Published online Sep 28, 2007. doi: 10.3748/wjg.v13.i36.4865
Revised: July 2, 2007
Accepted: July 9, 2007
Published online: September 28, 2007
Apoptosis is central for the control and elimination of viral infections. In chronic hepatitis C virus (HCV) infection, enhanced hepatocyte apoptosis and upregulation of the death inducing ligands CD95/Fas, TRAIL and TNFα occur. Nevertheless, HCV infection persists in the majority of patients. The impact of apoptosis in chronic HCV infection is not well understood. It may be harmful by triggering liver fibrosis, or essential in interferon (IFN) induced HCV elimination. For virtually all HCV proteins, pro- and anti-apoptotic effects have been described, especially for the core and NS5A protein. To date, it is not known which HCV protein affects apoptosis in vivo and whether the infectious virions act pro- or anti-apoptotic. With the availability of an infectious tissue culture system, we now can address pathophysiologically relevant issues. This review focuses on the effect of HCV infection and different HCV proteins on apoptosis and of the corresponding signaling cascades.
- Citation: Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol 2007; 13(36): 4865-4872
- URL: https://www.wjgnet.com/1007-9327/full/v13/i36/4865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i36.4865
Hepatitis C virus (HCV) infection persists in approximately. Eighty percent of patients and is a leading cause of liver cirrhosis and hepatocellular carcinoma[1-4]. Worldwide, about 300 million individuals are HCV infected. The only antiviral treatment available to date with PEG-INF and ribavirin does not eliminate HCV infection in a large proportion of patients, especially in HCV genotype 1 infection, and, at the same time, has multiple severe side effects. With the availability of an infectious tissue culture system, we now can address pathophysiologically relevant issues for new treatment options[1-3]. HCV belongs to the flaviviridae. It has an enveloped, positive strand RNA genome of 9.6 kb length containing one open reading frame translated into a single polyprotein. Posttranslational cleavage yields 4 structural (E1, E2, core, p7 (probably) and 6 nonstructual proteins (NS2, NS3, NS4A, NS4B, NS5A, NS5B). Six different genotypes (1 [a, b, c], 2 [a, b, c], 3 [a, b], 4a, 5a, 6a) and 52 subtypes have been described. Due to the lack of proofreading function of the RNA-dependent RNA-polymerase (NS5B), HCV has a high mutation rate and exists as genetically heterogeneous quasispecies in individual patients[5-7]. The different genotypes differ genetically from one another by at least 30%, and the different subtypes within a genotype by more than 20%. This genetic heterogeneity makes it difficult to compare apoptotic pathways obtained with different HCV genotypes. In general, apoptosis is central to viral clearance. In HCV-infected liver, however, despite enhanced hepatocyte apoptosis, viral persistence is observed.
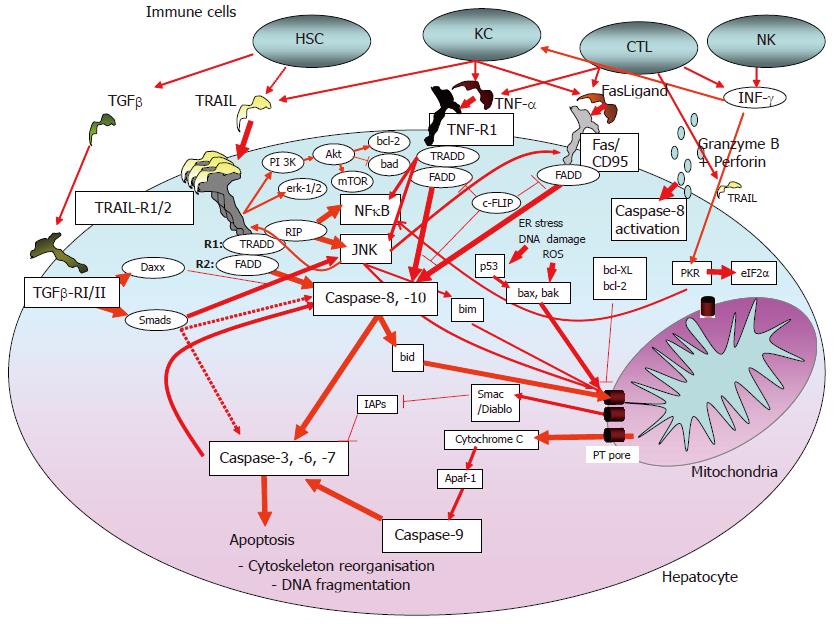
The immune response to viral infections includes different components of the innate and the acquired immune system. They induce apoptosis as a host defense against viral infections. The innate immune system as the first line of defense directly activates inflammatory cells, such as macrophages (e.g., granulocytes, Kupffer cells in the liver) and natural killer (NK) cells which may directly cause death of the infected cells. On the other hand, viral RNA or proteins can bind to intracellular molecules that modulate or directly induce cell death[8]. In this immune cell-independent, virus-induced apoptosis of the host cell protein kinase R (PKR)[9,10] and the cytoplasmic RNA helicase RIG-I[11] play central roles. RIG-I activates Cardif, a cytosolic protein that localizes to the mitochondrial membrane where it acts pro-apoptotic[12,13]. PKR is also activated by interferons (Figure 1) and acts via the downstream transcription factor eIF-2α[14,15]. In HCV infection, the activated innate immune system alone is obviously insufficient to eliminate the virus[16]. The acquired immune system consists of the humoral (antibody-secreting B-lymphocytes/plasma cells) and the cellular immune system (CD4+- and CD8+-T-lymphocyctes). This system is essential for the clearance of most viral infections and depends on complex intercellular interactions and the recognition of viral antigens presented by specific cells (e.g., dendritic cells). CD4+-T-lymphocytes activate CD8+-T-lymphocytes, cytotoxic T lymphocytes (CTLs), macrophages and B-lymphocytes[16]. The antigen-primed CD8+-T-lymphocytes/CTLs directly kill infected cells via direct cell-cell-contact, and release of cytotoxic and/or antiviral cytokines (e.g., IFNγ, TNFα), whereas IFNγ and INFα are also able to eliminate the virus without killing the host cell[17,18]. In chronic HCV infection, the acquired immune system is, among others, impaired by T cell failure, dysfunction and exhaustion[19]. This failure includes CD4+- as well as CD8+-T-lymphocytes.
Most of the cytotoxic effects mentioned above occur via programmed cell death, with activation of the intracellular suicide program through specific signals. Because chronic viral infection may reflect a failure of the immune system, specific apoptosis induction may not occur. In chronic HCV infection, however, enhanced hepatocyte apoptosis has been described, independent from the HCV genotype[20]. Apoptosis varies between 0.54%[20] and 20.00% of hepatocytes[21], depending on the methods used. Typical pathomorphological features of apoptosis (e.g., nuclear fragmentation, cell shrinkage) may be seen only in a minority of hepatocytes. The close physical proximity of apoptotic hepatocytes and infiltrating lymphocytes suggests an immune cell-mediated apoptosis[20,22]. Apoptosis correlates with liver pathology[20,21] and may contribute to fibrogenesis[23]. Due to the difficulty to identify HCV infected hepatocytes, it is unknown whether apoptotic hepatocytes are indeed HCV infected. The number of HCV infected hepatocytes is in the range between 1% and 10%[24]. Therefore, we actually do not know whether apoptosis is indeed related to HCV clearance. In an animal model of cholestasis, inhibition of hepatocyte apoptosis reduced fibrogenesis[25] and excessive apoptosis lead to fulminant hepatitis[26,27]. Therefore, anti-apoptotic therapy to prevent HCV-related liver damage has been suggested[28,29]. By contrast, in a chimeric mouse-human model, pro-apoptotic gene therapy with proapoptotic Bid, engineered to contain a specific cleavage site for NS3/NS4A protease, results in a considerable decline of HCV RNA in serum[30]. The relation between PEG-IFN/ribavirin-induced viral clearance and apoptosis of infected hepatocytes is largely unknown. INFs induce apoptosis in hepatoma cells, activate pro-apoptotic PKR[10] and upregulate death receptor ligands. However, anti-apoptotic effects have also been described[7,31-33].
Hepatocytes most likely represent so-called type-II cells, for which external activation of the death signaling pathway often is insufficient to induce apoptosis. Here, apoptosis requires in addition amplification by the mitochondrial pathway (intrinsic apoptosis pathway). The latter is affected by oxidative stress, DNA damage, and viral proteins (Figure 1).
Targeted apoptosis induction via CTLs and macrophages largely occurs via the ligands and receptors of the TNFα family: TNFα/TNF-receptor 1, CD95/CD95Ligand and TRAIL/Trail receptor-1 and -2, respectively (Figure 1). Ligand binding induces the formation of a death-inducing signaling complex, resulting in the activation of caspase-8 (caspases are the proteases involved in the apoptosis signaling cascade[34]. Active caspase-8 can trigger two signaling pathways. The first pathway involves cleavage of bid, followed by mitochondria-dependent activation of caspase-9 via cytochrome C release and apaf-1[35] (Figure 1). Mitochondria-dependent apoptosis is amplified by pro-apoptotic bax, bad, bak and others, while molecules like bcl-2 or bcl-XL act anti-apoptotic. These proteins converge at the mitochondrial permeability transition (PT) pore that regulates release of apoptotic regulatory proteins, e.g., procaspase-9, cytochrome C, apoptosis inducing factor (AIF) or Smac/Diablo[36-38]. The second pathway involves caspase-8 activation that may bypass mitochondria resulting in the direct activation of effector caspases (caspase-3, -6, -7). Cellular inhibitors of apoptosis (IAPs, survivin, c-FLIP) are able to block caspase activation and apoptosis[39] (Figure 1).
Growth-factor activated MAP-kinases Erk-1/2 and PKB/Akt inhibit apoptosis directly (e.g., through inactivation of pro-apoptotic bad) or via upregulation of anti-apoptotic proteins (e.g., bcl-2). By contrast, sustained stress activation of c-jun kinase (JNK) enhances death ligand-induced apoptosis via bim activation and consecutive mitochondrial apoptosis or via enhanced death-receptor membrane trafficking[40-42]. Most death ligands, especially TNFα and TRAIL, activate NFκB, which has anti-apoptotic effects in hepatocytes by upregulation of anti-apoptotic proteins, e.g., c-FLIP and bcl-XL[43].
Death receptor ligands may be secreted by immune cells (e.g., macrophages) or may be membrane-bound. The latter form induces apoptosis more efficiently[44]. In the normal liver, INFγ-activated Kupffer cells can kill neighbouring cells via TRAIL and CD95Ligand[37,44]. By contrast, in injured liver, activated hepatic stellate cells release TGF-β that may induce apoptosis of hepatocytes[45,46]. While TGF-β1 expression is increased in the HCV-infected liver[22], the impact of TGF-β on hepatocyte apoptosis in HCV-infected patients remains elusive. Apart from apoptosis induction, TGF-β is a key molecule in the pathogenesis of liver fibrosis[47].
Hepatocytes undergo apoptosis in response to CD95Ligand and TNFα, whereas TRAIL presumably only induces apoptosis in infected or malignantly transformed hepatocytes/hepatoma cells, but not in normal liver cells. For all three death ligands, in chronic HCV infection, upregulation has been described[20,48-51]. Further, HCV-specific CTL clones induced CD95Ligand-, TNFα- and perforin-dependent hepatocyte apoptosis[52,53]. In HCV-infected liver, CD8+ T cells express CD95Ligand[49] and TRAIL[54] (Fischer, Blum Schmitt-Gräff et al, unpublished data). Interestingly, CD95Ligand-induced apoptosis did not depend on HCV infection/antigen presentation, because bystander killing of non-HCV infected hepatocytes was observed. TRAIL-induced apoptosis seems especially important in viral defense. Adenoviral-infected murine and human hepatocytes are sensitized to TRAIL-induced apoptosis, while CD95Ligand-induced cell death is not affected[50,55]. In TRAIL knock-out mice resolution of pulmonary influenza infection is TRAIL-dependent[56], and CMV infected colon epithelial cells or skin fibroblasts become sensitive to TRAIL-induced apoptosis[57]. Further, in mice infected with encephalomyocarditis virus, blocking of TRAIL resulted in higher viral titers and early death[58]. In concanamycin- and listeria-induced hepatitis, liver cell apoptosis is TRAIL-dependent[59]. PEG-INF/ribavirin therapy of patients with chronic HCV infection results in a rapid and sustained TRAIL elevation, suggesting a role of TRAIL in viral clearance[60]. Similar observations have been made for soluble CD95Ligand[61,33]. Therefore, TRAIL-induced apoptosis may play a major role in HCV defense and elimination.
Another mechanism of apoptosis involves the release of granzyme B and perforin by CTLs[62,63]. Exocytosed perforins form transmembrane channels in the target cell that allow the entry of granzyme B. Similar to death-ligand induced apoptosis, granzyme B-mediated apoptosis largely depends on caspase activation and the sensitivity of the target cell. Hepatocytes seem resistant to granzyme B mediated cell death, and CTL killing of infected hepatocytes is perforin/granzyme B- independent[29,64]. Therefore, a contribution of this apoptosis mechanism in patients with viral hepatitis is very unlikely.
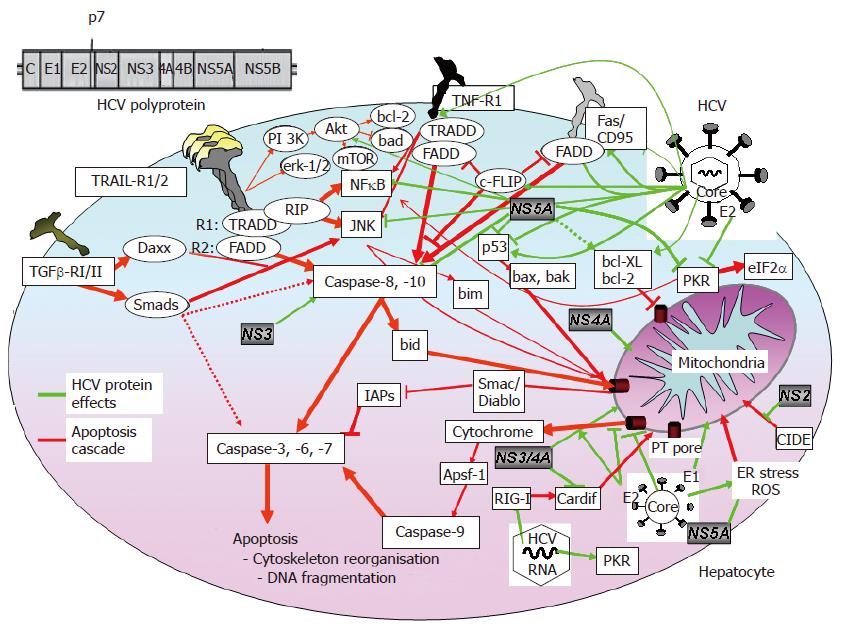
Viral proteins interfere with the cellular apoptotic signaling pathway and block key cellular elements of the host cell. Until recently, the lack of an infectious HCV tissue culture system did not allow to study the impact of HCV infection on hepatocyte apoptosis. Overall, the data regarding the role of different HCV proteins are controversial and ascribe to a given viral protein pro- and anti-apoptotic effects, depending on the experimental system used. Since in most models viral proteins are overexpressed by non-viral promoters, for virtually all HCV proteins a pro-apoptotic effect has been described. Apart from the unphysiological expression of viral proteins, these models further lack the balance of intracellular viral expression of the different HCV proteins and their interactions. Especially in HCV infection, intracellular viral protein expression is very low.
Further, HCV is genetically highly variable and exists as quasispecies in a given patient. Different pro- and anti-apoptotic effects of the HCV core protein from an individual patient have been described[65], suggesting special properties of different quasispecies proteins. These protein differences may explain in part the different effects of viral proteins on apoptosis. Studies of the contribution of genotypes or quasispecies to the effects on apoptosis are largely missing. Further, experiments designed to study the impact of HCV infection on hepatocyte apoptosis must also consider the interactions between the different HCV proteins. Therefore, only models based on the complete and infectious virus may reflect to some extent the in vivo situation.
The structural HCV core protein makes up the virion nucleocapsid[1,5,66]. The core protein has been shown to affect various cellular signaling pathways[67] and to activate different promoters, e.g., c-myc, c-fos[68-70]. It has further been shown to have pro- and anti-apoptotic effects in death ligand-mediated hepatocyte apoptosis. Core-dependent inhibition of TNF-α-[71] and CD95Ligand- induced apoptosis[72] has been described in a hepatoma cell line. In other models, overexpressed HCV core protein did not prevent CD95Ligand-induced apoptosis in hepatoma cells[73] or transgenic mice expressing HCV core protein, E1, E2 and NS2, respectively. HCV core protein inhibits CD95Ligand-mediated apoptosis by prevention of cytochrome C release from mitochondria and consecutive activation of caspase-9, -3 and -7[74]. Direct physical and pro-apoptotic interaction of the core protein with the cytoplasmatic domains of CD95, TNF-R1[75] and lymphotoxin-β[76] receptors have been reported. Further, direct binding to the downstream death domain of FADD and c-FLIP[77] has been shown to result in anti-apoptotic effects. Recently, inhibition of the TGF-β-pathway by direct interaction of the core protein with the DNA-binding domain of Smad3, important apoptosis mediators of TGF-β-receptor-I/II, has been demonstrated[65].
Several studies demonstrated binding of the HCV core protein to p53, either inhibiting or activating p53[69,78-80] with consecutive anti- or pro-apoptotic effects. In some studies apoptosis was inhibited in hepatoma through core-dependent phosphorylation and activation of STAT3 that induces the anti-apoptotic bcl-XL[81,82]. Other studies showed core-induced apoptosis through mitochondrial cytochrome C release and indirect activation of bax[83,84]. TRAIL-induced apoptosis in hepatoma cells seems enhanced by core-dependent bid-cleavage[83]. Mitochondrial functions are altered by core-induced oxidative stress, making cells more susceptible to apoptosis[85]. Machida et al[86] showed HCV-dependent production of reactive oxygen species (ROS), lowering of the mitochondrial transmembrane potential and consecutive caspase-independent cell death.
Taken together, it remains unclear whether HCV core protein inhibits or induces death receptor-mediated apoptosis of hepatocytes (Figure 2).
HCV proteins E1 and E2 are envelope proteins, that mediate viral binding and entry[7,87]. In a transgenic mouse model expressing HCV proteins, CD95Ligand-mediated hepatocyte apoptosis is inhibited by E1, E2, NS2 and core, respectively. The activation of mitochondrial apoptosis (intrinsic pathway) is involved, because release of cytochrome C and caspase-9, but not caspase-8 activation are inhibited. To date, the contribution of the individual HCV proteins was not investigated[74]. In E1-expressing hepatoma cells, apoptosis depends on the presence of the C-terminal transmembrane domain of E1, presumably altering membrane permeability of E1[88,89].
Inhibition of TRAIL-induced apoptosis in hepatoma cells by E2, presumably through inhibition of mitochondrial cytochrome C release has been demonstrated[90], while E1 had no effect and core did not counteract the anti-apoptotic effect of E2. Comparable results were obtained in core-E1-E2 transfected hepatoma cells or transgenic mice. In both models, core-E1-E2 induced less apoptosis than core-transfected cells/transgenic mice and controls, respectively[91]. By contrast, E2 induces mitochondria-related and caspase-dependent apoptosis in the same hepatoma cell line[92]. These controversial data may reflect the use of different promoters that overexpress E2, while at the same time, the HCV genotype or the individual sequence of E2 have not been considered. Therefore, it still remains unclear whether HCV E1 has apoptosis-modulating activity in vivo, and whether HCV E2 acts anti- or pro-apoptotic (Figure 2).
The non-structural HCV proteins NS2 and NS3 are the two viral proteases required for posttranslational cleavage of non-structural proteins. NS2 is a transmembrane protein localized in the endoplasmatic reticulum (ER) that directly binds and inhibits CIDE-B-induced apoptosis (cell death-inducing DFF45 (DNA-fragmentation-factor)-like effector[93]). CIDE-B-induced apoptosis is assumed to occur via the mitochondrial pathway[94,95]. Its role in hepatocyte apoptosis and viral hepatitis remain to be determined, however.
NS3 has a helicase and NTPase activity that are involved in RNA replication[7]. Importantly, NS3 prevents viral RNA-induced pro-apoptotic RIG-I effects by specific cleavage of downstream Cardif, a protein that translocates to the mitochondrial membrane when activated[13]. The precise role of Cardif in hepatocyte apoptosis and viral hepatitis is unknown, however. In contrast, NS3 induces caspase-8 dependent apoptosis in hepatocytes[96] and in dendritic cells[97]; the underlying mechanism remains unknown.
HCV NS4A is a cofactor that binds to NS3. NS4A alone and complexed with NS3 is localized in mitochondria and induces the release of cytochrome C and caspase-8 independent apoptosis[98]. NS4B is an integral ER membrane protein that may play a role in anchoring the replication complex[6,7]. A role in the apoptotic signaling pathway has not yet been described.
The function of NS5A is not yet well defined. NS5A interferes with the response to IFN and seems to play an important role in viral replication[5,7]. NS5A has sequence homologies with bcl-2 and binds to FKBP38, thereby augmenting the anti-apoptotic effect of bcl-2[99] and inhibiting the pro-apoptotic action of bax in hepatoma cells[100]. Anti-apoptotic effects of NS5A are further mediated by cytoplasmatic sequestering of p53[101], activation of PI3-kinase-Akt/PKB survival pathway[102], activation of STAT3 with enhanced expression of bcl-XL and p21[103] and activation of NFκB[104]. By contrast, the direct inhibition of pro-apoptotic bin1, a tumor suppressor protein with a SH3 domain, has been described in hepatoma cells[105], and a direct NS5A-induced apoptosis has also been shown[97,106]. NS5B is the viral RNA-dependent RNA polymerase[5-7]. There are no studies demonstrating a role of NS5B in apoptotisis of hepatocytes/hepatoma cells, while a pro-apoptotic effect of NS5B has been demonstrated in dendritic cells[97].
In conclusion, similar to HCV structural proteins, the effect of non-structural proteins on hepatocyte apoptosis in vivo remains unclear.
The role of apoptosis in HCV infection is not well defined. Kinetics and extent of hepatocyte apoptosis as well as the pro- and anti-apoptotic mechanisms involved remain unclear. It remains further unclear whether enhanced hepatocyte apoptosis in HCV infection is related to viral clearance, and whether it has a therapeutic benefit.
Most experimental models have fundamental shortcomings and there are no data from primary hepatocytes, tissue cultures or animal models. The majority of the data were obtained with different tumor cell lines that may in themselves be inhomogeneous. Different HCV genotypes and quasispecies may induce different effects, and most studies employ nonphysiologically overexpressed viral proteins. In HCV infected patients, by comparison, only very low quantities of HCV proteins are detectable, and the balanced expression of these proteins may be essential. Therefore, the results obtained to date have to be interpreted with great cautious. The now available infectious tissue culture systems[1-3] as well as future in vivo model systems may give answers to these questions, may better reflect the in vivo situation and may help to clarify the interference of HCV with apoptotic pathways and its role in the pathogenesis of HCV infection and clearance.
S- Editor Ma N L- Editor Alpini GD E- Editor Ma WH
| 1. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2275] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 2. | Lohmann V, Hoffmann S, Herian U, Penin F, Bartenschlager R. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J Virol. 2003;77:3007-3019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1849] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 4. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2042] [Cited by in RCA: 2025] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 5. | Bartenschlager R. Hepatitis C virus molecular clones: from cDNA to infectious virus particles in cell culture. Curr Opin Microbiol. 2006;9:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Pavio N, Lai MM. The hepatitis C virus persistence: how to evade the immune system? J Biosci. 2003;28:287-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74:1513-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Zhang P, Samuel CE. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J Virol. 2007;81:8192-8200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 656] [Cited by in RCA: 627] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 11. | Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282:15315-15318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Herzer K, Sprinzl MF, Galle PR. Hepatitis viruses: live and let die. Liver Int. 2007;27:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1976] [Cited by in RCA: 1921] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 14. | Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279-3283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 329] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis. 2000;5:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669-15674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 530] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Frese M, Schwärzle V, Barth K, Krieger N, Lohmann V, Mihm S, Haller O, Bartenschlager R. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology. 2002;35:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 768] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 19. | Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661-15668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 478] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Calabrese F, Pontisso P, Pettenazzo E, Benvegnù L, Vario A, Chemello L, Alberti A, Valente M. Liver cell apoptosis in chronic hepatitis C correlates with histological but not biochemical activity or serum HCV-RNA levels. Hepatology. 2000;31:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Bantel H, Lügering A, Poremba C, Lügering N, Held J, Domschke W, Schulze-Osthoff K. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Lau JY, Xie X, Lai MM, Wu PC. Apoptosis and viral hepatitis. Semin Liver Dis. 1998;18:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 24. | Hiramatsu N, Hayashi N, Haruna Y, Kasahara A, Fusamoto H, Mori C, Fuke I, Okayama H, Kamada T. Immunohistochemical detection of hepatitis C virus-infected hepatocytes in chronic liver disease with monoclonal antibodies to core, envelope and NS3 regions of the hepatitis C virus genome. Hepatology. 1992;16:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 27. | Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation. 1999;67:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Feldstein AE, Gores GJ. An apoptosis biomarker goes to the HCV clinic. Hepatology. 2004;40:1044-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 30. | Hsu EC, Hsi B, Hirota-Tsuchihara M, Ruland J, Iorio C, Sarangi F, Diao J, Migliaccio G, Tyrrell DL, Kneteman N. Modified apoptotic molecule (BID) reduces hepatitis C virus infection in mice with chimeric human livers. Nat Biotechnol. 2003;21:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Curr Med Chem. 2007;14:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Yano H, Ogasawara S, Momosaki S, Akiba J, Kojiro S, Fukahori S, Ishizaki H, Kuratomi K, Basaki Y, Oie S. Growth inhibitory effects of pegylated IFN alpha-2b on human liver cancer cells in vitro and in vivo. Liver Int. 2006;26:964-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Yoneyama K, Goto T, Miura K, Mikami K, Ohshima S, Nakane K, Lin JG, Sugawara M, Nakamura N, Shirakawa K. The expression of Fas and Fas ligand, and the effects of interferon in chronic liver diseases with hepatitis C virus. Hepatol Res. 2002;24:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 624] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 35. | Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: a multifunctional cytokine. Front Biosci. 2007;12:3813-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Eichhorst ST. Modulation of apoptosis as a target for liver disease. Expert Opin Ther Targets. 2005;9:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Fischer R, Schmitt M, Bode JG, Häussinger D. Expression of the peripheral-type benzodiazepine receptor and apoptosis induction in hepatic stellate cells. Gastroenterology. 2001;120:1212-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Brenner C, Grimm S. The permeability transition pore complex in cancer cell death. Oncogene. 2006;25:4744-4756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1910] [Cited by in RCA: 1905] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 40. | Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, Kassahn D, Torgler R, Mueller C, Schneider P. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Graf D, Kurz AK, Fischer R, Reinehr R, Häussinger D. Taurolithocholic acid-3 sulfate induces CD95 trafficking and apoptosis in a c-Jun N-terminal kinase-dependent manner. Gastroenterology. 2002;122:1411-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1154] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 43. | Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Fischer R, Cariers A, Reinehr R, Häussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Lee KY, Bae SC. TGF-beta-dependent cell growth arrest and apoptosis. J Biochem Mol Biol. 2002;35:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA. 1992;89:5408-5412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 482] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 48. | Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M. Apoptosis in liver diseases--detection and therapeutic applications. Med Sci Monit. 2005;11:RA337-RA345. [PubMed] |
| 49. | Mita E, Hayashi N, Iio S, Takehara T, Hijioka T, Kasahara A, Fusamoto H, Kamada T. Role of Fas ligand in apoptosis induced by hepatitis C virus infection. Biochem Biophys Res Commun. 1994;204:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94-96. [PubMed] |
| 51. | Zylberberg H, Rimaniol AC, Pol S, Masson A, De Groote D, Berthelot P, Bach JF, Bréchot C, Zavala F. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283-5291. [PubMed] |
| 53. | Gremion C, Grabscheid B, Wölk B, Moradpour D, Reichen J, Pichler W, Cerny A. Cytotoxic T lymphocytes derived from patients with chronic hepatitis C virus infection kill bystander cells via Fas-FasL interaction. J Virol. 2004;78:2152-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Saitou Y, Shiraki K, Fuke H, Inoue T, Miyashita K, Yamanaka Y, Yamaguchi Y, Yamamoto N, Ito K, Sugimoto K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand and tumor necrosis factor-related apoptosis-inducing ligand receptors in viral hepatic diseases. Hum Pathol. 2005;36:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, Kühnel F, Kubicka S. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Ishikawa E, Nakazawa M, Yoshinari M, Minami M. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J Virol. 2005;79:7658-7663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Sträter J, Walczak H, Pukrop T, Von Müller L, Hasel C, Kornmann M, Mertens T, Möller P. TRAIL and its receptors in the colonic epithelium: a putative role in the defense of viral infections. Gastroenterology. 2002;122:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Sedger LM, Shows DM, Blanton RA, Peschon JJ, Goodwin RG, Cosman D, Wiley SR. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920-926. [PubMed] |
| 59. | Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Pelli N, Torre F, Delfino A, Basso M, Picciotto A. Soluble tumor necrosis factor-related ligand (sTRAIL) levels and kinetics during antiviral treatment in chronic hepatitis C. J Interferon Cytokine Res. 2006;26:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Toyoda M, Kakizaki S, Horiguchi N, Sato K, Takayama H, Takagi H, Nagamine T, Mori M. Role of serum soluble Fas/soluble Fas ligand and TNF-alpha on response to interferon-alpha therapy in chronic hepatitis C. Liver. 2000;20:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 796] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 63. | Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1161] [Cited by in RCA: 1179] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 64. | Kafrouni MI, Brown GR, Thiele DL. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J Immunol. 2001;167:1566-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Pavio N, Battaglia S, Boucreux D, Arnulf B, Sobesky R, Hermine O, Brechot C. Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene. 2005;24:6119-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827-3836. [PubMed] |
| 67. | Lai MM, Ware CF. Hepatitis C virus core protein: possible roles in viral pathogenesis. Curr Top Microbiol Immunol. 2000;242:117-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Ray RB, Lagging LM, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995;37:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 188] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Ray RB, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983-10986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Ray RB, Steele R, Meyer K, Ray R. Hepatitis C virus core protein represses p21WAF1/Cip1/Sid1 promoter activity. Gene. 1998;208:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Ray RB, Meyer K, Steele R, Shrivastava A, Aggarwal BB, Ray R. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J Biol Chem. 1998;273:2256-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Ruggieri A, Harada T, Matsuura Y, Miyamura T. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology. 1997;229:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 73. | Dumoulin FL, vsn dem Bussche A, Söhne J, Sauerbruch T, Spengler U. Hepatitis C virus core protein does not inhibit apoptosis in human hepatoma cells. Eur J Clin Invest. 1999;29:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Machida K, Tsukiyama-Kohara K, Seike E, Toné S, Shibasaki F, Shimizu M, Takahashi H, Hayashi Y, Funata N, Taya C. Inhibition of cytochrome c release in Fas-mediated signaling pathway in transgenic mice induced to express hepatitis C viral proteins. J Biol Chem. 2001;276:12140-12146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai MM. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691-3697. [PubMed] |
| 76. | Matsumoto M, Hsieh TY, Zhu N, VanArsdale T, Hwang SB, Jeng KS, Gorbalenya AE, Lo SY, Ou JH, Ware CF. Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-beta receptor. J Virol. 1997;71:1301-1309. [PubMed] |
| 77. | Saito K, Meyer K, Warner R, Basu A, Ray RB, Ray R. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J Virol. 2006;80:4372-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Herzer K, Weyer S, Krammer PH, Galle PR, Hofmann TG. Hepatitis C virus core protein inhibits tumor suppressor protein promyelocytic leukemia function in human hepatoma cells. Cancer Res. 2005;65:10830-10837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Kao CF, Chen SY, Chen JY, Wu Lee YH. Modulation of p53 transcription regulatory activity and post-translational modification by hepatitis C virus core protein. Oncogene. 2004;23:2472-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Otsuka M, Kato N, Lan K, Yoshida H, Kato J, Goto T, Shiratori Y, Omata M. Hepatitis C virus core protein enhances p53 function through augmentation of DNA binding affinity and transcriptional ability. J Biol Chem. 2000;275:34122-34130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Otsuka M, Kato N, Taniguchi H, Yoshida H, Goto T, Shiratori Y, Omata M. Hepatitis C virus core protein inhibits apoptosis via enhanced Bcl-xL expression. Virology. 2002;296:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196:641-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Chou AH, Tsai HF, Wu YY, Hu CY, Hwang LH, Hsu PI, Hsu PN. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Lee SH, Kim YK, Kim CS, Seol SK, Kim J, Cho S, Song YL, Bartenschlager R, Jang SK. E2 of hepatitis C virus inhibits apoptosis. J Immunol. 2005;175:8226-8235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 679] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 86. | Machida K, Cheng KT, Lai CK, Jeng KS, Sung VM, Lai MM. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. 2006;80:7199-7207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 87. | Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Ciccaglione AR, Marcantonio C, Costantino A, Equestre M, Rapicetta M. Expression of HCV E1 protein in baculovirus-infected cells: effects on cell viability and apoptosis induction. Intervirology. 2003;46:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Ciccaglione AR, Marcantonio C, Tritarelli E, Equestre M, Magurano F, Costantino A, Nicoletti L, Rapicetta M. The transmembrane domain of hepatitis C virus E1 glycoprotein induces cell death. Virus Res. 2004;104:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Lee SK, Park SO, Joe CO, Kim YS. Interaction of HCV core protein with 14-3-3epsilon protein releases Bax to activate apoptosis. Biochem Biophys Res Commun. 2007;352:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Kamegaya Y, Hiasa Y, Zukerberg L, Fowler N, Blackard JT, Lin W, Choe WH, Schmidt EV, Chung RT. Hepatitis C virus acts as a tumor accelerator by blocking apoptosis in a mouse model of hepatocarcinogenesis. Hepatology. 2005;41:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Chiou HL, Hsieh YS, Hsieh MR, Chen TY. HCV E2 may induce apoptosis of Huh-7 cells via a mitochondrial-related caspase pathway. Biochem Biophys Res Commun. 2006;345:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Viswakarma N, Yu S, Naik S, Kashireddy P, Matsumoto K, Sarkar J, Surapureddi S, Jia Y, Rao MS, Reddy JK. Transcriptional regulation of Cidea, mitochondrial cell death-inducing DNA fragmentation factor alpha-like effector A, in mouse liver by peroxisome proliferator-activated receptor alpha and gamma. J Biol Chem. 2007;282:18613-18624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Inohara N, Koseki T, Chen S, Wu X, Núñez G. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 1998;17:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 95. | Erdtmann L, Franck N, Lerat H, Le Seyec J, Gilot D, Cannie I, Gripon P, Hibner U, Guguen-Guillouzo C. The hepatitis C virus NS2 protein is an inhibitor of CIDE-B-induced apoptosis. J Biol Chem. 2003;278:18256-18264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Prikhod'ko EA, Prikhod'ko GG, Siegel RM, Thompson P, Major ME, Cohen JI. The NS3 protein of hepatitis C virus induces caspase-8-mediated apoptosis independent of its protease or helicase activities. Virology. 2004;329:53-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Siavoshian S, Abraham JD, Thumann C, Kieny MP, Schuster C. Hepatitis C virus core, NS3, NS5A, NS5B proteins induce apoptosis in mature dendritic cells. J Med Virol. 2005;75:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Nomura-Takigawa Y, Nagano-Fujii M, Deng L, Kitazawa S, Ishido S, Sada K, Hotta H. Non-structural protein 4A of Hepatitis C virus accumulates on mitochondria and renders the cells prone to undergoing mitochondria-mediated apoptosis. J Gen Virol. 2006;87:1935-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Wang J, Tong W, Zhang X, Chen L, Yi Z, Pan T, Hu Y, Xiang L, Yuan Z. Hepatitis C virus non-structural protein NS5A interacts with FKBP38 and inhibits apoptosis in Huh7 hepatoma cells. FEBS Lett. 2006;580:4392-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Chung YL, Sheu ML, Yen SH. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int J Cancer. 2003;107:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Lan KH, Sheu ML, Hwang SJ, Yen SH, Chen SY, Wu JC, Wang YJ, Kato N, Omata M, Chang FY. HCV NS5A interacts with p53 and inhibits p53-mediated apoptosis. Oncogene. 2002;21:4801-4811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 102. | Street A, Macdonald A, Crowder K, Harris M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232-12241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | Sarcar B, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A mediated STAT3 activation requires co-operation of Jak1 kinase. Virology. 2004;322:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 498] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 105. | Nanda SK, Herion D, Liang TJ. The SH3 binding motif of HCV [corrected] NS5A protein interacts with Bin1 and is important for apoptosis and infectivity. Gastroenterology. 2006;130:794-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Macdonald A, Harris M. Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol. 2004;85:2485-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |