90) | 12 (92.3) | 12 (85.7) | 17 (94.4) |
| Regurgitation | 15 (75) | 10 (76.9) | 12 (85.7) | 13 (72.2) |
Statistical analysis was performed between (1) 24-h pH monitoring subgroups, (2) esophageal manometry subgroups, and (3) categorized groups according to combined 24-h pH monitoring and esophageal manometry findings as acid reflux/abnormal, acid reflux/normal, no acid reflux/abnormal, and no acid reflux/normal with the following parameters: Age, sex, body mass index (BMI), LES localization defined during manometry, and TCEUS findings (WT, ED, ELD).
There were no significant differences in BMI, LES localization, and typical and extraesophageal symptoms between subjects grouped according to 24-h pH monitoring and according to esophageal manometric findings. There was no correlation between the TCEUS findings and sex or presence of hiatal hernia or esophagitis.
When 24-h pH monitoring subgroups were compared according to TCEUS findings, the ELD measurement was greater in group A + F than group E (P = 0.023). No significant differences were determined between the other subgroups when compared regarding TCEUS findings.
There was a positive significant correlation between ED and ELD (r = 0.889, P = 0.000) and ED and WT (r = 0.499, P = 0.001) (Pearson correlation analysis).
There were 2 patients with hypertensive LES. Excluding this group, when the nonspastic esophageal motor disorder group (hypotensive LES, IEM) was compared with the normal manometric group, there was no significant difference with regard to TCEUS findings and the LES localization between subgroups.
There was no significant difference according to TCEUS findings when 24-h pH monitoring and esophageal manometry subgroups were evaluated together (Table 4). Table 5 shows the detailed 24-h pH monitoring and manometric findings. In 27/38 (71.1%) patients, there was at least an acidic reflux and/or pathologic manometry finding. 11/38 (28.9%) with refluxate in CE had no acid reflux and normal esophageal manometric findings.
| Manometric findings (abnormal) | Manometric findings (normal) | |
| 24-h pH monitoring (Acid reflux) | 7 (18.4) | 13 (34.3) |
| 24-h pH monitoring (No acid reflux) | 7 (18.4) | 11 (28.9) |
| 24-h pH monitoring | IEM (n = 5) | Hypo LES (n = 7) | Hyper LES (n = 2) | Normal manometry (n = 24) |
| PR + DR | 2 | 2 | 4 | |
| PR | 6 | |||
| DR | 3 | 3 | ||
| Reflux negatives | 3 | 2 | 1 | 7 |
| HSE | 1 | 4 |
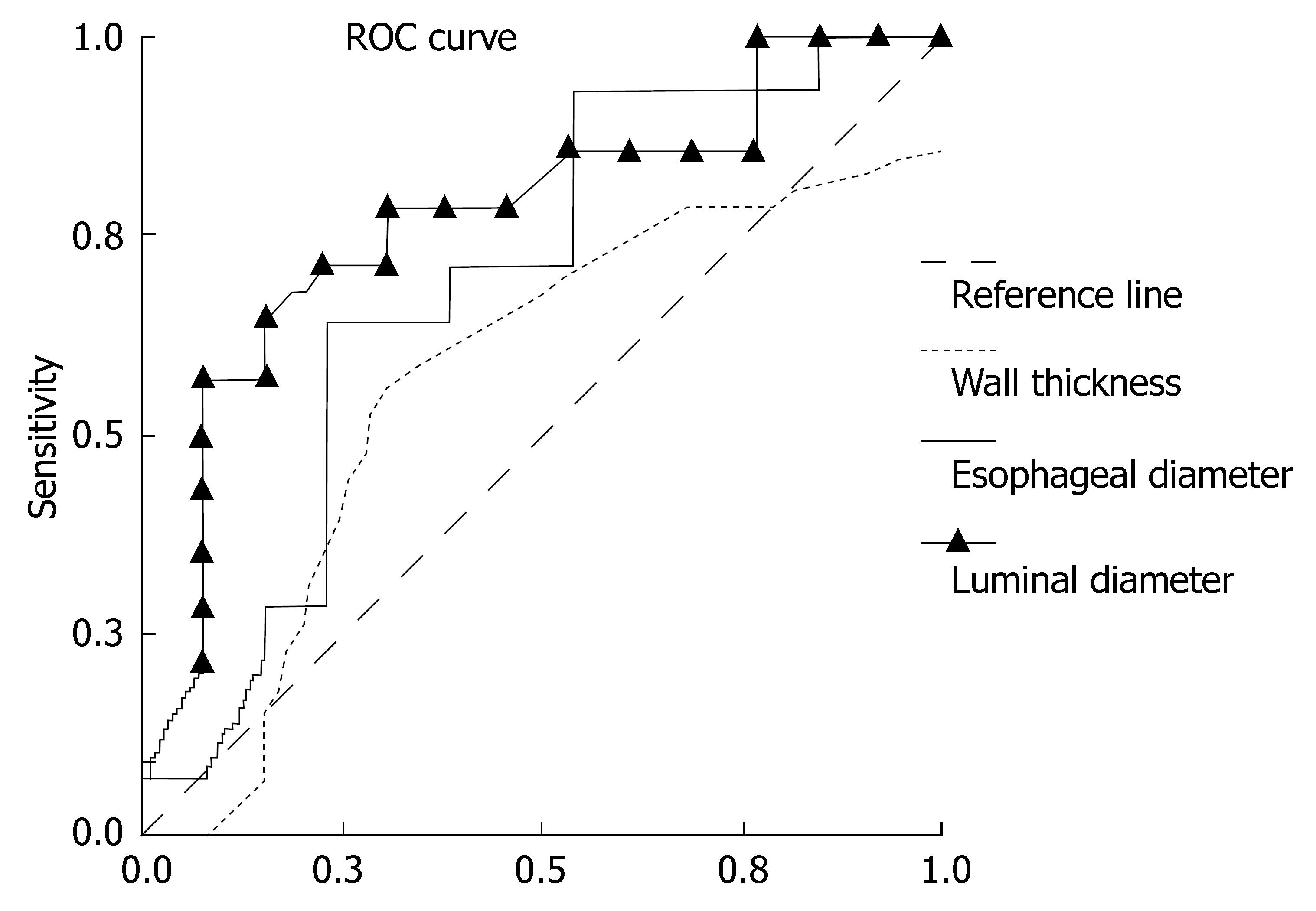
We tried to find cut-off values in order to differentiate total GER, PR or the other reflux subgroups from the reflux-negatives and to differentiate each manometry subgroup according to TCEUS parameters. The groups which had significant cut-off values (AUC, P < 0.05) are given in Table 6 with their sensitivity and specificity rates for ELD in determining reflux. The ROC curve is given in Figure 2 according to TCEUS findings in patients with total PR (group B + D) (n = 14) and in patients without reflux (group E) (n = 13).
| Between groups | Cut off (mm) | AUC | P | Sensitivity (%) | Specificity (%) |
| Group B + D (total PR) (n = 14)/Group E (reflux negative) (n = 13) | 4.83 | 0.775 | 0.015a | 79 | 61 |
| Group B + D (total PR) (n = 14)/Group E + F (reflux negative with HSE) (n = 18) | 4.95 | 0.708 | 0.046 | 71 | 77 |
| Group A (n = 20)/Group E (reflux negative) (n = 13) | 4.95 | 0.721 | 0.034 | 60 | 77 |
The esophagus is a 23-24 cm muscular channel. The longitudinal scan of the esophagus shows a tubular structure with hypoechogenic muscular layer on the wall and one or two echogenic inner layer(s) representing the mucosa and the collapsed lumen of the esophagus[3,10,15,16,20,22].
US evaluation is performed at four sites of the esophagus: GEJ[4,9,27,28], thoracal esophagus[22], CE[15,16], and upper esophageal sphincter[29].
GERD arises from increased exposure and/or sensitivity of the esophageal mucosa to gastric contents[30,31], and affects 5%-40% of the population[32,33]. The content of refluxate can be isolated liquid (acid or non-acid nature), isolated gas, or gas/liquid mixture. 24-h pH monitoring and multichannel intraluminal impedance (MII) are the gold standard techniques to evaluate GER[19,34,35].
US has been used in GERD since 1984[36]. The GEJ was first described by Westra[6] and Gomes[1] during US by transabdominal route. The first-line use of esophageal GEJ US in GERD for infants and children was established by multiple studies[2,4,6,37]. Sonographic sensitivity was 81%-94%[3,4]. US provides a morphologic and functional approach. In infants and children, 24-h pH monitoring and esophageal US are the complementary techniques of choice[2,4].
Zhu points out the importance of conventional US to evaluate the GEJ, but the use of the CE was defined to be restricted. Zhu defined the normal sonographic parameters of the CE (7.5-12 MHz transducer) transcutaneously[15]. Mateen et al used a modified technique which differed from the normal neck US to evaluate the CE. Visualization failure of the right lateral two-thirds CE was decreased from 36% to 2% using this modified technique[16]. In our study, use of this modified technique resulted in failed visualization in only 1 (0.2%) of 500 patients due to the deformed anatomy of the patient.
GERD was diagnosed in 26% of a healthy population of infants and children according to US[3]. In our study, postprandial refluxate was seen in 45 of 500 (9%) adults. Furthermore, 20/38 (52.63%) of the patients who admitted to the hospital for other than gastrointestinal symptoms had refluxate in TCEUS and acidic reflux according to the 24-h pH monitoring. Since non-acid reflux was not evaluated, the 18 other patients were not evaluated in this respect.
Cool et al[38] showed that respiratory and ear, nose and throat symptoms were especially related with gas reflux with weak acidity and not abnormal proximal acid reflux. We did not find any correlation in our study between proximal acid reflux, GER and any symptoms.
During the first hour after a meal, 20% more reflux episodes reach a higher proximal extent than during the fasting period and the late postprandial period (after 1 h). Acid reflux can reach 15 cm above the LES in approximately 6.8-21 s[39,40]. We performed the sonography at the postprandial 1st-2nd h.
The content of liquid refluxate, whether acidic or not, did not affect the sonographic appearance[41]. The new studies have pointed out that gas reflux with weak acidity is quite often determined in PR studies performed by pharyngeal impedance-pH recordings. Mixed reflux of gas and fluid is more frequent than pure fluid reflux[39].
In the study of Mittal performed simultaneously with high frequency endoscopic US (HFEUS) and pH-metry, five US refluxate patterns were identified, as fluid, gas, first gas later fluid transition, first liquid later gas transition, or no luminal opening[19]. In our study, we observed these patterns as fluid with or without gas and its to-and-fro movement during a period patients did not swallow. During swallowing, the comet-tail artifact was detectable in 42.8% of the cases[15,19]. We observed it in approximately half of the patients. A few patients had reverse movement of refluxate to the esophagus after swallowing. This could be US documentation of a new terminology, “re-reflux”[23].
Jang et al found no correlation between reflux number in 15 min counted during US and the reflux index in the 24-h pH monitoring. Sonographic reflux number was not considered as a specific indicator of disease severity[2]. We did not count the reflux episodes in our study. Only one reflux episode in CE during US was included. Dickmann et al[42] had shown that the acid reflux period below pH 4 was significantly lower when the distal pH probe was located 16 cm above the LES than 1, 6 or 11 cm above the LES in non-erosive reflux disease (NERD).
Transcutaneous CE WT has been reported as 2.3 ± 0.3 (1.3-4.1) mm in healthy adults[15]. Mateen reported right-side thickness as 2.8 ± 0.4 mm (upper limit of normal, 3.6 mm) and left-side thickness as 2.9 ± 0.2 mm (upper limit of normal, 3.3 mm). We determined the left anterior WT as 2.29 ± 0.38 mm (1.57-4.0) in TCEUS (Table 1).
Dogan et al[43] reported an increase in distal esophageal WT in conjunction with increasing age (1.56 ± 0.32 mm vs 1.29 ± 0.24 mm). In our study, we found no correlation between age, sex and TCEUS findings in subjects with refluxate.
Endosonographically, GEJ and 10 cm above thicknesses were given as 2.43 ± 0.16 and 2.28 ± 0.21 mm in healthy adults, respectively. The distal esophagus wall was thicker than the proximal[28]. In reflux esophagitis, total esophageal WT and the smooth muscle layer were observed to be thicker than in normal subjects[19,28,32]. Changchien measured normal GEJ WT as 3.8 ± 1.2 (2-5) mm using real time US. During acute severe inflammation in reflux esophagitis, the GEJ wall was observed as 7.6 ± 2.1 (5-10) mm, which was significantly thicker than normal[9]. The submucosal healing due to lansoprazole in GERD was evaluated by US and the WT had decreased significantly in the GEJ region[32,44]. We did not determine any significant difference in WT between patients with or without reflux according to 24-h pH monitoring. The distal esophageal WT increased with reflux according to the literature as described above, but we could not confirm this observation for the proximal esophagus in GER. Although HSE is a new terminology in the GERD spectrum, there was no significant difference between the HSE subgroup and the other subgroups with regard to CE WT.
Zhu reported normal transverse ED as 11.1 ± 1.6 (7.1-13.9) mm and anteroposterior diameter as 7.5 ± 1.2 mm (4.9-10.1)[15]. Mateen measured the transverse diameter as 6.8 ± 2.7 mm (max 12.2)/10.7 ± 4.0 mm (18.7) and anteroposterior diameter as 6.5 ± 1.1 (max 8.7) mm/7.4 ± 1.5 (10.4) mm with right and left approaches, respectively, using the modified technique[16]. We measured the cervical anteroposterior ED as 5.5-12.4 mm, and the ELD with refluxate was 1.5-7.9 mm in patients longitudinally (Tables 1 and 2). No significant differences were determined between proximal and total reflux patient groups and the other subgroups with regard to ED. ELD measurements with refluxate were statistically greater in group A + F than group E (P = 0.023). There was no difference between the other groups regarding ELD. Peak ED was given as 22 mm during physiologic swallows with 15 mL water[19]. PR volume has not been accurately diagnosed to date, though esophageal continuous aspiration and scintigraphic studies have been used in an effort to obtain results about the reflux volume[39]. In our study, the ED and ELD measurements may be an indirect indicator of reflux amount.
The distal esophageal distension and the cross-sectional area (CSA) are known to be wider than the proximal esophagus[19]. Mittal reported that healthy asymptomatic individuals had comparable esophageal diameter and CSA measurements according to spontaneous fluid GER and 5 mL swallow. It is difficult to differentiate between the ingested fluid and the refluxate of esophageal content. Mittal made the differentiation by looking at transient LES relaxations synchronously. In our study, our patients did not drink water during the TCEUS measurements. We measured the esophageal refluxate during a non-swallowing period. Our ED measurements (Table 1) were compatible with the 5 mL water intake in Mittal’s studies[19].
Mittal et al observed many reflux episodes determined by pH probe but not concomitant sonographic reflux by HFEUS. Similar observations were also made using impedance techniques. They concluded the gas-dominant or mixed reflux episodes could be the contributory factor[19]. In our study, we did not perform TCEUS and the 24-h pH monitoring concurrently. This finding points out that US in GERD has some shortcomings. In contrast to this finding is the short reflux period which was determined by color Doppler (CD) US but not by pH monitoring[45].
There was a positive significant correlation between ED and ELD (r = 0.889, P = 0.000) and ED and WT (r = 0.499, P = 0.001) (Pearson correlation analysis). The positive relation could be explained by presence of refluxate in the esophageal lumen. Although there was positive correlation between ED and WT, no significant difference was found between groups. This could be explained by the small patient groups.
Esophageal motor disorders can cause abnormal fluid or viscous bolus transit[37,46]. Esophageal dysmotility can cause reflux esophagitis and reflux can cause esophageal dysmotility[12,37]. Patients with normal esophageal motility, diffuse esophageal spasm (DES) and achalasia had 35%, 67%, and 100% abnormal fluid or viscous bolus transit, respectively[37]. The possible manometric disorders that could be responsible for the PR were also evaluated in our study. We did not observe any patients with achalasia, DES or nutcracker esophagus. The manometric abnormality prevalence in patients with cervical refluxate during TCEUS was 36.84% (14/38 patients) [5 (13.16%) IEM, 7 (18.4%) hypotensive LES, 2 (5.4%) hypertensive LES] (Table 2).
There is a gradual increase in muscle thickness, thickening of the muscularis propria and increase in CSA from the proximal to distal esophagus in primary spastic esophageal motor disorders like achalasia, DES, nutcracker esophagus, hypertensive LES, and atypical LES relaxation, and in non-spastic esophageal motor disorders like hypotensive LES, IEM, and incomplete LES relaxation[12,15,37,41]. WT according to disorder was achalasia > DES > nutcracker esophagus[19,41,43]. The normal basal esophageal WTs at 2 cm and 10 cm above the GEJ were measured as 1.45 ± 0.31 mm and 1.24 ± 0.23 mm, respectively, by Dogan et al. The corresponding abnormal values were 2.08 mm and 1.71 mm[43].
We found no difference in anterior CE WT in patient subgroups divided according to esophageal manometry. In our study, the esophageal measurements were taken at the thyroid gland level, while corresponding values in the literature were measured at the GEJ or 2 or 10 cm above the GEJ. IEM is characterized by low amplitude esophageal contractions, which could cause ineffective acid clearance and aid the reflux pathogenesis[37]. In our study, 7 of 14 (50%) patients with abnormal esophageal manometry had acid reflux (3 had DR and 4 had both PR and DR) in 24-h pH monitoring. Two of 5 patients who had IEM disorder, 5 of 7 patients with hypotensive LES and 0 of 2 patients with hypertensive LES had reflux (Table 5). The PR rate was higher than DR rate (70%, 30%) in group A. None of the patients with isolated PR had manometric impairment. Twenty-seven patients (71.1%) had at least one pathology in pH monitoring (acid reflux) and/or manometry. We did not observe any pathology which could cause impairment in esophageal transit in 11 of 38 patients (28.9%). Since we did not investigate non-acid reflux, the probable reflux patterns in these 11 patients are unknown.
We aimed to determine the possible cut-off values for TCEUS findings in patients with refluxate as a predictor of GER or pathologic manometry finding. ELD but not WT and ED showed cut-off values (AUC, P < 0.05). ELD (with refluxate) of 4.95 mm had 71% sensitivity and 77% specificity in the estimation of total PR patients (Table 6).
The fact that 24-h pH monitoring and manometry were not performed in subjects without refluxate during TCEUS is a limitation of this study.
Esophageal refluxed material can be recognized in ultrasonographic images. TCEUS can not substitute for 24-h pH monitoring or esophageal manometry, but it can serve as a complementary technique by aiding in the estimation of proximal reflux, GER and motility disorders which could cause impairment in bolus transit.
To our knowledge, there is no study in the available literature showing refluxate presence in the cervical esophageal lumen and measuring the TCEUS parameters at the thyroid gland level transcutaneously while correlating pH monitoring and esophageal manometry findings in adults.
We express our thanks to Eylul Ozturk and Mahmut Kacar for their guidance and help.
Esophageal refluxed material can be recognized in ultrasonographic images. The content of refluxate can be isolated liquid (acid or non-acid nature), isolated gas, or gas/liquid mixture. The content of liquid refluxate, whether acidic or not, did not affect the sonographic appearance. Liquid can be present in the esophageal lumen in gastroesophageal reflux (GER) and esophageal motility disorders. Esophageal ultrasonography is currently being used to evaluate the gastroesophageal junction by transabdominal route, especially in newborns and children, and endosonographic studies have been used especially for motility disorders. The relation between the presence of refluxate in the cervical esophageal lumen and the esophageal pH-metry/manometry findings using transcervical esophageal ultrasonography (TCEUS) has not been studied previously in children and adults.
We evaluated the possible pathologies in 24-h pH monitoring and esophageal manometry in patients with refluxate in the lumen of the cervical esophagus during TCEUS. In 27/38 (71.1%) patients with refluxate in TCEUS, there was at least one pathologic acid reflux and/or pathologic manometry finding. 24 h pH-metry and esophageal manometry subgroups were compared statistically according to TCEUS findings [anterior wall thickness (WT) of the esophagus, esophageal diameter (ED), esophageal luminal diameter (ELD)].
Our study is distinct from other studies evaluating the GER and esophageal manometry pathologies with ultrasonographic methods due to our usage of TCEUS. We performed esophageal manometry and 24-h pH monitoring in patients with refluxate in the esophageal lumen. The shortcomings of the study were that 1) we did not perform manometry or pH monitoring in patients without refluxate and 2) we did not evaluate the non-acid reflux.
Different patient groups and volunteers without refluxate can be evaluated for different study designs.
The presence of refluxate in esophageal lumen in TCEUS: The luminal anechoic fluid and/or linear bright stratifying small lines indicating gas in refluxate with the patients not swallowing; The presence of comet-tail artifact: During swallowing, the presence of saliva mixed with air and downward movement of refluxate generated a strong echogenic appearance; TCEUS parameters: Wall thickness (WT) of the esophagus: Distance between adventitia and the mucosa, with 5-7 esophageal wall layers; Esophageal diameter (ED): Distance between the adventitia (outer to outer); Esophageal luminal diameter (ELD) with or without refluxate: Distance between the mucosa (inner to inner).
The authors studied transcutaneous cervical esophageal ultrasound (TCEUS) as a possible diagnostic procedure in gastroesophageal reflux. TCEUS has been forgotten in the diagnosis of esophageal diseases, however, it is a non-invasive, available and high sensitive technique. This manuscript is in principle an interesting topic for the readers of World Journal of Gastroenterology.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Lu W
| 1. | Gomes H, Menanteau B. Gastro-esophageal reflux: comparative study between sonography and pH monitoring. Pediatr Radiol. 1991;21:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Jang HS, Lee JS, Lim GY, Choi BG, Choi GH, Park SH. Correlation of color Doppler sonographic findings with pH measurements in gastroesophageal reflux in children. J Clin Ultrasound. 2001;29:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Halkiewicz F, Kasner J, Karczewska K, Rusek-Zychma M. Ultrasound picture of gastroesophageal junction in children with reflux disease. Med Sci Monit. 2000;6:96-99. [PubMed] |
| 4. | Koumanidou C, Vakaki M, Pitsoulakis G, Anagnostara A, Mirilas P. Sonographic measurement of the abdominal esophagus length in infancy: a diagnostic tool for gastroesophageal reflux. AJR Am J Roentgenol. 2004;183:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Riccabona M, Maurer U, Lackner H, Uray E, Ring E. The role of sonography in the evaluation of gastro-oesophageal reflux--correlation to pH-metry. Eur J Pediatr. 1992;151:655-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Westra SJ, Wolf BH, Staalman CR. Ultrasound diagnosis of gastroesophageal reflux and hiatal hernia in infants and young children. J Clin Ultrasound. 1990;18:477-485. [PubMed] [DOI] [Full Text] |
| 7. | Westra SJ, Derkx HH, Taminiau JA. Symptomatic gastroesophageal reflux: diagnosis with ultrasound. J Pediatr Gastroenterol Nutr. 1994;19:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Milocco C, Salvatore CM, Torre G, Guastalla P, Ventura A. Sonography versus continuous 24 hours oesophageal pH-monitoring in the diagnosis of infant gastroesophageal reflux. Pediatr Med Chir. 1997;19:245-246. [PubMed] |
| 9. | Changchien CS, Hsu CC. Use of sonography in the evaluation of the gastroesophageal junction. J Clin Ultrasound. 1996;24:67-72. [PubMed] [DOI] [Full Text] |
| 10. | Esposito F, Lombardi R, Grasso AC, Dolezalova H, Sodano A, Tarantino L, Giorgio A. Transabdominal sonography of the normal gastroesophageal junction in children. J Clin Ultrasound. 2001;29:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Tanomkiat W, Chongchitnan P. Transabdominal sonography of gastroesophageal junctions. J Clin Ultrasound. 1999;27:505-512. [PubMed] [DOI] [Full Text] |
| 12. | Manabe N, Haruma K, Hata J, Kusunoki H, Yoshida S, Futagami K, Tanaka S, Chayama K. Evaluation of esophageal motility by endosonography using a miniature ultrasonographic probe in patients with reflux esophagitis. Scand J Gastroenterol. 2002;37:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Mittal RK. Motor and sensory function of the esophagus: revelations through ultrasound imaging. J Clin Gastroenterol. 2005;39:S42-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Holloway RH. Esophageal ultrasonography: A new view on esophageal motility. Am J Gastroenterol. 2007;102:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Zhu SY, Liu RC, Chen LH, Yang H, Feng X, Liao XH. Sonographic anatomy of the cervical esophagus. J Clin Ultrasound. 2004;32:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Mateen MA, Kaffes AJ, Sriram PV, Rao GV, Reddy DN. Modified technique of high-resolution ultrasonography of the normal cervical esophagus. J Gastroenterol Hepatol. 2006;21:1660-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Doldi SB, Lattuada E, Zappa MA, Cioffi U, Pieri G, Massari M, Peracchia A. Ultrasonographic imaging of neoplasms of the cervical esophagus. Hepatogastroenterology. 1997;44:724-726. [PubMed] |
| 18. | Chen MH, Zhu Q, Kiyoshi C, Yan K, Wang B, Cao JF, Yue JL, Dong BW. Transcutaneous ultrasound of the cervical esophagus in patients with esophageal carcinoma. Chin Med J (Engl). 1994;107:332-337. [PubMed] |
| 19. | Mittal RK. Measuring esophageal distention by high-frequency intraluminal ultrasound probe. Am J Med. 2003;115 Suppl 3A:130S-136S. [PubMed] |
| 20. | Malinger G, Levine A, Rotmensch S. The fetal esophagus: anatomical and physiological ultrasonographic characterization using a high-resolution linear transducer. Ultrasound Obstet Gynecol. 2004;24:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 779] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Zhu SY, Liu RC, Chen LH, Luo F, Yang H, Feng X, Liao XH. Sonographic demonstration of the normal thoracic esophagus. J Clin Ultrasound. 2005;33:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci. 1992;37:849-856. [PubMed] |
| 25. | Dent J. Definitions of reflux disease and its separation from dyspepsia. Gut. 2002;50 Suppl 4:iv17-iv20; discussion iv21-iv22. [PubMed] |
| 26. | Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 27. | Gomes H, Lallemand A, Lallemand P. Ultrasound of the gastroesophageal junction. Pediatr Radiol. 1993;23:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Caletti GC, Ferrari A, Mattioli S, Zannoli R, Di Simone MP, Bocus P, Gozzetti G, Barbara L. Endoscopy versus endoscopic ultrasonography in staging reflux esophagitis. Endoscopy. 1994;26:794-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Miller LS, Dai Q, Sweitzer BA, Thangada V, Kim JK, Thomas B, Parkman H, Soliman AM. Evaluation of the upper esophageal sphincter (UES) using simultaneous high-resolution endoluminal sonography (HRES) and manometry. Dig Dis Sci. 2004;49:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 604] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 31. | Fox M, Forgacs I. Gastro-oesophageal reflux disease. BMJ. 2006;332:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Mine S, Fujisaki T, Tabata T, Matsuoka H, Iida T, Yamada S, Tanaka Y, Morimoto I, Eto S, Aibe T. Ultrasonographic evaluation of lansoprazole-induced improvement of submucosal injury in patients with gastroesophageal reflux. Am J Gastroenterol. 2000;95:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Modlin IM, Malfertheiner P, Hunt RH, Armstrong D, Holtmann G, Quigley EM, Spechler SJ. GERD evaluation: time for a new paradigm? J Clin Gastroenterol. 2007;41:237-241. [PubMed] |
| 34. | Frazzoni M, Manno M, De Micheli E, Savarino V. Pathophysiological characteristics of the various forms of gastro-oesophageal reflux disease. Spectrum disease or distinct phenotypic presentations? Dig Liver Dis. 2006;38:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Wise JL, Murray JA. Utilising multichannel intraluminal impedance for diagnosing GERD: a review. Dis Esophagus. 2007;20:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Naik DR, Moore DJ. Ultrasound diagnosis of gastro-oesophageal reflux. Arch Dis Child. 1984;59:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Dogan I, Mittal RK. Esophageal motor disorders: recent advances. Curr Opin Gastroenterol. 2006;22:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Cool M, Poelmans J, Feenstra L, Tack J. Characteristics and clinical relevance of proximal esophageal pH monitoring. Am J Gastroenterol. 2004;99:2317-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Sifrim D. Relevance of volume and proximal extent of reflux in gastro-oesophageal reflux disease. Gut. 2005;54:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Emerenziani S, Zhang X, Blondeau K, Silny J, Tack J, Janssens J, Sifrim D. Gastric fullness, physical activity, and proximal extent of gastroesophageal reflux. Am J Gastroenterol. 2005;100:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Mittal RK, Kassab G, Puckett JL, Liu J. Hypertrophy of the muscularis propria of the lower esophageal sphincter and the body of the esophagus in patients with primary motility disorders of the esophagus. Am J Gastroenterol. 2003;98:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Dickman R, Bautista JM, Wong WM, Bhatt R, Beeler JN, Malagon I, Risner-Adler S, Lam KF, Fass R. Comparison of esophageal acid exposure distribution along the esophagus among the different gastroesophageal reflux disease (GERD) groups. Am J Gastroenterol. 2006;101:2463-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Dogan I, Puckett JL, Padda BS, Mittal RK. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol. 2007;102:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Mine S, Iida T, Tabata T, Kishikawa H, Tanaka Y. Management of symptoms in step-down therapy of gastroesophageal reflux disease. J Gastroenterol Hepatol. 2005;20:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Hirsch W, Preiss U, Kedar R. Color coded Doppler ultrasound in diagnosis of gastroesophageal reflux. Klin Padiatr. 1997;209:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: Esophageal impedance monitoring. Am J Gastroenterol. 2007;102:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |