Published online Sep 28, 2007. doi: 10.3748/wjg.v13.i36.4903
Revised: June 15, 2007
Accepted: June 18, 2007
Published online: September 28, 2007
AIM: To study the outcomes of patients with compensated hepatitis C virus-related cirrhosis.
METHODS: Twenty-four grade A5 and 11 grade A6 of Child-Pugh classification cirrhotic patients with active virus replication, treated for a mean period of 31.3 ± 5.1 mo with moderate doses of interferon-alpha and ribavirin, were compared to a cohort of 36 patients with similar characteristics, without antiviral treatment. Salivary caffeine concentration, a liver test of microsomal function, was determined at the starting and thrice in course of therapy after a mean period of 11 ± 1.6 mo, meanwhile the resistive index of splenic artery at ultra sound Doppler, an indirect index of portal hypertension, was only measured at the beginning and the end of study.
RESULTS: Eight out of the 24 A5- (33.3%) and 5 out of the 11 A6- (45.45%) treated-cirrhotic patients showed a significant improvement in the total overnight salivary caffeine assessment. A reduction up to 20% of the resistive index of splenic artery was obtained in 3 out of the 8 A5- (37.5%) and in 2 out of the 5 A6- (40%) cirrhotic patients with an improved liver function, which showed a clear tendency to decrease at the end of therapy. The hepatitis C virus clearance was achieved in 3 out of the 24 (12.5%) A5- and 1 out of the 11 (0.091%) A6-patients after a median period of 8.5 mo combined therapy. In the cohort of non-treated cirrhotic patients, not only the considered parameters remained unchanged, but 3 patients (8.3%) had a worsening of the Child-Pugh score (P = 0.001).
CONCLUSION: A prolonged antiviral therapy with moderate dosages of interferon-alpha and ribavirin shows a trend to stable liver function or to ameliorate the residual liver function, the entity of portal hypertension and the compensation status at acceptable costs.
- Citation: Tarantino G, Gentile A, Capone D, Basile V, Tarantino M, Minno MNDD, Cuocolo A, Conca P. Does protracted antiviral therapy impact on HCV-related liver cirrhosis progression? World J Gastroenterol 2007; 13(36): 4903-4908
- URL: https://www.wjgnet.com/1007-9327/full/v13/i36/4903.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i36.4903
Hepatitis C virus (HCV) infection is a leading cause of liver disease worldwide. Infected patients usually develop chronic hepatitis, which may progress producing diffuse disorganization of normal hepatic structure, to liver cirrhosis over a long period. Approximately 10%-20% of patients with chronic HCV infection have cirrhosis at the first clinical presentation, and near 20%-30% of those who do not present liver cirrhosis will eventually develop this illness and its complications[1-5].
To date, the most effective treatment to prevent disease progression and eventually cure chronic hepatitis C (CHC) virus infection is the combined therapy of pegylated (PEG) interferon (IFN) alpha and ribavirin (RBV). The rate of sustained viral response (SVR) in immuno-competent patients undergoing this antiviral regimen ranges from 42% to approximately 80%, depending on the HCV genotype, with an acceptable safety profile[6,7]. Thus, PEG-IFN alpha has substituted standard IFN alpha in the regimens used to treat CHC. However, the advantage of such an approach, with the additional cost it entails, still remains to be demonstrated[8]. In patients with compensated HCV-related cirrhosis, standard IFN alpha or combination therapy offers an interesting SVR[9]. Neither substantial difference has been reported between PEG-IFN alpha and standard IFN alpha in combination with RBV, nor PEG-IFN has a higher risk of complications[10]. Indeed, also in the decompensated form of liver cirrhosis, favourable results have been achieved after a 24 wk-course of combined therapy[11]. Since liver cirrhosis progression is related to other factors beyond the viral infection, the therapeutic efficacy should be evaluated under a wider perspective. Actually, data on the liver residual function and hepatic hemodynamics assessment, following the route of compensated liver cirrhosis after antiviral therapy, are limited, having prevalently studied the survival rate and hepato-carcinoma appearance.
Expecting a modification of the liver parenchymal structure and vascular bed (as partial gain following the reduced cellular damage, fibrosis and nodular regeneration) after a long-term antiviral treatment at a moderate-dosage, our principal aim was to investigate the impact of this schedule on eventually modifying the progression of liver cirrhosis. Then, we started weighing three characteristic prognostic aspects of the illness, i.e., the residual liver function (index of intact cellular mass) by a quantitative liver function test (QLFT), the presence of liver decompensation by regular assessment of Child-Pugh (C-P) severity class, and the altered hemodynamics (consequence of a cyto-architectural alteration) by a non-invasive parameter reflecting the portal hypertension entity.
Among the 121 patients (regularly followed up at our out-patient clinic) suffering from HCV-related liver cirrhosis, with HCV load > 800 000 IU/mL and genotype 1, 71 were enrolled in the study, well matched for sex, age and severity of the disease. These 71 patients were divided into treated group (35) and non-treated group (36) (Table 1). Of the patients (including 13 females) in the first group, 24 (mean age 58.9 ± 5.7 years) belonged to C-P grade A5 cirrhotics and 11 (mean age 61 ± 5.0 years, 7F) to C-P grade A6 cirrhotics. A further characteristic was that 18 underwent previous antiviral therapy during the past years (14 non-responders and 4 relapsers). These patients were treated for a mean period of 31.3 ± 5.1 mo with moderate doses of IFN alpha (8 with 1.5 MU daily, 13 with 3 MU on alternate days and 14 with 1 mcg/kg body weight of Peg-IFN alpha 2b weekly) in combination with oral RBV (400 or 600 mg per day for body weight < or > 75 kg). One was excluded from the study because he spontaneously ended the therapy and two had a suspension period of more than six weeks. Collaterally, a cohort (without antiviral treatment) of 36 initially compensated cirrhotics (25, A5 C-P patients including 12 females, and 11including 6 females, A6 C-P patients) was studied for the same period (9 patients were suffering from various grades of mood disorders, 4 were affected by microcythemia, 6 presented with thyroid laboratory abnormalities, 17 refused the treatment by choice. Of these patients, 15 were non-responders and 2 relapsers to previous antiviral therapy).
No patient in the two groups received mono- or combined treatment in the last two years.
The diagnosis of liver cirrhosis was made by histology (25 patients), laboratory data (34 patients), and combined clinical and laboratory parameters plus ultra sound (US) findings (12 patients).
As QLFT, we chose to probe the microsomial activity of enzymes, e.g., cytochrome P450 1A2, with the total overnight salivary caffeine assessment (TOSCA), comparable to the well-known caffeine clearance according to a recent study[12] and with the normal values set at ≤1 mcg/mL. Briefly, patients were allowed to drink coffee in late afternoon and on the day after overnight intake-washout, a sample of saliva, was collected in the morning, roughly centrifuged, frozen and stored until analysis. TOSCA was performed at first and then thrice during the course of therapy, at a mean interval period of 11 ± 1.6 mo. Caffeine was determined by an enzyme multiplied immunoassay technique (Dade Behring Liederbach, Germany) at the end of the follow-up period. Clinical assessment combined with other laboratory parameters, i.e., serum albumin, prothrombin time and bilirubin levels, was performed between periods of TOSCA evaluation. Occult blood in stools was detected to exclude gastrointestinal haemorrhage due to portal hypertension, as a possible cause of decompensation.
The resistive index of the splenic artery (SARI) at US Doppler was used to indirectly ascertain the entity of portal hypertension as previously described[13]. SARI was taken at the beginning and the end of the observation period using a 3.5 convex probe of an ESAOTE (Genoa, Italy), by two operators who were blind to each other and the laboratory results.
A slightly modified C-P classification was adopted to define the severity of liver cirrhosis (Table 2). A worsening total score was set at three points when patients belonged to the A5 class and two points in case of the A6 class.
The exclusion criteria included: (a) alcohol abuse screening according to recent recommendation[14], (b) metabolic syndrome following the adult treatment panel III classification[15], (c) detection of iron overload, (d) serum positivity for HBsAg, (e) previous chronic use of potentially toxic drugs as amiodadarone, methotrexate and high doses of vitamin A.
Chi square test was used to study the frequencies. Differences between means were studied by unpaired T test or Mann-Whitney test. Wilcoxon signed ranks test was carried out for paired observations. ANCOVA (repeated measure analysis of variance corrected for the basal values) was carried out to analyze the differences in TOSCA data at various time intervals. Association was evaluated by applying Pearson correlation. Logistic regression was used to verify if the IFN formulation and the schedule of antiviral therapy affected the severity of liver cirrhosis, based on C-P classification. The inter-observer agreement at US was appraised by the Cohen’s Kappa. Kaplan-Meier (K-M) survival curves were used for comparing C-P classification worsening probability curves in the two groups of patients. Cox proportional-hazards regression was employed to analyze the effect of non treatment (risk factor) on the C-P score aggravation. MedCalc version 9.0.2.1. and SYSTAT version 11.00.01 were used.
The study was conducted following the International Conference on Harmonization for Good Clinical Practice, in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
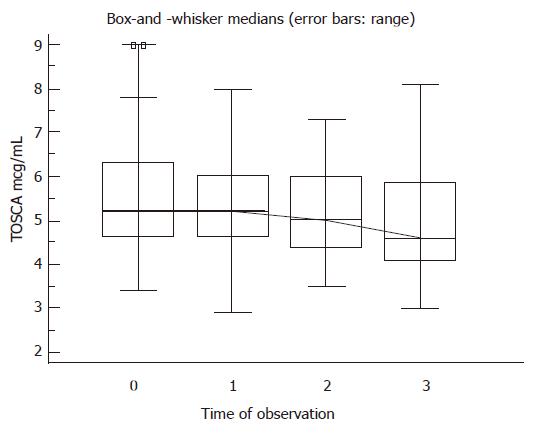
Whenever neutrophil count was < 750 cells/μL or haemoglobin < 10 g/dL, granulocyte colony-stimulating factor (filgrastim, Neupogen, Amgen Inc., California, 300 μg to eight patients, weekly) or erythropoietin analog (Eprex, Janssen-Cilag, 10 000 units to seven patients, twice/thrice weekly) was administered (after the blood count increased, the growth factor administration was prolonged for at least two weeks). In the treated group, six out of the 24 A5 C-P (25%) and four out of the 11 A6 C-P (36.4%) cirrhotic patients showed an improvement (> 20% in respect to the base-line determination) in TOSCA (Figure 1). A clear association was found between the post-treatment TOSCA values and the prothrombin time (r = -0.76, P = 0.0001). No patient showed a worsening liver function. In the comparative cohort of initially compensated cirrhotic patients without antiviral treatment, five out of the 36 patients (13.9%) had an exacerbation in respect to previous TOSCA values. A higher C-P score was found in non-treated than with treated patients (8/36 versus 1/35, P = 0.04).
Taking the US operator-dependent variability into consideration (although our inter-observer agreement was high, i.e., 89%), an appreciable reduction (> 20% compared with the pre-treatment measurements) in the SARI was obtained in 3 of 6 A5 C-P and 2 of 4 A6 C-P cirrhotic patients, showing an improvement in liver function. Furthermore, a significant decrease in the median SARI was present in the whole population compared to the pre- and post- treatment values.
HCV viral clearance was achieved in 3 out of the 24 (12.5%) A5 C-P and 1 out of the 11 (.91%) A6 C-P patients after a median (range) period of 8.5 (8-10) mo combined therapy.
No independent risk factor for C-P worsening was highlighted among the type of IFN, doses and period of treatment.
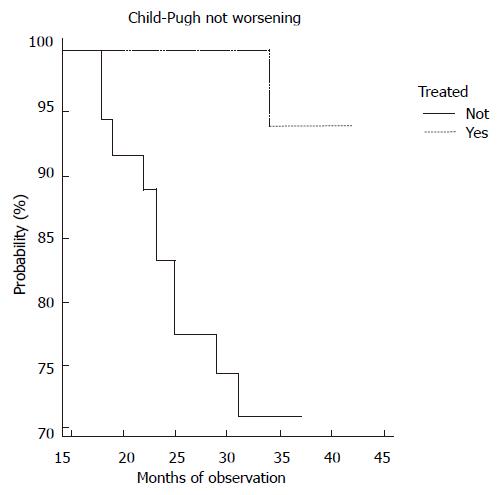
In our series during the entire period of study, no liver failure, hepato-cellular carcinoma and non-Hodgkin’s lymphoma were discovered. There was a significant difference in the K-M curves of liver cirrhosis during the four year-observation period between the two groups. About 30% of patients in the non-treated group experienced a worsening C-P score than the opposite cohort (Figure 2).
A small percentage of side-effects was registered in the treated group, e.g., two patients showed onset or enlargement of psoriatic plaques, three asthenia, one repeated low urinary tract infection, six anorexia, two low-grade fever, seven local erythema, one mild alopecia.
The cost of combined therapy plus growth factors per patient was estimated to be approximately 6000 US$ for standard IFN and 15 000 US$ for Peg-IFN, respectively.
The final values of TOSCA in samples were assessed for each group of patients (α = 0. 05, β = 0.01).
The main outcomes of antiviral therapy for chronic hepatitis C detected are the viral clearance and its lasting time. Actually, IFN therapy seems also be able to determine a decrease in the rate of functional disease progression in patients with the same disease, even in non-responders and relapsers[16].
On the other hand, previous data have emphasised the scarce response to IFN therapy when dealing with advanced chronic liver diseases. This finding has not been further supported by a recent study[11]. However, an uncertainty still exists, because it is not clear if a reduction in viral load or its clearance is of some benefit for any of the main processes, i.e., fibrosis and nodular regeneration, the extent of which determines the severity of liver cirrhosis. Variation exists among individuals regarding the rate and time of the progression of fibrosis to cirrhosis. There is evidence that the Ito cells are activated in response to HCV-induced hepatocellular damage initiating fibrosis[17]. These cells proliferate and become myofibroblasts, enhancing degradation of the normal matrix and producing excess abnormal matrix. Reactive oxygen species (ROS)[18] and inflammatory cytokines (mainly transforming growth factor and platelet-derived growth factor) speed up fibrosis. These cells play a key role in the alteration of metalloproteinase enzymes that regulate matrix collagen metabolism[19]. Fibrous tracts join branches of afferent portal veins and efferent hepatic veins, bypassing the hepatocytes and limiting their blood supply. Since the same myofibroblasts stimulated by endothelin-1, contribute to the increased portal vein resistance, fibrosis leads to “hepatocyte ischemia” with successive hepatocellular dysfunction and portal hypertension. Cirrhosis resulting from HCV infection has a slowly progressing course. Then, using the classic ones as therapeutic end-points[20], could result in the short-medium ones. Furthermore, an eventual change in some prognostic aspects of liver cirrhosis has not been reported, especially if long-term, moderate-dosage schedules were used.
Having this in mind, we tracked the progression of liver cirrhosis, using three combined parameters, all indices of the severity of the illness.
We found an improvement in residual liver function as assessed by TOSCA, a lack of C-P classification variation and a concomitant constancy of the indirect assessment of portal hypertension grade. All of them showed a stable severity of the illness in treated patients. Antiviral therapy could affect the ongoing liver cirrhosis by influencing the inflammation- or apoptosis-mediated fibrosis process, working on HCV viral replication, lessening ROS formation. The last mechanism is of paramount importance because it alters reparative processes. Interestingly, a reduced cellular damage could also justify the improvement in QLFT following therapy. Obviously, SARI is a surrogate marker, reflecting the hyper-dynamic, high-flow circulatory state as a consequence of splanchnic vasodilatation caused by portal hypertension. Actually, the only supra-hepatic vein catheterization could ultimately give valid information on hypertensive status. In any case, this non-invasive tool offers its best resources for serial measurements of hypertension and it has not yet been criticized as other Doppler US parameters[19]. In our study, a stable sonographic parameter or a slightly-reduced sonographic parameter which non invasively weighs the portal hypertension, was evidenced. Furthermore, the present study has other limitations in its methodology. The first is that it was not a randomized study, but a pilot study. In fact, only patients who accepted the treatment or had no contra-indications were enrolled. The second is that a four-year-interval was not representative of the natural history of this chronic disease, although it could foresee a certain trend. The third is that even if we tried to exclude the presence of co-factors, we were not sure that occult causes such as low intake of alcohol or episodic drug toxicity could have influenced the progression of liver cirrhosis in the non-treated patients. Moreover, we could have measured a serum fibrosis marker, a procollagen-III peptide, even if a consolidated study has failed to asses its utility[20]. We stress that the follow-up and work-up intervals were similar in the two groups. The fourth is that the quality of life was not opportunely evaluated, which is very important for the patient, although the non-standard dosage plays a key role in reducing side-effects. One positive aspect of this approach could be the health-care-cost in light of a likely procrastination of the need for hospitalization and liver transplantation.
In conclusion, our results favour the hypothesis that a stability (lack of fatal progression) or a trend to improve the severity of liver cirrhosis based on the clinical status and laboratory-instrumental profile is a reliable end-point after antiviral therapy. A prolonged combined antiviral course at reduced doses may slow down the progression of liver disease to cirrhosis. Further well-designed studies are needed to corroborate the present observations.
Hepatitis C virus (HCV) infection is a major health care problem. Recently combined antiviral therapy has been ascertained to improve the non-progressive form of chronic liver disease, eliminating the viral load and reducing fibrosis. However, whether HCV-related-liver cirrhosis characterized by a reduced hepato-cellular mass and an increased pressure of portal vein can be cured still remains a challenge.
The hot spots discussed in the article are the main outcomes of this controlled study, i.e., the assessment of real liver function with a simple and repeatable test (TOSCA) that probes the microsomial enzymatic activity and the evaluation of an Ultrasonographic index of portal hypertension.
The differences from the other related or similar studies relay on the particular schedule of combined antiviral therapy for the treatment of HCV-related liver cirrhosis (low long-protracted doses).
The perspectives of future application of this research could be expanded by larger and double-blinded studies to validate the good cost/benefit ratio.
Total overnight salivary caffeine assessment (TOSCA) is comparable to the well-known caffeine clearance. Briefly, patients were allowed to drink coffee up to late afternoon and after overnight intake-washout, a sample of saliva, was collected in the morning of the next day, roughly centrifuged, frozen and stored until analysis.
The manuscript describes that antiviral therapy in patients with hepatitis C-related cirrhosis shows a trend to stabilize or even to ameliorate residual liver function. Although the number of patients was rather small, the study provides evidence for how to treat these patients.
S- Editor Liu Y L- Editor Wang XL E- Editor Yin DH
| 1. | Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis. 1995;15:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 957] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 4. | Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 439] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 7. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 8. | Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, Bourliere M, Boucher E, Miguet JP, Parlier D. Treatment of hepatitis C virus-related cirrhosis: a randomized, controlled trial of interferon alfa-2b versus no treatment. Hepatology. 1999;29:1870-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Shepherd J, Brodin HF, Cave CB, Waugh NR, Price A, Gabbay J. Clinical- and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Int J Technol Assess Health Care. 2005;21:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, Accadia L, Bombardieri G, Andriulli A. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Tarantino G, Conca P, Capone D, Gentile A, Polichetti G, Basile V. Reliability of total overnight salivary caffeine assessment (TOSCA) for liver function evaluation in compensated cirrhotic patients. Eur J Clin Pharmacol. 2006;62:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Bolognesi M, Sacerdoti D, Merkel C, Gerunda G, Maffei-Faccioli A, Angeli P, Jemmolo RM, Bombonato G, Gatta A. Splenic Doppler impedance indices: influence of different portal hemodynamic conditions. Hepatology. 1996;23:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Giannini E, Fasoli A, Botta F, Testa E, Romagnoli P, Ceppa P, Testa R. Long-term follow up of chronic hepatitis C patients after alpha-interferon treatment: a functional study. J Gastroenterol Hepatol. 2001;16:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Novo E, Marra F, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cannito S, Antonaci C, Colombatto S, Pinzani M, Parola M. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Gut. 2006;55:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Nieto N, Dominguez-Rosales JA, Fontana L, Salazar A, Armendariz-Borunda J, Greenwel P, Rojkind M. Rat hepatic stellate cells contribute to the acute-phase response with increased expression of alpha1(I) and alpha1(IV) collagens, tissue inhibitor of metalloproteinase-1, and matrix-metalloproteinase-2 messenger RNAs. Hepatology. 2001;33:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Gramenzi A, Andreone P, Fiorino S, Cammà C, Giunta M, Magalotti D, Cursaro C, Calabrese C, Arienti V, Rossi C. Impact of interferon therapy on the natural history of hepatitis C virus related cirrhosis. Gut. 2001;48:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | O'Donohue J, Ng C, Catnach S, Farrant P, Williams R. Diagnostic value of Doppler assessment of the hepatic and portal vessels and ultrasound of the spleen in liver disease. Eur J Gastroenterol Hepatol. 2004;16:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bayerdörffer E, Lamerz R, Fliege R, Köpcke W, Mannes GA. Predictive value of serum procollagen-III-peptide for the survival of patients with cirrhosis. J Hepatol. 1991;13:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |