Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5127
Revised: August 3, 2007
Accepted: August 26, 2007
Published online: October 14, 2007
AIM: To evaluate attenuating properties of N-acetylcysteine (NAC) on oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis (NASH).
METHODS: Male Sprague-Dawley rats were randomly divided into three groups. Group 1 (control, n = 8) was free accessed to regular dry rat chow (RC) for 6 wk. Group 2 (NASH, n = 8) was fed with 100% fat diet for 6 wk. Group 3 (NASH + NAC20, n = 9) was fed with 100% fat diet plus 20 mg/kg per day of NAC orally for 6 wk. All rats were sacrificed to collect blood and liver samples at the end of the study.
RESULTS: The levels of total glutathione (GSH) and hepatic malondialdehyde (MDA) were increased significantly in the NASH group as compared with the control group (GSH; 2066.7 ± 93.2 vs 1337.5 ± 31.5 μmol/L and MDA; 209.9± 43.9 vs 3.8 ±1.7 μmol/g protein, respectively, P < 0.05). Liver histopathology from group 2 showed moderate to severe macrovesicular steatosis, hepatocyte ballooning, and necroinflammation. NAC treatment improved the level of GSH (1394.8 ± 81.2 μmol/L, P < 0.05), it did not affect MDA (150.1 ± 27.0 μmol/g protein), but led to a decrease in fat deposition and necroinflammation.
CONCLUSION: NAC treatment could attenuate oxidative stress and improve liver histology in rats with NASH.
- Citation: Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol 2007; 13(38): 5127-5132
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5127
Non-alcoholic steatohepatitis (NASH) is a liver disease characterized by macrovesicular steatosis, hepatocyte necrosis, inflammation, Mallory bodies, and fibrosis[1]. NASH is closely associated with the metabolic or insulin resistance syndrome[2]. This is a cluster of disorders, such as obesity, diabetes mellitus, dyslipidemia, arteriosclerosis, and hypertension, with insulin resistance as a common feature[3]. In initial phases, during which fat accumulates in the liver, no clinical symptoms are evident. In advanced stages, fibrosis is detectable, which might progress into cirrhosis in some patients[4].
There are many models of NASH-like liver injuries in animals as the genetic model of ob/ob mice[5], the methionine and choline deficient diet model[6,7], and a model with high-fat liquid diet in which 71% of energy is derived from fat, 11% from carbohydrates, and 18% from protein[8].
Oxidative stress is believed to play an important role in pathogenesis of NASH. It is likely involved in the progression of disease from steatosis to NASH and potentially cirrhosis. It has been shown that chronic oxidative stress, generated through the oxidation of cytotoxic free fatty acids, can lead to upregulation of cytokines[9], induction of the liver cytochrome P450 enzyme 2E1 (CYP2E1), and depletion of hepatic antioxidant concentration[10]. In addition, enhanced lipid peroxidation leads to the generation of byproducts, such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA), which have been shown to further stimulate cytokine production. They are involved in hepatic stellate cell activation[11], fibrogenesis, and enhanced extracellular matrix protein deposition.
According to the concepts of pathogenesis of NASH, these might make a wise basis for the use of antioxidants or drugs that could protect hepatocytes from oxidative stress. N-acetylcysteine (NAC) is a glutathione precursor which increases glutathione levels in hepatocytes[12]. Increased glutathione levels, in turn, limit the production of reactive oxygen species (ROS) which cause hepatocellular injury[13]. Oral NAC treatment (1 g/d) of 11 NASH patients for 3 mo was demonstrated to improve liver function test significantly at the end of treatment period[12]. In a controlled study, NAC (600 mg/d) was administered to NASH patients for 4 wk, and a significant improvement in aminotransferase levels was found[14]. Although NAC was shown to improve liver function test in NASH patients, the mechanism remained unclear. Treatment of NASH with diet or diet plus NAC could attenuate oxidative stress as well as improve biochemical parameters and liver histopathology. However, the result of addition of NAC is not better than diet treatment alone[15]. Therefore, this study was conducted to determine the effects of NAC on oxidative stress and liver pathology in a rat model of 100% fat diet induced NASH[16].
This study was approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Male Sprague-Dawley rats weighing 220-260 g from the National Laboratory Animal Center, Mahidol University, Salaya, Nakorn Pathom were used. The animals were allowed to rest for a week after arrival at the Animal Center, Department of Physiology, Faculty of Medicine, Chulalongkorn University. They were kept at a controlled temperature of 25 ± 1°C under standard conditions (12 h dark: 12 h light cycle), fed with regular dry rat chow ad libitum, and had freely access to drinking water.
Rats were randomly divided into three experimental groups. Group 1: Fed ad libitum with regular dry rat chow for 6 wk (control group, n = 8). Group 2: Fed ad libitum with 100% fat diet for 6 wk to induce NASH (NASH group, n = 8). Group 3: Fed ad libitum with 100% fat diet plus 20 mg/kg per day of NAC orally (NASH + NAC20 group, n = 9) for 6 wk.
All rats were weighed weekly. They were sacrificed to collect blood, serum, and liver samples at the end of the study, 20 h after the last NAC treatment. The diagram of the experiment was shown as follow.
At the end of the study, all rats were anaesthetized using intraperitoneal injection of an overdose (45 mg/kg) of sodium pentobarbital, and the abdominal walls were opened. Blood was drawn by cardiac puncture for total glutathione assay and biochemical assay. The livers were excised quickly and cleaned in iced-cold NSS. One lobe of the liver was collected for MDA measurement, the remaining liver was fixed in 40 g/L formaldehyde solution for histological examination.
Total glutathione levels were quantified using Cayman’s GSH assay kit. This assay uses glutathione reductase for determination of glutathion. The sulfhydryl group of glutathion reacts with DTNB (5, 5’-dithiobis-2-nitrobenzoic acid, Ellman’s reagent) and produces a yellow colored 5-thio-2-nitrobenzoic acid (TNB). The mixed disulfide, GSTNB (between glutathion and TNB) that is concomitantly produced, is reduced by glutathione reductase to recycle glutathion and to produce more TNB. The rate of TNB production is directly proportional to this recycling reaction which is in turn directly proportional to the concentration of glutathionn in the sample. Measurement of the absorbance of TNB at 405 nm provides an accurate estimation of glutathion in the sample.
One lobe of the liver was removed and weighed. One gram of the tissue was placed in a test tube containing 2.25 mL homogenization buffer (11.5 g/L KCl) and homogenized in an ice box using a homogenizer at a rotational speed of 12 000 r/min for 1 min. MDA was quantified by using the thiobarbituric acid reaction as described by Ohgawa et al[17]. MDA levels in the samples were determined the linear regression equation from a standard curve. The content of lipid peroxide is expressed as nmol of MDA/g of wet weight, and the total protein was determined by the Lowry method[18] to correct the MDA level which is expressed in terms of μmol/g protein.
The remaining liver samples were fixed in 40 g/L formaldehyde solution at room temperature. They were processed by standard methods. Briefly, tissues were embedded in paraffin, sectioned at 5 μm, stained with HE, and then picked up on glass slides for light microscopy. An experienced pathologist blinded to the experiment evaluated all samples. All fields in each section were examined for grading of steatosis and necroinflammation according to the criteria described by Brunt et al[19].
The severity of steatosis was scored on the basis of the extent of involved parenchyma as 1 if fewer than 33% of the hepatocytes were affected, as 2 if 33%-66% of the hepatocytes were affected, as 3 if more than 66% of the hepatocytes were affected, and as 0 if no hepatocytes were affected.
Hepatic necroinflammation was graded from 0 to 3; score 1 (mild) = sparse or mild focal zone 3 hepatocyte injury/inflammation, score 2 (moderate) = noticeable zone 3 hepatocyte injury/inflammation, score 3 (severe) = severe zone 3 hepatocyte injury/inflammation, and score 0 = no hepatocyte injury/inflammation.
The data were expressed as mean ± SEM using the SPSS version 11.5 for Windows program. Statistical comparisons between groups were analyzed by ANOVA and post hoc comparisons were done with Bonferroni correction. P < 0.05 were considered significant.
The body mass at 6 wk of the NASH group and NASH + NAC20 group were decreased compared to the control (197.0 ± 8.1 g, 207.8 ± 6.9 g vs 438.4 ± 9.7 g, P < 0.05). Despite weight loss, the general condition of 100% fat diet-fed rats remained good throughout the observation periode. After the first 6 wk, rats were fed with regular dry rat chow for additional 4 wk. The body mass was significantly increased in all groups (Table 1).
| Parameter(mean ± SEM) | Control(n = 8) | NASH(n = 8) | NASH + NAC20(n = 9) |
| Body mass (g) at the beginning | 239.0 ± 2.27 | 245.1 ± 1.0 | 251.4 ± 1.7 |
| at 6 wk | 438.4 ± 9.7 | 197.0 ± 8.1a | 207.8 ± 6.9a |
| AST (U/L) | 86.8 ± 4.3 | 53.6 ± 9.3a | 65.6 ± 8.7 |
| ALT (U/L) | 40.2 ± 2.4 | 23.0 ± 1.9a | 25.4 ± 5.7a |
| Cholesterol (g/L) | 71.8 ± 1.8 | 94.8 ± 3.1a | 91.4 ± 3.5a |
| Triglycerides (g/L) | 90.3 ± 19.1 | 147.8 ± 32.6 | 89.2 ± 28.2 |
Serum biochemical parameters in the control and the experimental groups are given in Table 1. Serum AST and ALT activities decreased significantly in the NASH group when compared to the control group (AST; 53.7 ± 9.3 U/L vs 86.8 ± 4.3 U/L, ALT; 23.0 ± 1.9 U/L vs 40.1 ± 2.4 U/L, P < 0.05). Serum ALT but not AST activity returned to control levels in the NASH + NAC20 group (ALT 25.4 ± 5.7 U/L; AST 65.6 ± 8.7 U/L). Serum cholesterol was significantly higher in the NASH group and NASH + NAC20 group than that in the control group (94.8 ± 3.1 g/L, 91.4 ± 3.5 g/L vs 71.8 ± 1.8 g/L, P < 0.05), whereas there were no significant differences in serum triglycerides (Table 1).
Whole blood total glutathione levels were significantly higher in the NASH group compared to the control group (2066.7 ± 93.8 μmol/L vs 1337.5 ± 31.5 μmol/L, P < 0.05). Glutathion in NASH + NAC20 group was significantly lower than in the NASH group (1394.8 ± 81.2 μmol/L vs 2066.7 ± 93.8 μmol/L, P < 0.05.
MDA was elevated significantly in the NASH group when compared to the control group (209.9 ± 43.8 μmol/g protein vs 3.8 ± 1.7 μmol/g protein, P < 0.05). There was no statistical significant difference in MDA levels in NASH + NAC20 group (150.1 ± 27.0 μmol/g protein).
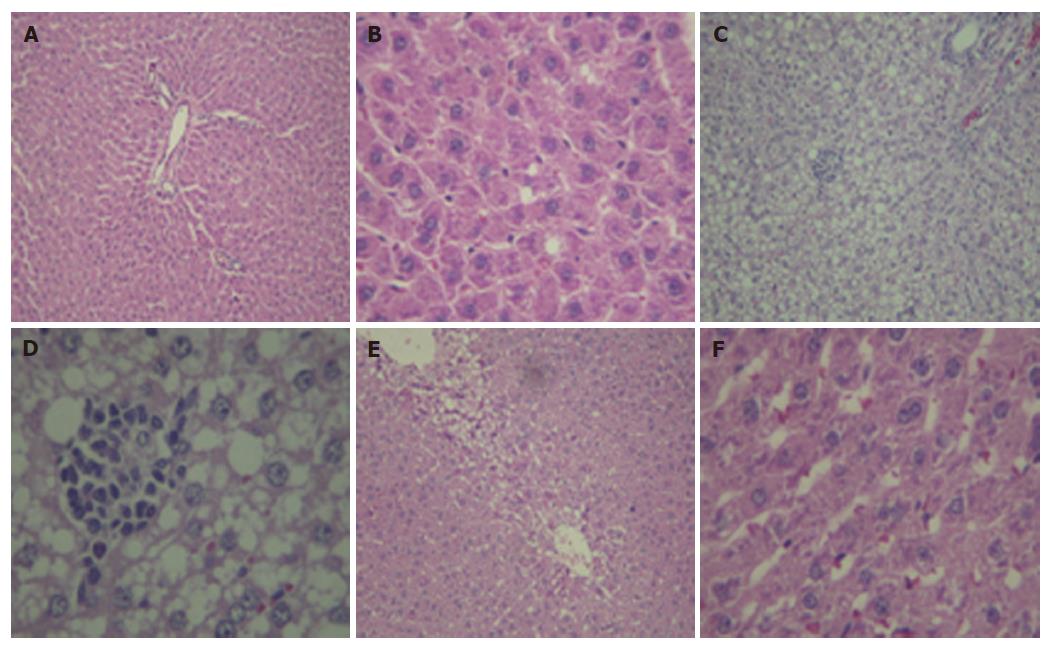
Liver sections from rats fed with the regular dry rat chow had normal morphological appearance. In the NASH group, all animals developed moderate to severe macrovesicular steatosis, hepatocyte ballooning, mild to moderate inflammation, and regeneration of hepatocytes (Table 2). NAC treatment improved steatosis and necroinflammation scores in animals of the NASH + NAC20 group when compared with the NASH group (Figure 1).
| Group | n | Steatosis | Necroinflammation | ||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Control | 8 | 8 | - | - | - | 8 | - | - | - |
| NASH | 8 | - | - | 5 | 3 | - | 5 | 2 | 1 |
| NASH + NAC20 | 9 | - | 6 | 2 | 1 | 3 | 4 | 1 | 1 |
Histopathology of NASH is similar to that of ethanol-induced hepatitis with the presence of macrovesicular steatosis, hepatocyte ballooning, necroinflammation, Mallory bodies, and fibrosis[1]. To study the pathogenesis of or therapeutic options for NASH, there are many models that can be used including a genetic model (obese rats), a model of methionine and choline deficient diet, a model of high fat liquid diet, and a 100% fat diet[5-8,16]. In this study, 100% fat diet was chosen to induce NASH in Sprague-Dawley rats as this procedure is fast, easy, and provides a comparable pattern of pathological changes as in humans although this model represents malnutrition induced steatohepatitis.
By feeding rats with 100% fat diet, the hepatic lesions of NASH were apparent within 6 wk. Histopathological examination showed macrovesicular steatosis, hepatocyte ballooning, Mallory bodies, and mild to moderate inflammation. One hundred percent fat diet caused mobilization of free fatty acid (FFA) from adipose tissue and transport into hepatocytes. In this condition, the liver failed to synthesize apolipoprotein that is required for packaging and exporting fat from the liver, triglycerides (TG) thus accumulate in the liver[20]. β-oxidation of FFA in hepatocytes produces reactive oxygen species (ROS) which activate lipid peroxidation[21]. ROS and lipid peroxidation cause direct damage to hepatocytes by disrupting membranes, protein, and DNA[22,23]. Hepatocyte damage and lipid peroxidation products induce an inflammatory response.
AST and ALT are useful screening tests for detecting liver injury[24]. They are found in hepatocytes and can not diffuse out of the cells in the physiological condition. When the hepatocyte is injured, plasma membrane can be disrupted and the leakage through extracellular fluid of the enzyme occurs where they can be detected at abnormal levels in the serum[25]. AST and ALT activities have been found to be increased in NASH rats[10,26-29]. In contrast, AST and ALT activities decreased significantly with 6 wk of 100% fat diet in this study. The decreased serum transaminases may be due to poor nutrition or hepatocyte death. Rats fed with 100% fat diet derived main energy from fat, when there were low in vitamin and mineral contents. The decreased AST and ALT levels were probably due to nutritional deficiency of pyridoxal phosphate which is a cofactor for both AST and ALT to catalyze the transfer of the α amino group from aspartate or alanine to α-ketoglutarate with made the release of pyruvate, oxaloacetate, and glutamate[24]. In addition, oxidative stress condition may be a cause of hepatocyte death, therefore, aminotransferases can not be produced.
In 100% fat diet-fed rats, body mass decreased significantly (P < 0.05) as compared to the control group. While serum cholesterol significantly increased, serum TG level was unchanged. Feeding with 100% fat diet for 6 wk caused a loss of body mass that may be due to a metabolic imbalance of carbohydrate, protein, and fat. Moreover, 100% fat diet contained highly saturated fat which may increase blood cholesterol concentration by 15% to 25%[30]. This result was from an increase of fat deposition in the liver which then provides the increased quantities of acetyl CoA in the liver cell for production of cholesterol[30]. The increased cholesterol was found in this experiment and had been observed in another study that used 10% lard oil and 2% cholesterol supplement adding into the standard diet[30].
FFA causes oxidative stress that has the potential to induce NASH[2]. FFA in the body is increased and this is associated with state of starvation[2]. Stored FFA can be mobilized from adipose tissue through lipolysis[2]. FFA metabolism increases the production of ROS which activated lipid peroxidation. Consequences are the disruption of membranes and the production of reactive metabolites such as MDA[21]. This study found high hepatic MDA levels in 100% fat-diet fed rats in accordance with studies by others[26-29]. Glutathione is the major intracellular non-protein antioxidant and plays a crucial role in the detoxification of free radicals[31,32]. Serum level of glutathione was increased in patients with NASH[33]. Similarly in this experiment, an increasing in total glutathione in whole blood with 100% fat diet feeding could be explained by compensatory protection mechanism against oxidative stress.
NAC is a thiol compound that acts directly as free radical scavenger and as a precursor of reduced glutathione[34]. Therefore, treatment with 20 mg/kg of NAC improved the total glutathione level to normal level in NASH + NAC20 group and improved necroinflammation score. Because of some limitations of our study, such as dose of NAC, time for treatment, and the number of animals, the effect of NAC on reducing hepatic MDA level remained unclear. In our previous study, diet treatment alone and diet plus NAC groups, total glutathione, serum AST, ALT, cholesterol, TG, and hepatic MDA returned to normal levels as in the control group. In addition, the pathological changes of liver in these groups were improved[15]. These results emphasized how crucial the nutritional composition of the diet is. Good proportion of nutrients (i.e., carbohydrate, lipid, and protein) is essential for growth and maintenance. These nutrients supply energy, promote growth, repair body tissues, and regulate metabolic processes[34].
In conclusion, feeding with 100% fat diet for 6 wk induced macrovesicular steatosis, hepatocyte ballooning, and inflammation in rats similar to histopathology of NASH. Treatment with NAC in NASH could improve oxidative stress and liver histopathology.
Non-alcoholic steatohepatitis (NASH), in advanced stages, can cause liver fibrosis, eventually progressing to cirrhosis in some patients. Oxidative stress is believed to play an important role in pathogenesis of NASH. N-acetylcysteine (NAC) is a glutathione precursor which increases glutathione levels in hepatocytes. Increased glutathione levels, in turn, limit the production of reactive oxygen species (ROS) which cause hepatocellular injury that could protect hepatocytes from oxidative stress.
NAC is a thiol compound that acts directly as free radical scavenger. In the pathogenesis of NASH, prevention of oxidative stress could protect hepatocytes from injury. The hotspots of this study indicate that NAC treatment could attenuate oxidative stress and improve liver histology in rats with NASH.
According to a previous report, oral NAC treatment of NASH patients for several months was found to significantly improve aminotransferase levels. However, the mechanism remained unclear. This study is a novel and well conducted experimental study showing the efficacy of NAC on improvement of total glutathion level and hepatic MDA in rats with NASH. Furthermore, treatment with NAC showed improvement in steatosis and necroinflammation.
Our data indicate that NAC treatment could attenuate oxidative stress and improve liver histology in rats with NASH.
NASH is a liver disease characterized by macrovesicular steatosis, hepatocyte necrosis, inflammation, Mallory bodies, and fibrosis. In initial phases, during which fat accumulates in the liver, no clinical symptoms are evident. In advanced stages, fibrosis is detectable, eventually progressing to cirrhosis. NAC is a glutathione precursor which increases glutathione levels in hepatocytes. Increased glutathione levels, in turn, limit the production of ROS which cause hepatocellular injury.
This is an experimental work on a steatosis model in the rat, induced by 100% fat diet in which the co-administration of NAC protects against fat induced liver injury. This is a very interesting and well conducted experimental study showing the efficacy of NAC in preventing biochemical and histological alterations secondary to a fat rich diet.
S- Editor Ma N L- Editor Mihm S E- Editor Yin DH
| 1. | Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Te Sligte K, Bourass I, Sels JP, Driessen A, Stockbrugger RW, Koek GH. Non-alcoholic steatohepatitis: review of a growing medical problem. Eur J Intern Med. 2004;15:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Medina J, Fernández-Salazar LI, García-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway--a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 332] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 303] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 7. | Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 358] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502-509. [PubMed] |
| 9. | García-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernández-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825-834. [PubMed] |
| 10. | Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561-40567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Gulbahar O, Karasu A, Ersoz G, Akarca US, Musoglu A. Treatment of non-alcoholic steatohepatitis with N- acetyl cysteine. Gastroenterology. 2000;118:A1444. [DOI] [Full Text] |
| 12. | Pastor A, Collado PS, Almar M, González-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Gulbahar O, Karasu A, Ersoz G, Akarca US, Musoglu A. N-acetyl cysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterology. 2003;18:1220-1221. |
| 14. | Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Patumraj S, Klaikeaw N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis. J Med Assoc Thai. 2007;90:788-797. [PubMed] |
| 15. | Thong-Ngam D, Samuhasaneeto S, Suyasunanont D, Wisedopas N. Development of a simple rat model of nonalcoholic steatohepatitis. Thai J Gastroenterol. 2005;6:144-148. |
| 16. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18761] [Article Influence: 407.8] [Reference Citation Analysis (0)] |
| 17. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 18. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2880] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 19. | Brody T. Nutritional biochemistry. 2nd ed. The United States: Academic Press 1994; 243-245. |
| 20. | Benzie IF. Lipid peroxidation: a review of causes, consequences, measurement and dietary influences. Int J Food Sci Nutr. 1996;47:233-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | de Knegt RJ. Non-alcoholic steatohepatitis: clinical significance and pathogenesis. Scand J Gastroenterol Suppl. 2001;234:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 295] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Kaplowitz N. Liver and biliary diseases. Baltimore: Williams & Wilkins 1992; 383. |
| 24. | Robbins SL. Pathologic basic of disease. London: W. B. Soder Company 1974; 25-30. |
| 25. | Fan JG, Zhong L, Xu ZJ, Tia LY, Ding XD, Li MS, Wang GL. Effects of low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2003;9:2045-2049. [PubMed] |
| 26. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 590] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall Pde L. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol. 2003;18:1272-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Guyton AC, Hall JE. Textbook of Medical physiology. 10th ed. The United States W. B. Saunders. 2000;788. |
| 30. | Meister A, Larsson A. Glutathione synthetase deficiency and other disorders of the γ-glutamyl cycle. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. New York McGraw-Hill 1989; 855-868. |
| 31. | Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1053] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 32. | Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57-62. [PubMed] |
| 33. | Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 279] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Guthrie HA. Introductory nutrition. 6th ed. St Louis: Times Mirror/Mosby College Publishing 1986; 11. |