Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5139
Revised: July 26, 2007
Accepted: August 6, 2007
Published online: October 14, 2007
AIM: To investigate the effects of Cromolyn Sodium (CS) pretreated prior to reperfusion on the activity of intestinal mucosal mast cells (IMMC) and mucous membrane of the small intestine in ischemia-reperfusion (IR) injury of rats.
METHODS: Thirty-two Sprague-Dawley (SD) rats were randomly divided into four groups: sham group (group S), model group (group M), high and low dosage of CS groups, (treated with CS 50 mg/kg or 25 mg/kg, group C1 and C2). Intestinal IR damage was induced by clamping the superior mesenteric artery for 45 min followed by reperfusion for 60 min. CS was intravenouly administrated 15 min before reperfusion. Ultrastructure and counts of IMMC, intestinal structure, the expression of tryptase, levels of malondisldehyde (MDA), TNF-α, histamine and superoxide dismutase (SOD) activity of the small intestine were detected at the end of experiment.
RESULTS: The degranulation of IMMC was seen in group M and was attenuated by CS treatment. Chiu’s score of group M was higher than the other groups. CS could attenuate the up-regulation of the Chiu’s score, the levels of MDA, TNF-α, and expression of tryptase and the down-regulation of SOD activity and histamine concentration. The Chiu’s score and MDA content were negatively correlated, while SOD activity was positively correlated to the histamine concentration respectively in the IR groups.
CONCLUSION: Pretreated of CS prior to reperfusion protects the small intestine mucous from ischemia-reperfusion damage, the mechanism is inhibited IMMC from degranulation.
- Citation: Hei ZQ, Gan XL, Luo GJ, Li SR, Cai J. Pretreatment of cromolyn sodium prior to reperfusion attenuates early reperfusion injury after the small intestine ischemia in rats. World J Gastroenterol 2007; 13(38): 5139-5146
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5139
Mast cell degranulation is an important component of inflammatory tissue responses[1]. Bortolotto proved that ischemia-reperfusion injury was depending upon the presence of mast cells in skeletal muscle[2].
Intestinal mucosal mast cells (IMMCs) are particularly frequent in close proximity to epithelial surfaces where they are strategically located for optimal interaction with the environment and for their putative functions for host defense[3].
Some studies on the role of IMMC in small intestine ischemia-reperfusion injury have been reported[4-7]. Lindestrom and his colleagues[4] found the eosinophils and mast cells in rat ileum gradually increased after intestine ischemia (60 min)/reperfusion (60 min), and reported that the epithelium permeability increased significantly after ischemia-reperfusion. The researches of Kanwar and Schramm[5,6] were focused on mast cells stimulating neutrophil adherence, resupination, recruitment, and the intestinal mucosal injury. Boros[7] reported that mast cell degranulation prior to ischemia may induce a potentially protective mechanism in the small bowel mucosa and decrease ischemia-reperfusion injury in the dog.
Cromolyn Sodium (CS) is a MC membrane stabilizer. MC-stabilization protocols were proved to reduce the leukocyte recruitment[8]. Kimura[9] reported that the intestine ischemia and reperfusion could induce a decrease in the mucosal histamine content, an increase in plasma histamine levels, and an significantly enhance in mucosal permeability. However, MAR-99, another mast cell stabilizer, can prevented these changes by pretreatment prior to ischemia. Kalia[10] found that all ketotifen-pretreated animals (1 mg/kg orally twice daily for 3 d before ischemia) survived after ischemia-reperfusion and ketotifen could abrogate the leukocyte adherence induced by ischemia-reperfusion within the villus mucosal capillaries and supplying arterioles and largely prevented pulmonary injury.
The above studies proved that IMMC are associated with the small intestine injury after ischemia-rreperfusion, and MC membrane stabilizer pretreatment prior to ischemia can protects against the injury, such as CS and MAR-99. While the studies about the intestinal mucosal injury with CS pretreatment after the small intestine ischemia before reperfusion were few. Oxidative stress is one of the mechanism about the small intestine ischemia-reperfusion injury has been generally acknowledged. We hypothesized that CS have an influence on the oxidative stress during the small intestine ischemia-reperfusion, and the purpose of our present study was to investigate whether CS pretreatment prior to reperfusion could protect against early intestinal mucosal damage induced by ischemia-reperfusion through inhibition of IMMC degranulation or oxidative stress. To test our hypothesis, we showed in a rat IR gut injury model: (1) the ultrastructure and counts of IMMC in the early reperfusion; (2) mucosal damage with CS pretreatment; (3) expression of tryptase in the IMMC; and (4) the levels of malondisldehyde (MDA), TNF-α, histamine and superoxide dismutase (SOD) activity of the small intestine in rats.
Thirty-two healthy Sprague-Dawley rats (200-250 g, provided by Animal Center of Sun Yat-Sen University and approved by the University Animal Study Committee) were randomly divided into four groups each of which contained 8 rats. Laboratory temperature was kept at 25°C-27°C. Surgery was conducted under general anesthesia with intra-peritoneal sodium pentobarbital (45 mg/kg) after they were fasted for 18 h. Tracheotomy was performed for ventilation. The right femoral vein was cannulated for fluid infusion and drugs.The rat abdomen was opened and its superior mesenteric artery (SMA) was found and clamped for 45 min. Then the clamp was released and reperfusion of the splanchnic region was maintained for 60 min (in M group). In control group, SMA was found but not clamped and i.v. saline solution via the right femoral vein at 30th min after the start of experiment (sham group). In another two groups, the same operation was done and CS (50 mg/kg or 25 mg/kg, The dosage and method of CS pretreatment according to Cordeiro et al[11] for Cromolyn sodium is poorly absorbed by oral.) was given via right femoral vein 15 min before the opening of the clamp (C1 and C2 groups).
After ischemia-reperfusion, the rats were killed and paunched rapidly. A segment of 0.5-1.0 cm intestine was cut from 5 cm to terminal ileum and fixed in 4% formaldehyde polymerisatum, then embedded in paraffin for section. Another segment of small intestine was washed with frozen saline and dried with suction paper and at -70°C.
The segment of small intestine was stained with hematoxylin-eosin. The damages of intestinal mucosa were evaluated by two different pathologist according to the criteria of Chiu’s method[12]. Criteria of Chiu grading system consists from 5 subdivisions according to the changes of villus and gland of intestinal mucosa: grade 0, normal mucosa; grade 1, development of subepithelial Gruenhagen’s space at the tip of villus; grade 2, extension of the space with moderate epithelial lifting; grade 3, massive epithelial lifting with a few denuded villi; grade 4, denuded villi with exposed capillaries; and grade 5, disintegration of the lamina propria, ulceration and hemorrhage.
Intestines were immersed and fixed in 2.5% glutaraldehyde overnight at 4°C and washed three times in PBS. Then they were postfixed in aqueous 1% OsO4 and 1% K3Fe (CN)6 for 1 h. After three times of PBS washes, the tissue was dehydrated through a graded series of 30% to 100% ethanol and 100% propylene oxide and then infiltrated in 1:1 mixture of propylene oxide and Polybed 812 epoxy resin for 1 h. The infiltration solution was changed to 100% resin. After 24 h of infiltration, the tissue was embedded in molds and cured at 37°C overnight, followed by additional hardening at 65°C for 2 d. Ultrathin (70 nm) sections were collected on 200-mesh copper grids and stained with 2% uranyl acetate in 50% methanol for 10 min, followed by 1% lead citrate for 7 min. Sections were photographed using a Hitachi H-600 transmission electron microscope (TOSHIBA, Japan) at 80 kV onto electron microscope film.
Intestinal tissues were homogenized with normal saline. Intestinal protein quantitation was by the Bradford method[13] with a BSA standard using kits were provided by Shenerg Biocolor BioScience & Technolgy Company, Shanghai, China.
Intestinal tissues were homogenized with normal saline. MDA content was determined by the TBA method (Jiancheng Bioengineering Ltd, Nanjing, China). Homogenate (0.1 mL) was taken to detect MDA content. Briefly, 0.1 mL 8.1% SDS, 0.8 mL acetic acid buffer, 0.8 mL 0.8% TBA and 0.2 mL distilled water were added into the sample tubes and one standard tube (containing 0.1 mL tetraethoxypropane). All the tubes were then incubated at 100°C for 1 h. After cooled at -20°C for 5 min, 2 mL of n-butyl alcohol was added into the sample, which was then vibrated for 1 minute and centrifuged for 10 min at 3000 r/min.The supernatant of the samples were assayed to detect absorbance at 532 nm; and the results were expressed as nmol/mL. The content of MDA in intestine was calculated as millimicromole per milligram of protein.
Intestinal tissues were made into a homogenate with normal saline, frozen at -20°C for 5 min and centrifuged for 15 min at 4000 r/min. Supernatants were transferred into fresh tubes for evaluation of SOD activity. SOD activity was evaluated with an SOD detection kit according to the manufacturer’s instructions (Jiancheng Bioengineering Ltd, Nanjing, China). Results were expressed as nmol/mL. The activity of SOD in the intestine was calculated as U per milligram of protein.
Detection of the concentration of TNF-α in the intestine
Intestinal tissues were made into a homogenate with normal saline, frozen at -20°C for 5 min and centrifuged for 15 min at 4000 r/min. Supernatants were transferred into fresh tubes for evaluation of concentration of TNF-α (Biosource, USA) using a commercially available ELISA kit in accordance with the manufacturer’s instructions, results were expressed as pg/mL. The concentration of TNF-α in the intestine was calculated as picogram per milligram of protein.
Intestinal tissues were made into a homogenate with normal saline, frozen at -20°C for 5 min and centrifuged for 15 min at 4000 r/min. Supernatants were transferred into fresh tubes for evaluation of concentration of histamine (RapidBio Lab, USA) using a commercially available ELISA kit in accordance with the manufacturer’s instructions, results were expressed as ng/mL. The concentration of histamine in the intestine was calculated as nanogram per milligram of protein.
Five μm thick sections were prepared from paraffin-embedded tissue. After deparaffinization, endogenous peroxidase was quenched with 3% H2O2 in deionised water for 10 min. Nonspecific binding sites were blocked by incubating the sections in 10% normal rabbit serum for 1 h. The sections were then incubated with polyclonal rat anti-mast cell tryptase (dilution 1: 50) for 30 min at 37°C, followed by incubation with biotinylated goat-anti-rat IgG at room temperature for 10-15 min. After 3 × 5 min PBS rinses, the horseradish-peroxidase-conjugated streptavidin solution was added and incubated at room temperature for 10-15 min. The antibody binding sites were visualized by incubation with a diaminobenzidine-H2O2 solution. The sections incubated with PBS instead of the primary antibody were used as negative controls. Brown-yellow granules in the cytoplasm were recognized as positive staining for tryptase. We calculated the tryptase positive mast cells and their intensity in 5 representative areas at × 400 magnification by Image-Pro Plus 5.0 (USA).
Data were expressed as mean ± SD and analysis of variance was performed using SPSS 11.0 software. One-way analysis of variance was used for multiple comparison, least significant difference test (LSD-t) was used for intra-group comparison or Tamhane’s T2 test was used if equal variances was not assumed. Pearson analysis was used for the correlation in the ischemia and reperfusion groups. Differences were considered significant when P was < 0.05.
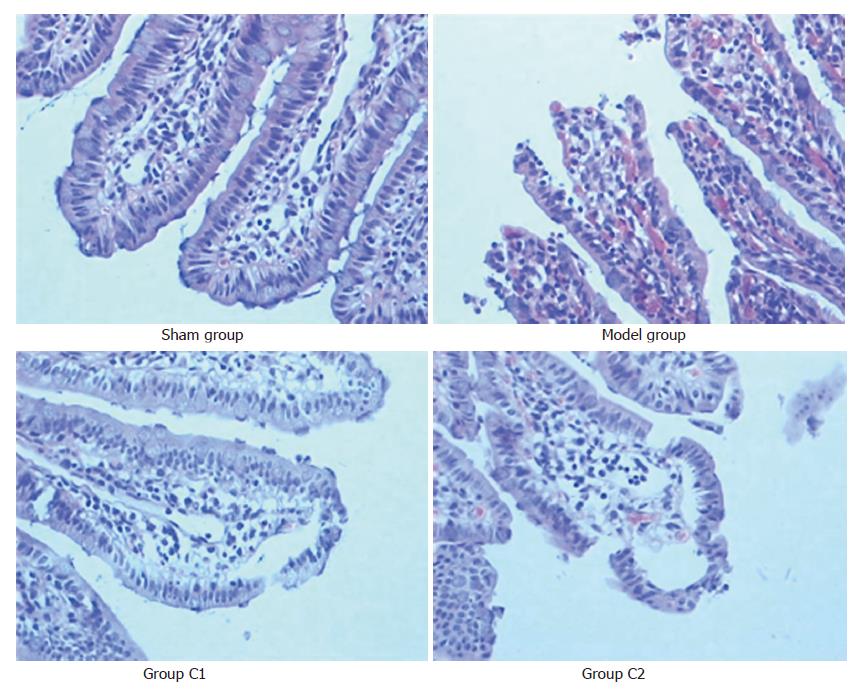
The villus and glands were normal and no inflammatory cell infiltration was observed in mucosal epithelial layer in sham group. Multiple erosions and bleeding were observed in model group. Light edema of mucosa villus and infiltration of few necrotic epithelial inflammatory cells neutrophil leukomonocyte were found in mucosa epithelial layer in C2 and C1 groups (Figure 1).
The Chiu’s score in sham group was the lowest, while in the model group it was the highest in the four groups (P < 0.05). The Chiu’s score in C1 group was significantly lower than in C2 group after treated with CS (P < 0.05) (Table 1).
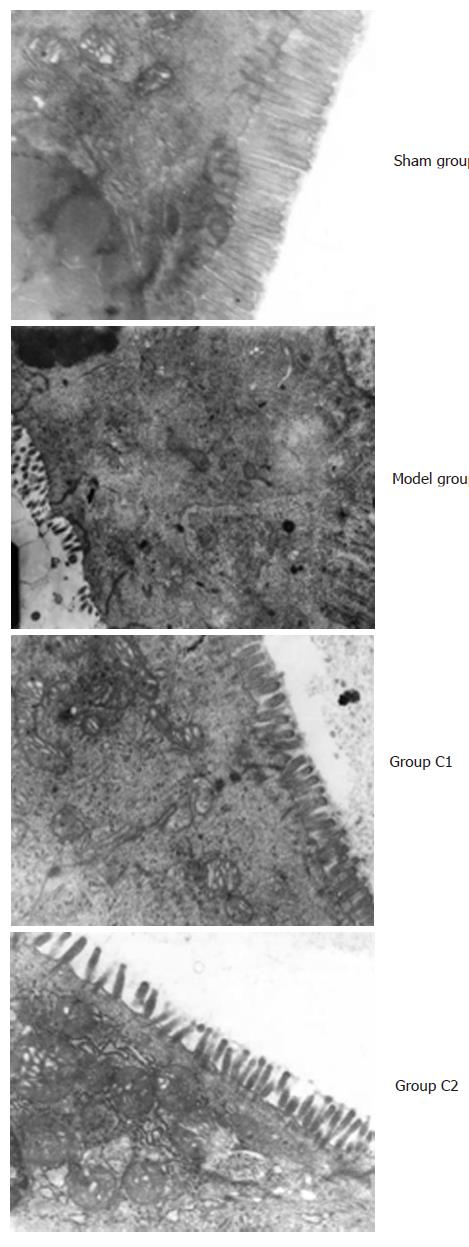
The ultrastructure of small intestinal was normal in group S. There was seen the karyopyknosis of epithelial cell of small intestine in group M, the nuclear membrane was more irregularity, and the swelling microvillus became shorter and thicker, most of the microvillus were shedding. The nucleus of epithelial cell of small intestine in group C1 and C2 was deflated, the nuclear membrane was irregularity, and the light swelling microvillus became shorter (Figure 2).
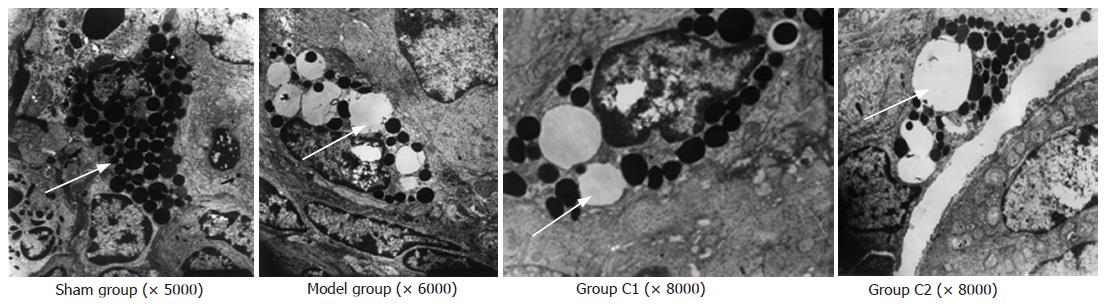
The ultrastructure of IMMC was normal in sham group. There were abundant vacuolus with a reduction granulation in their endochylema in model group. There were few swollen granules with a reduction in IMMC homogeneity in C1 and C2 group (Figure 3).
The content of MDA in intestine of model group was the highest in all experimental groups, it decreased significantly compared with the model group after treated with CS (P < 0.05) and there was no significant difference compared with the sham group (P > 0.05) ( Table 2).
The activity of intestinal SOD decreased significantly in ischemia-reperfusion injury groups compared with the sham group (P < 0.05), treated with CS it increased significantly compared with the model group (P < 0.05), and there was no significant difference between group C1 and C2 (P > 0.05) ( Table 2).
The concentration of TNF-α of intestine in model group rats was higher than the other three groups (P < 0.05). There were no significant difference in sham group, C1 group and C2 group (P > 0.05) (Table 2). There was a positive correlation between the Chiu’s score and the concentration of TNF-α in the ischemia and reperfusion groups (r = 0.734, P < 0.05).
The histamine concentration of intestine in the model and C2 groups decreased significantly compared with the sham group (P < 0.05), it increased significantly after pretreated with CS compared with the model group (P < 0.05). There were no significant difference between C1 and C2 groups (P > 0.05) (Table 2). The Chiu’s score and MDA content were negatively correlated to the histamine concentration respectively (r = -0.676, P < 0.05 or r = -0.452, P < 0.05), while the SOD activity was positively correlated to the concentration of histamine in the ischemia and reperfusion groups (r = 0.579, P < 0.05).
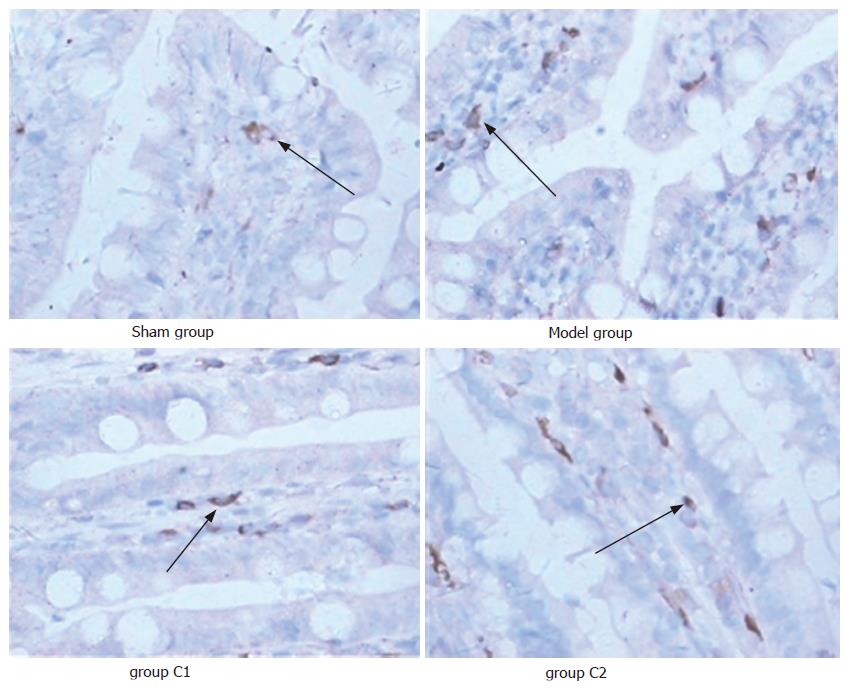
Expression of tryptase in sham group was the lowest, while in the model group it was the highest in the four groups (P < 0.05), and the expression of tryptase in C1 group was significantly lower than in C2 group (P < 0.05). The number of IMMC increased significantly in ischemia-reperfusion injury groups compared with the sham group (P < 0.05), no difference was compared among the three groups (P > 0.05) (Table 1, Figure 4).
IMMC are located in close proximity to submucosal collecting venules, which are primary targets of leukocyte-endothelial interactions during ischemia-reperfusion injure. IMMC are particularly frequent in close proximity to epithelial surfaces where they are strategically located for optimal interaction with the environment and for their putative functions for host defense. They sense the foreign material invading the mucosa in an appropriate inflammatory response, and were considered as one of components of the fourth level of mucosal defense[14]. Acute inflammation could lead to increase of IMMC counts and release of a multi-faceted spectrum of proinflammatory mediators by IMMC such as cytokines and chemokines, and MC have the capacity to coordinate trafficking of leukocytes[15]. Boros[16] proved that intestinal ischemia induced the release of a variety of IMMC-derived inflammatory compounds and resulted in a spectrum of injury ranging from reversible permeability changes to structural mucosal damage.
The ischemia time of small intestine rats’ model is from 30 min to 60 min[17-19], here we used the median time (45 min). Cizova reported that the concentration of thiobarbituric acid reactive substances was increased at the end of the ischemia lasting from 30 to 90 min[20], and CS plasma life in vivo is very short. Thus the reperfusion time in our study was watched in 60 min, it was the early reperfusion according to Hamar et al[21]. All of previous studies were focused on the MC membrane stabilizer pretreatment prior to ischemia, and had proved that IMMC were associated with the damage to intestinal mucosal after the small intestine ischemia-reperfusion. While the main purpose of our study was to see whether pretreatment with CS prior to reperfusion also have the protective effects during early reperfusion after the small intestine ischemia.
Tryptase is one of the specificity markers of IMMC[22]. We counted the IMMC counts through the expression of tryptase using immunohistochemical methods which is more accuracy than oluidine blue staining. Our study found that the expression of tryptase and IMMC counts increased significantly in 60 min reperfusion injury in model group. There were abundant vacuolus in IMMCs in the model group after they were degranulated by electron microscope. IMMC is the main source of histamine in intestine. The levels of intestinal histamine includes the concentration of histamine intra- and extro-IMMC. The level of intestinal histamine is mainly represent of the concentration of histamine intra-IMMC as the extracellular histamine released from IMMC in the gastrointestinal tract is rapidly cleared and degraded[23] and the more IMMC degranulate, the lower concentration of histamine is found in intestine[24]. Our study found the histamine level decreased significantly in model group while IMMC counts increased, suggesting that IMMC may degranulate and release histamine in 60 min reperfusion.
Histamine has many pathophysiological roles, and it is an important messenger in the gut[25]. Akerstrom et al[26] reported that anti-histaminergic pretreatment could decrease the trauma-induced leakage of albumin by mechanisms which may involve readjustments of pressures and flows in capillaries as well as a prevention of histamine effects on capillary permeability on a model of mechanical intra-abdominal trauma in rats. Our results found that the intestinal histamine concentration decreased after early ischemic-reperfusion, and there was a negative corrletion between the small intestinal Chiu’s score and the level of histamine in the intestine. This result suggested that histamine took part in the ischemia-reperfusion intestinal mucosal damage.
TNF-α is an inflammatory cytokine that may be an important mediator in the development of reperfusion-induced tissue injury and lethality[27]. Grewal[28] demonstrated that treatment of rats with anti-TNF antiboies could prevent neutrophil influx, tissue injury. Our study found that the intestinal TNF-α concentration increased after ischemic-reperfusion; and there was a positive correlation between the small intestinal Chiu’s score and the level of TNF-α in the intestine. This result suggested that TNF-α also took part in the ischemia-reperfusion intestinal mucosal damage. Althrough the most intestinal TNF-α is considered by some one from the mast cells[29], it has been proved that many sorts of cells also release the TNF-α besides the mast cells. We believe the increase of the TNF-α is contributed by many factors.
CS is a stabilizing agent of mast cell which prevents histamine and TNF-α released from IMMC[30]. Szabo et al[31] reported that 30 min segmental ischemia and 120 min reperfusion induced significant tissue injury, elevated the segmental vascular resistance, and decreased intramucosal pH (pHi), and CS pretreatments prior to ischemia significantly inhibited the permeability changes, but did not influence the pHi and morphological alterations induced by ischemia-reperfusion, they conclude that intestinal mast cells and mast cell-induced reactions contribute to the mucosal permeability alterations during reperfusion, but play only a minor role in ischemia-reperfusion-induced structural injury. Pretreatment with CS protecting against degranulation, caused a significant impairment of plasma exudation at 30 min of inflammation corresponding to a significantly decreased level of histamine, one of the most potent vasoactive factors released from activated mast cells[32].
The results in our study showed that the injury of small intestinal villus and microvillus was alleviated after CS pretreatment prior to reperfusion and that the ultrastructure of IMMC was basically normal. The expression of tryptase and TNF-α concentration were also alleviated by CS pretreatment prior to reperfusion, and the concentration of histamine in intestine was increased compared with the model group after CS pretreatment prior to reperfusion. The results indicated that CS decreased ischemia-reperfusion injury by prevention of IMMC degranulation, thus it decreased the release of histamine and TNF-α. This protection may be dose-dependent as high dose of CS with more powerful effect.
There were many reports about intestinal ischemia and reperfusion resulted in the increase of MDA and decrease of SOD activity, toxic-free oxygen radicals are produced in the ischemic tissue[33,34]. Our study also demonstrated that ischemia-reperfusion injury elevated the oxygen radicals and lipid radicals. Frossi[35] reproted that oxidative stress could induce a pro-type 2 inflammatory response and degranulation of mast cells. Fukuishi[36] found the compound 48/80, a typical histamine liberator elicited superoxide anion generation in mast cells in a dose-dependent fashion. These studies indicated that degranulation of mast cells was able to induce oxidative stress injury and oxygen radicals could make mast cells to degranulate. In this study we found there were correlations among the MDA, SOD activity and the concentration of histamine, the other findings of our study were that MDA content increased and SOD activity decreased remarkably in the model group, while pretreatment by cromolyn sodium prior to reperfusion could attenuate the up-regulation of MDA content and the down-regulation of SOD activity. The results were indicating that IMMC degranulation and oxidative stress can affect each other, and the less IMMC degranulation can make less oxidative stress. Future studies are need to focus on the relationships in vitro.
In conclusion, pretreatment of Cromolyn Sodium prior to reperfusion could attenuate early reperfusion injury after the small intestine ischemia in rats. The mechanisms includes: inhibitied IMMC from degranulation, decreased the release of histamine and TNF-α from IMMC, and decreased oxidative stress.
Intestinal mucosal mast cells (IMMCs) is associated with the mucosal damage. The aim of this study was to investigate the effects of Cromolyn Sodium (CS) pretreated prior to reperfusion on the activity of IMMC and mucous membrane of the small intestine in ischemia-reperfusion (IR) injury of rats.
Previous studies proved that IMMC are associated with the small intestine injury after ischemia-reperfusion, and MC membrane stabilizer pretreatment prior to ischemia can protects against the injury, such as CS and MAR-99.
While the studies about the intestinal mucosal injury with CS pretreatment after the small intestine ischemia before reperfusion were few. Oxidative stress is one of the mechanism about the small intestine ischemia-reperfusion injury has been generally acknowledged. We hypothesized that CS have an influence on the oxidative stress during the small intestine ischemia-reperfusion, and the purpose of our present study was to to investigate whether CS pretreatment prior to reperfusion could protect against early intestinal mucosal damage induced by ischemia-reperfusion through inhibition of IMMC degranulation or oxidative stress.
Pretreated of CS prior to reperfusion protects the small intestine mucous from ischemia-reperfusion damage, the mechanism is inhibited IMMC from degranulation.
Restoration of blood supply to tissue which is ischemic due to decrease in normal blood supply. The decrease may result from any source including atherosclerotic obstruction, narrowing of the artery, or surgical clamping. It is primarily a procedure for treating infarction or other ischemia, by enabling viable ischemic tissue to recover, thus limiting further necrosis. However, it is thought that reperfusion can itself further damage the ischemic tissue, causing REPERFUSION INJURY.
This paper reports an experimental study very well designed and performed and very elegant results and discussion. The final conclusions are nicely shown. Their english is of good quality. The purpose of this paper was to determine whether cromolyn sodium reduces or prevents injury of the small intestine of rats following ischemia-reperfusion. To this end the authors perform a number of biochemical measurements (MDA, TNFalpha, histamine, SOD) as well as ultrastructural studies of mast cells and microscopical investigations of the small intestine.
S- Editor Liu Y L- Editor Li M E- Editor Li JL
| 1. | Boros M, Takaichi S, Masuda J, Newlands GF, Hatanaka K. Response of mucosal mast cells to intestinal ischemia-reperfusion injury in the rat. Shock. 1995;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Bortolotto SK, Morrison WA, Messina A. The role of mast cells and fibre type in ischaemia reperfusion injury of murine skeletal muscles. J Inflamm (Lond). 2004;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kanwar S, Kubes P. Mast cells contribute to ischemia-reperfusion-induced granulocyte infiltration and intestinal dysfunction. Am J Physiol. 1994;267:G316-G321. [PubMed] |
| 4. | Lindeström LM, Ekblad E. Structural and neuronal changes in rat ileum after ischemia with reperfusion. Dig Dis Sci. 2004;49:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222-G229. [PubMed] |
| 6. | Schramm R, Thorlacius H. Neutrophil recruitment in mast cell-dependent inflammation: inhibitory mechanisms of glucocorticoids. Inflamm Res. 2004;53:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Boros M, Kaszaki J, Ordögh B, Nagy S. Mast cell degranulation prior to ischemia decreases ischemia-reperfusion injury in the canine small intestine. Inflamm Res. 1999;48:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Holian A, Hamilton R, Scheule RK. Mechanistic aspects of cromolyn sodium action on the alveolar macrophage: inhibition of stimulation by soluble agonists. Agents Actions. 1991;33:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kimura T, Fujiyama Y, Sasaki M, Andoh A, Fukuda M, Nakajima S, Bamba T. The role of mucosal mast cell degranulation and free-radical generation in intestinal ischaemia-reperfusion injury in rats. Eur J Gastroenterol Hepatol. 1998;10:659-666. [PubMed] |
| 10. | Kalia N, Brown NJ, Wood RF, Pockley AG. Ketotifen abrogates local and systemic consequences of rat intestinal ischemia-reperfusion injury. J Gastroenterol Hepatol. 2005;20:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cordeiro PG, Lee JJ, Mastorakos D, Hu QY, Pinto JT, Santamaria E. Prevention of ischemia-reperfusion injury in a rat skin flap model: the role of mast cells, cromolyn sodium, and histamine receptor blockade. Plast Reconstr Surg. 2000;105:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1416] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 13. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157165] [Article Influence: 3207.4] [Reference Citation Analysis (0)] |
| 14. | Penissi AB, Rudolph MI, Piezzi RS. Role of mast cells in gastrointestinal mucosal defense. Biocell. 2003;27:163-172. [PubMed] |
| 15. | Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 283] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Boros M. Microcirculatory dysfunction during intestinal ischemia-reperfusion. Acta Physiol Hung. 2003;90:263-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ito K, Ozasa H, Horikawa S. Edaravone protects against lung injury induced by intestinal ischemia/reperfusion in rat. Free Radic Biol Med. 2005;38:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Andoh A, Fujiyama Y, Araki Y, Kimura T, Tsujikawa T, Bamba T. Role of complement activation and mast cell degranulation in the pathogenesis of rapid intestinal ischemia/reperfusion injury in rats. Digestion. 2001;63 Suppl 1:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Tomatsuri N, Yoshida N, Takagi T, Katada K, Isozaki Y, Imamoto E, Uchiyama K, Kokura S, Ichikawa H, Naito Y. Edaravone, a newly developed radical scavenger, protects against ischemia-reperfusion injury of the small intestine in rats. Int J Mol Med. 2004;13:105-109. [PubMed] |
| 20. | Cízová H, Lojek A, Kubala L, Cíz M. The effect of intestinal ischemia duration on changes in plasma antioxidant defense status in rats. Physiol Res. 2004;53:523-531. [PubMed] |
| 21. | Hamar J, Rácz I, Cíz M, Lojek A, Pállinger E, Furész J. Time course of leukocyte response and free radical release in an early reperfusion injury of the superior mesenteric artery. Physiol Res. 2003;52:417-423. [PubMed] |
| 22. | Marone G, Triggiani M, Genovese A, De Paulis A. Role of human mast cells and basophils in bronchial asthma. Adv Immunol. 2005;88:97-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Boros M, Ordögh B, Kaszaki J, Nagy S. The role of mast cell degranulation in ischaemia-reperfusion-induced mucosal injury in the small intestine. Ann Acad Med Singapore. 1999;28:79-84. [PubMed] |
| 25. | Rangachari PK. Histamine: mercurial messenger in the gut. Am J Physiol. 1992;262:G1-G13. [PubMed] |
| 26. | Akerström G, Lisander B. Antihistaminergic pretreatment prevents tissue extravasation of albumin from intra-abdominal trauma in rats. Acta Anaesthesiol Scand. 1994;38:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, Dias AA. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214-28; discussion 214-28;. [PubMed] |
| 29. | Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1464] [Cited by in RCA: 1509] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 30. | Shin HY, Kim JS, An NH, Park RK, Kim HM. Effect of disodium cromoglycate on mast cell-mediated immediate-type allergic reactions. Life Sci. 2004;74:2877-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Szabó A, Boros M, Kaszaki J, Nagy S. The role of mast cells in mucosal permeability changes during ischemia-reperfusion injury of the small intestine. Shock. 1997;8:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Dib M, Zhao X, Wang X, Andersson R. Mast cells contribute to early pancreatitis-induced systemic endothelial barrier dysfunction. Pancreatology. 2002;2:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Yoshimaru T, Suzuki Y, Inoue T, Niide O, Ra C. Silver activates mast cells through reactive oxygen species production and a thiol-sensitive store-independent Ca2+ influx. Free Radic Biol Med. 2006;40:1949-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Olguner C, Koca U, Kar A, Karci A, Işlekel H, Canyilmaz M, Mavioĝlu O, Kizildaĝ S, Unlü G, Elar Z. Ischemic preconditioning attenuates the lipid peroxidation and remote lung injury in the rat model of unilateral lower limb ischemia reperfusion. Acta Anaesthesiol Scand. 2006;50:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003;33:2168-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Fukuishi N, Sakaguchi M, Matsuura S, Nakagawa C, Akagi R, Akagi M. The mechanisms of compound 48/80-induced superoxide generation mediated by A-kinase in rat peritoneal mast cells. Biochem Mol Med. 1997;61:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |