INTRODUCTION
The interruption of hepatic blood flow followed by reperfusion, called ischemia/reperfusion, causes liver injury in a number of clinical interventions such as liver transplantation, hepatic resection, abdominal surgery with hepatic vascular occlusion, and hypovolemic shock[1-4]. Especially in liver transplantation, ischemia/reperfusion injury can lead to the primary dysfunction of liver allografts; still the most feared complication after liver transplantation because of the associated high mortality, and the fact that there is no treatment other than retransplantation[5-7]. The pathophysiology and substantial mechanisms of hepatic ischemia/reperfusion injury are increasingly well understood, and recent studies have drawn attention to ischemic preconditioning as one therapeutic strategy to decrease any injury[4,8-10]. Ischemic preconditioning is a phenomenon by which brief periods of ischemia followed by reperfusion render tissues resistant to subsequent prolonged ischemia/reperfusion. This phenomenon was originally characterized in a canine model of myocardial ischemia[11], and has since been recognized in hepatic injury after ischemia/reperfusion in animal models[12-14], as well as in humans[15-18].
We have recently reported that hepatoprotection of ischemic preconditioning in rats is mediated by reactive oxygen species (ROS) produced by Kupffer cells. Moreover, we have shown that hepatic pretreatment with a sublethal dose of H2O2 mimics the hepatoprotective effect of ischemic preconditioning[19]. Since H2O2 is produced as a consequence of ROS metabolism, it may be possible that H2O2 resulting from ROS production in Kupffer cells directly affects hepatocytes, and induces their adaptive response to ischemia/reperfusion injury. Therefore, in this study we sought to elucidate the mechanisms of H2O2 preconditioning to better understand the pathophysiology of ischemic preconditioning. We report here that H2O2 preconditioning did not protect hepatocytes directly, but via Kupffer cells, as in ischemic preconditioning. We also noted that the activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in Kupffer cells may play an important role in hepatoprotection of H2O2, as well as in ischemic preconditioning.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 180-240 g were maintained on a commercial pellet diet and water ad libitum, in a room under normal lighting conditions. All animals received humane care in compliance with our institutional and National Institutes of Health guidelines.
in vitro experiments
Isolation of hepatocytes: Rat hepatocytes were isolated and cultured as previously described[20]. Rat livers were minced after perfusion with 0.8 mg/mL collagenase (type I; Worthington Biochemical Corporation, Lakewood, NJ, USA) through the portal vein. Hepatocytes were separated from non-parenchymal cells by centrifugation at 50 ×g for 2 min at 4°C. The viability of the collected hepatocytes was ≥ 95%, as determined by the trypan blue exclusion test. Hepatocytes were resuspended in William’s E medium containing 100 mL/L fetal calf serum, 27 mmol/L NaHCO3, 100 nmol/L insulin, and 10 nmol/L dexamethasone at pH 7.4. Hepatocytes were then plated on type I collagen-coated 96-multiwell plates (Asahi Techno Glass Corporation, Tokyo, Japan) at a density of 4 × 104 cells/well and incubated overnight in an atmosphere of 95% air/5% CO2 at 37°C.
Treatment of isolated hepatocytes: Isolated hepatocytes were treated by incubation for 10 min at 37°C with 100 μL Krebs-Ringer N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) buffer (KRH) at pH 7.4 containing various concentrations of H2O2, and washed twice with KRH. We set doses of H2O2 up to 1 mmol/L in accordance with earlier liver perfusion experiments[19]. Hepatocytes were then incubated in an anaerobic chamber under anoxic conditions using an AnaeroPack (Mitsubishi Gas Chemical Corporation, Tokyo, Japan), and subsequently subjected to reoxygenation at 37°C. The oxygen concentration in the chamber decreased to < 0.005% within 30 min after insertion of AnaeroPack. Control hepatocytes were cultured under normoxic conditions for the same length of time at 37°C.
Cell viability assay: Cell viability was determined by propidium iodide fluorometry as previously described, with some modification[21]. Hepatocytes were incubated in 100 μL KRH containing 30 μmol/L propidium iodide, after H2O2 treatment. Fluorescence from each well was measured immediately before anoxic incubation and then at given times after reoxygenation. Cell viability after reoxygenation was calculated, with final fluorescence corresponding to the 100% cell death obtained by addition of 350 μmol/L digitonin. We used a Cytofluor S4000 fluorescence reader (Applied Biosystems, Stafford, TX, USA), employing 530 nm excitation and 645 nm emission filters.
Liver perfusion experiments
Preconditioning before warm ischemia/reperfusion injury: Rats were anesthetized intraperitoneally with 50 mg/kg pentobarbital sodium, and the abdomen was opened. Livers were perfused through the portal vein with Krebs-Henseleit bicarbonate buffer (KHB) saturated with 95% O2 and 5% CO2 at 30 mL/min for 10 min at 37°C. For H2O2 preconditioning, livers were perfused with KHB containing 1 mmol/L H2O2 for 10 min, and then perfused with KHB for 2 min to wash out H2O2, as previously described[19]. For ischemic preconditioning, the perfusion was stopped for 10 min, followed by reperfusion for 10 min. In control rats, livers were manipulated similarly except for adding H2O2 or stopping the perfusion, respectively.
Drug treatments: In some rats, 20 mg/kg gadolinium chloride (GdCl3; Wako Pure Chemical Industries, Tokyo, Japan) was injected intravenously at 24 h before H2O2 preconditioning, under light ether anesthesia to inhibit Kupffer cell function[22,23]. GdCl3 was dissolved in saline, and control rats were injected with the appropriate vehicle solution. The efficacy of GdCl3 injection was confirmed by immunohistochemical staining of liver sections with anti-ED2 antibody in the preliminary experiments. In other rats, diphenyleneiodonium chloride (DPI; Sigma-Aldrich, St. Louis, MO, USA), an inhibitor of NADPH oxidase, at a final concentration of 10 μmol/L, was added to the perfusate[24] during H2O2 preconditioning, or the reperfusion period after ischemic preconditioning. Livers were then perfused with KHB for 10 min to wash out DPI. DPI was dissolved in dimethyl sulfoxide (DMSO) at 20 mmol/L. Livers of vehicle-treated rats were perfused with KHB containing 500 μL/L DMSO during each preconditioning.
Warm ischemia/reperfusion: After H2O2 preconditioning or ischemic preconditioning, livers were stored in KHB at 37°C as previously described[19]. After 40 min storage, livers were reperfused in a recirculating system with 200 mL KHB saturated with 95% O2 and 5% CO2 at 37°C. The perfusate was collected after 60 min of reperfusion for determination of alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) activity.
Biochemical assays: Commercial kits (Wako Pure Chemical Industries) were used to determine ALT and LDH activity in the perfusates.
Determination of oxygen radical formation in Kupffer cells after H2O2 preconditioning
Livers were perfused through the portal vein with KHB for 10 min, and then perfused with KHB containing 1 mmol/L H2O2 for 10 min. Subsequently, the livers were perfused with KHB containing 500 mg/L nitro blue tetrazolium (NBT; Sigma-Aldrich) for 10 min. Livers were then fixed by infusion of 10% formalin, embedded in paraffin, sectioned and stained with nuclear fast red. As formazan deposition is formed by the reaction of NBT with oxygen radicals in Kupffer cells, the number of formazan-positive cells was determined as the mean in 10 different areas of each section, observed by light microscopy at × 400 magnification (high power field).
Statistical analysis
All values are expressed as means ± SE. The difference between the means was analyzed with Student’s t test, after confirming that the data passed normal distribution and equal-variance tests. P < 0.05 was considered significant.
RESULTS
Effect of H2O2 preconditioning on anoxia/reoxygenation injury of isolated hepatocytes
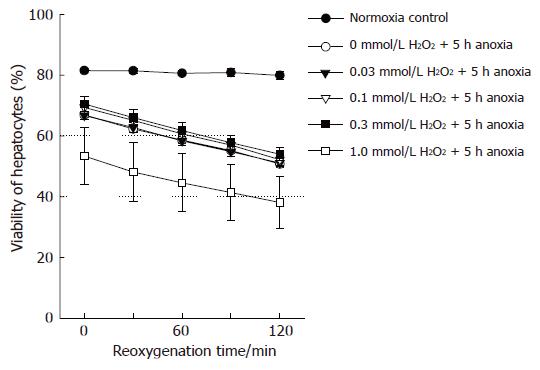
In the preliminary experiments, the viability of hepatocytes exposed to anoxia for > 3-4 h decreased after reoxygenation, compared to cells consistently cultured under normoxic conditions. Anoxic culture for 6 h resulted in a marked and rapid loss of cell viability after reoxygenation. Accordingly, we set the anoxic time to 5 h, and determined cell viability serially for 2 h after reoxygenation. As shown in Figure 1, anoxia/reoxygenation decreased hepatocyte viability linearly, though the viability of normoxic controls was not affected during the corresponding period. Preincubation with 0-1 mmol/L H2O2 did not improve such anoxia/reoxygenation injury, and the highest concentration of H2O2 somewhat worsened cell viability.
Figure 1 Viability of hepatocytes after anoxia/reoxygenation.
(●): Viability of hepatocytes cultured under normoxia consistently. Viability of hepatocytes, pretreated with (○): 0 mmol/L; (▼): 0.03 mmol/L; ( ): 0.1 mmol/L; (■): 0.3 mmol/L;
Contribution of Kupffer cells to hepatocyte protection by H2O2 preconditioning
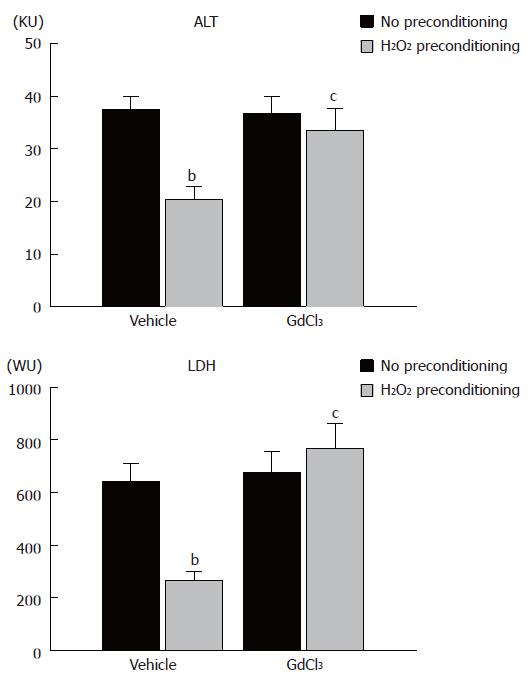
The results of the in vitro experiments suggested that H2O2 did not directly protect hepatocytes. We determined the contribution of Kupffer cells in H2O2 preconditioning, using an isolated perfused liver system. In untreated controls, which were perfused for the corresponding period without ischemia/reperfusion, ALT and LDH activity in the perfusate was 4.4 ± 0.6 and 35.1 ± 7.9, respectively (data not shown). As shown in Figure 2, warm ischemia/reperfusion markedly increased ALT and LDH activity in the perfusate to 37.4 ± 2.5 and 642 ± 68, respectively, with H2O2 preconditioning significantly reducing these values. GdCl3 pretreatment itself did not change ALT and LDH activity after warm ischemia/reperfusion. In the H2O2-preconditioned group, however, GdCl3 pretreatment reversed the decrease in ALT and LDH activity by H2O2 preconditioning.
Figure 2 ALT and LDH activities in perfusate after ischemia/reperfusion in an isolated perfused liver system from no preconditioning control, H2O2 preconditioning, GdCl3-no preconditioning, and GdCl3/H2O2 preconditioning rats.
Columns and bars represent means ± SE from six rats in each group. bP < 0.01 vs no preconditioning, cP < 0.05 vs vehicle treatment and H2O2 preconditioning. KU: Karmen unit; WU: Wróblewski unit.
Production of oxygen radicals in Kupffer cells after H2O2 preconditioning
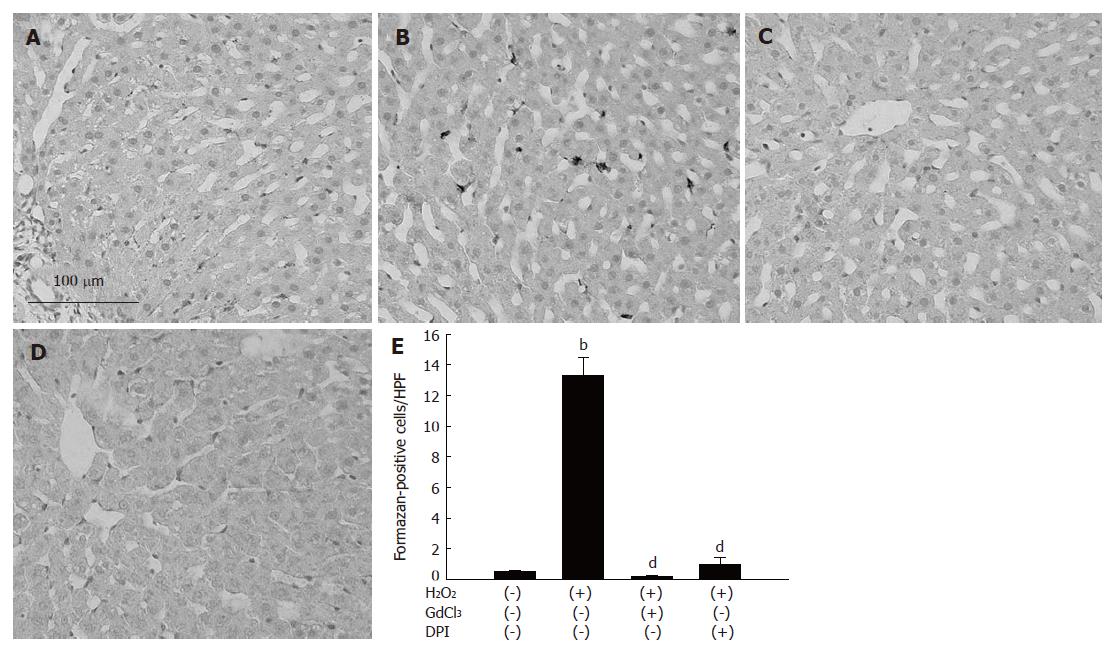
To elucidate the mechanisms of the contribution of Kupffer cells in H2O2 preconditioning, we determined oxygen radical production in Kupffer cells. Livers were perfused with H2O2 for 10 min and then perfused with NBT. When non-preconditioned livers were perfused with NBT, formazan deposition did not develop (Figure 3A). As shown in Figure 3B, H2O2 perfusion induced formazan deposition in non-parenchymal cells. When rats were pretreated with GdCl3 injection, H2O2 perfusion did not induce such dense deposition (Figure 3C). Moreover, formazan deposition did not develop after H2O2 perfusion in livers in which DPI was added to the perfusate during H2O2 perfusion (Figure 3D). The number of formazan-positive cells from three rats in each group is shown in Figure 3E; treatment with GdCl3 or DPI significantly reduced the number of formazan-positive cells in H2O2-perfused livers to the non-preconditioned control level.
Figure 3 Oxygen radical formation in Kupffer cells after H2O2 preconditioning.
A: Non-preconditioned livers; B: Livers after 10 min of H2O2 perfusion; C: Pretreatment with GdCl3 and H2O2 perfusion; D: Treatment with DPI and H2O2 perfusion; E: The number of formazan-positive cells. Columns and bars represent means ± SE from 3 rats in each group. bP < 0.01 vs non-preconditioned control; dP < 0.01 vs H2O2 perfusion.
Contribution of NADPH oxidase to hepatoprotection by H2O2 preconditioning
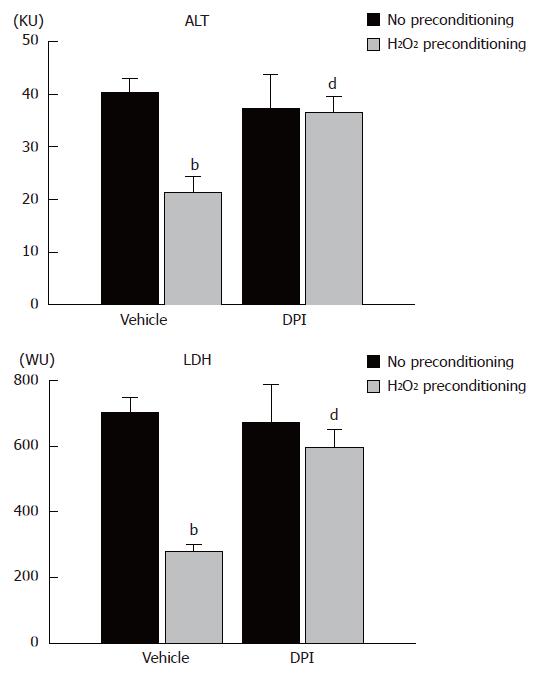
To determine the contribution of NADPH oxidase to the effect of H2O2 preconditioning, DPI was added to the perfusate during H2O2 preconditioning, and livers were then subjected to warm ischemia/reperfusion. We confirmed that H2O2 preconditioning decreased ALT and LDH activity after warm ischemia/reperfusion in a reproducible fashion. DPI treatment reversed this decrease in ALT and LDH activity induced by H2O2 preconditioning, although without H2O2 preconditioning, DPI alone did not change these values (Figure 4).
Figure 4 ALT and LDH activities in perfusate after ischemia/reperfusion in an isolated perfused liver system from no preconditioning control, H2O2 preconditioning, DPI-no preconditioning, and DPI/H2O2 preconditioning rats.
Columns and bars represent means ± SE from eight rats in each group. bP < 0.01 vs no preconditioning; dP < 0.01 vs vehicle treatment and H2O2 preconditioning.
Contribution of NADPH oxidase to hepatoprotection byischemic preconditioning
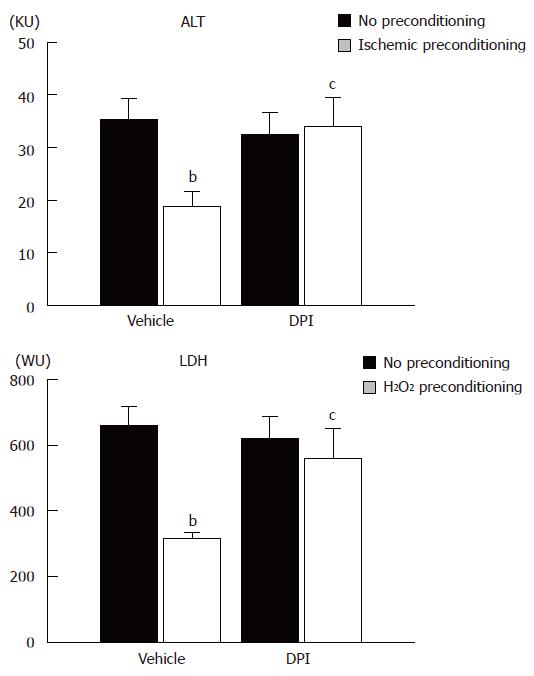
Finally, we determined whether DPI treatment could also reverse the protective effect of ischemic preconditioning. Ischemic preconditioning was performed with buffer containing DPI and livers were subjected to warm ischemia/reperfusion. As shown in Figure 5, ischemic preconditioning significantly reduced the increase in ALT and LDH activity after warm ischemia/reperfusion, with DPI reversing the hepatoprotection induced by ischemic preconditioning.
Figure 5 ALT and LDH activity in perfusate after ischemia/reperfusion in an isolated perfused liver system from no preconditioning control, ischemic preconditioning, DPI-no preconditioning, and DPI-ischemic preconditioning rats.
Columns and bars represent means ± SE from six rats in each group. bP < 0.01 vs no preconditioning; cP < 0.05 vs vehicle treatment and ischemic preconditioning.
DISCUSSION
We have previously reported that brief liver perfusion with a buffer containing a low dose of H2O2 reduces warm ischemia/reperfusion injury similar to that with ischemic preconditioning[19]. However, the mechanisms of such H2O2 preconditioning have not been clarified. Since H2O2 is produced as a consequence of ROS metabolism, and is now considered to be involved in not only pathological, but also physiological mechanisms[25-27], it may be that H2O2 derived from Kupffer cells induces adaptation of hepatocytes to ischemia/reperfusion injury. Accordingly, in the present study, we sought to determine whether pretreatment with H2O2 directly protects isolated hepatocytes against subsequent warm ischemia/reperfusion injury.
To simulate ischemia/reperfusion injury in vitro, isolated rat hepatocytes were incubated under anoxic conditions using an anaerobic chamber, and subsequently subjected to reoxygenation, following a widely used model[28,29]. Cell viability after reoxygenation was assessed with propidium iodide fluorometry. Propidium iodide binds to the double-stranded nucleic acids of permeable, non-viable cells[21], and the fluorescence is linearly related to LDH release[30]. Therefore, the changes in fluorescent intensity after anoxia/reoxygenation possibly represent in vitro ischemia/reperfusion injury. As shown in Figure 1, the viability of hepatocytes decreased slowly during anoxia and then rapidly after reoxygenation, compared with cells consistently maintained under normoxic conditions. These results were similar to those previously reported that showed necrotic hepatocytes after anoxia/reoxygenation[29]. We pretreated cells with H2O2 before such anoxia/reoxygenation injury; however, the viability of hepatocytes was not improved with any of the concentrations of H2O2 examined. Pretreatment with 1 mmol/L H2O2 for 10 min even worsened hepatocyte viability, although liver perfusion with the same concentration of H2O2 for 10 min reduced warm ischemia/reperfusion injury in our earlier study[19]. As some reports have indicated that isolated hepatocytes have lower tolerance to H2O2 than those in vivo, because of the absence of a sinusoidal structure[31-33], the present results might suggest that the effective dose of H2O2 in in vitro conditions is different from that during perfusion experiments. However, another interpretation is that sublethal H2O2 concentrations did not directly protect hepatocytes against warm ischemia/reperfusion injury.
Based on the hypothesis that Kupffer cells mediate hepatoprotection by H2O2 preconditioning, we performed liver perfusion experiments using rats in which Kupffer cell function was inhibited by GdCl3 injection[22,23]. As shown in Figure 2, GdCl3 alone did not change ischemia/reperfusion injury, which showed that Kupffer cells were not involved in the development of warm ischemia/reperfusion injury in our model. Accordingly, it is suggested that hepatoprotection by H2O2 preconditioning cannot be ascribed to the suppression of the deleterious function of Kupffer cells. Moreover, GdCl3 reversed the effect of H2O2 preconditioning. As previously recognized, inhibition of Kupffer cell function also reversed the effect of ischemic preconditioning[19]. Therefore, we concluded that H2O2 preconditioning protected hepatocytes via Kupffer cells, as in ischemic preconditioning.
Kupffer cells are the principal source of oxygen radicals after prolonged hepatic ischemia/reperfusion[34]. Moreover, even after a brief period of ischemia, Kupffer cells predominantly produce oxygen radicals[19]. If H2O2 preconditioning protects hepatocytes via stimulation of Kupffer cells, as in ischemic preconditioning, a brief period of perfusion with H2O2 may induce oxygen radical formation in Kupffer cells. As NBT reacts with oxygen radicals to form insoluble blue formazan, the NBT perfusion technique enables one to evaluate oxygen radical production in situ[35,36]. As shown in Figure 3, histological analysis indicated that H2O2 perfusion stimulated Kupffer cells to produce oxygen radicals. It has been reported that structural changes in Kupffer cells, or transient increases in the phagocytosis of Kupffer cells, is induced by perfusion with 0.7 mmol/L H2O2 for 10 min or 1 mmol/L H2O2 for 5 min, respectively[33,37]. These observations support our present findings; that stimulation of Kupffer cells with 1 mmol/L H2O2 for 10 min produced oxygen radicals.
Kupffer cells generate oxygen radicals by activating NADPH oxidase from a wide variety of stimulations[38-40]. However, it has never been determined whether H2O2 activates NADPH oxidase in Kupffer cells. Therefore, we sought to determine the contribution of NADPH oxidase to oxygen radical production in Kupffer cells by brief H2O2 perfusion. As shown in Figure 3, oxygen radical formation induced by H2O2 perfusion was eliminated by inhibition of NADPH oxidase with DPI. Some of these histological findings suggested that even brief H2O2 perfusion induced oxygen radical formation via NADPH oxidase in Kupffer cells. The active NADPH oxidase complex, which is bound to the plasma membrane in Kupffer cells, primarily produces superoxide anion radicals by reducing extracellular oxygen with electrons from cytosolic NADPH[40-42]. Since DPI acts on the cytosolic component of NADPH oxidase[43] and H2O2 permeates the cell membrane[41,44], an increase in intracellular H2O2 concentration by H2O2 treatment might act on NADPH oxidase in Kupffer cells. Subsequently, we examined the contribution of NADPH oxidase to the effect of H2O2 preconditioning against warm ischemia/reperfusion injury. As shown in Figure 4, inhibition of NADPH oxidase reversed the hepatoprotective effect of H2O2 preconditioning. Taken together with histological findings, the results indicate that NADPH oxidase in Kupffer cells mediates hepatocyte protection in H2O2 preconditioning.
Finally, we determined whether NADPH oxidase contributes to the effect of ischemic preconditioning. As shown in Figure 5, hepatocyte protection in ischemic preconditioning was also reversed by inhibition of NADPH oxidase. We concluded that H2O2 preconditioning, as well as ischemic preconditioning protects hepatocytes via NADPH oxidase in Kupffer cells. Moreover, the present results suggest that extracellular superoxide anion radicals produced by NADPH oxidase in Kupffer cells play a crucial role in hepatocyte protection by both H2O2 and ischemic preconditioning. Extracellular free radicals do not permeate the cell membrane[41,44] and directly oxidize the lipid bilayer of the cell membrane[45]. Thus, experiments to investigate the contribution of lipid peroxidation to hepatocyte preconditioning against ischemia/reperfusion injury are presently underway in our laboratory.
In conclusion, we report, to the best of our knowledge, a novel finding that hepatocyte protection against warm ischemia/reperfusion injury was achieved via NADPH oxidase in Kupffer cells. This was induced by preconditioning with a sublethal dose of H2O2 perfusion or brief ischemia/reperfusion. Based on the results of an earlier study, we assumed that a sublethal dose of H2O2 directly set off hepatocyte protection against ischemia/reperfusion injury. However, the present results reaffirmed the importance of Kupffer cells in induction of ischemic tolerance in liver. In general, Kupffer cells and their production of oxidative stress are considered to be a detrimental factor in hepatic ischemia/reperfusion injury[1,3]. However, under sublethal conditions, they actually could play a principal role in hepatocyte preconditioning against such injury. According to this concept, to obtain hepatoprotection, Kupffer cell activation may have to be strictly controlled between a steady-state level and a stimulated injurious level. Investigations to elucidate how sublethal oxidative stress induces these protective effects on hepatocytes are needed to determine any clinical application.
COMMENTS
Background
As a therapeutic strategy against hepatic ischemia/reperfusion injury, which can occur in a number of clinical settings such as liver transplantation and hepatic resection, recent studies have drawn attention to ischemic preconditioning. Ischemic preconditioning is a phenomenon in which brief periods of ischemia followed by reperfusion render tissues resistant to subsequent prolonged ischemia/reperfusion. The authors have previously shown that reactive oxygen species derived from Kupffer cells mediate the hepatoprotection induced by ischemic preconditioning, and pretreatment with a sublethal dose of H2O2 mimics such hepatoprotection. However, the mechanism of H2O2 preconditioning or the role of H2O2 in the preconditioning phenomenon has not been determined.
Research frontiers
Clinical investigations of ischemic preconditioning in human liver resection and transplantation have recently been reviewed (World J Gastroenterology 2007; 13: 657-670). New findings about the beneficial role of reactive oxygen species in some experimental settings are increasingly being reported.
Innovations and breakthroughs
Cell-specific investigations of ischemic preconditioning have rarely been reported. The authors focused on Kupffer cells and their production of reactive oxygen species, which are in general regarded as detrimental factors in hepatic injury, and showed that, paradoxically, they mediated hepatoprotection induced by ischemic preconditioning. In the present article, we also demonstrated that superoxide anion radicals produced by NADPH oxidase in Kupffer cells were implicated in the hepatoprotective effects of ischemic and H2O2 preconditioning.
Applications
Investigations into the mechanisms of ischemic preconditioning will enable the establishment of a pharmacological preconditioning regime before liver transplantation or hepatic surgery, leading to a reduction in ischemia/reperfusion injury.
Terminology
Interruption of tissue blood flow followed by reperfusion causes tissue injury, called ischemia/reperfusion injury. Ischemic preconditioning is a phenomenon whereby brief periods of ischemia followed by reperfusion render tissues resistant to subsequent ischemia/reperfusion injury.
Peer review
This is an interesting study, well written and well designed. The methods are sound. The discussions are to the point and not overstated.