Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4690
Revised: April 23, 2007
Accepted: April 29, 2007
Published online: September 21, 2007
Non-HFE hereditary haemochromatosis (HH) refers to a genetically heterogeneous group of iron overload disorders that are unlinked to mutations in the HFE gene. The four main types of non-HFE HH are caused by mutations in the hemojuvelin, hepcidin, transferrin receptor 2 and ferroportin genes. Juvenile haemochromatosis is an autosomal recessive disorder and can be caused by mutations in either hemojuvelin or hepcidin. An adult onset form of HH similar to HFE-HH is caused by homozygosity for mutations in transferrin receptor 2. The autosomal dominant iron overload disorder ferroportin disease is caused by mutations in the iron exporter ferroportin. The clinical characteristics and molecular basis of the various types of non-HFE haemochromatosis are reviewed. The study of these disorders and the molecules involved has been invaluable in improving our understanding of the mechanisms involved in the regulation of iron metabolism.
- Citation: Wallace DF, Subramaniam VN. Non-HFE haemochromatosis. World J Gastroenterol 2007; 13(35): 4690-4698
- URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4690
After the identification of the HFE gene in 1996[1] it became apparent that not all cases of haemochromatosis are caused by mutations in HFE. HFE-associated HH (HFE-HH) or type 1 HH is the most common form, especially in populations of Northern European origin, where the C282Y mutation has a high allele frequency[2]. Haemochromatosis that is unrelated to mutations in the HFE gene are collectively referred to as non-HFE haemochromatosis. Non-HFE haemochromatosis occurs in populations world wide and makes up a larger proportion of HH cases in areas where the C282Y mutation is less common, such as Southern Europe[3] and Asia[4]. Non-HFE HH can be further differentiated according to the gene mutated. There are four main types of non-HFE HH. The molecules mutated in all forms of HH are related in pathways involved in the regulation of iron homeostasis. Hepcidin the central regulator of iron homeostasis and hemojuvelin are mutated in juvenile or type 2 HH[5,6]. Transferrin Receptor 2 is mutated in type 3 HH[7] and the iron exporter ferroportin is mutated in the autosomal dominant type 4 HH or ferroportin disease[8,9]. The genetic, clinical and laboratory features of the various types of HH are outlined in Table 1. This review will describe in detail the four main types of non-HFE HH and review the current literature in this area.
| HHType | Gene | Inheritance | Clinical features | Laboratoryfindings | Liver pathology | Functional consequences ofmutations |
| 1 | HFE | Autosomal recessive | May include: fatigue, lethargy, arthropathy, skin pigmentation, liver damage, diabetes mellitus, endocrine dysfunction, cardiomyopathy, hypogonadotropic hypogonadism | ↑ serum ferritin, ↑ transferrin saturation | Hepatocyte iron loading, fibrosis, cirrhosis | Impaired hepcidin regulation by iron, leading to increased intestinal iron absorption and release of iron from reticuloendothelial cells |
| 2A | Hemojuvelin(HJV) | Autosomal recessive | As for HFE. Earlier onset (< 30 yr). Cardiomyopathy and hypogonadism more prevalent. | ↑ serum ferritin, ↑ transferrin saturation | Hepatocyte iron loading, fibrosis, cirrhosis | Loss of hepcidin regulation, leading to increased intestinal iron absorption and release of iron from reticuloendothelial cells |
| 2B | Hepcidin(HAMP) | Autosomal recessive | As for HFE. Earlier onset (< 30 yr). Cardiomyopathy and hypogonadism more prevalent. | ↑ serum ferritin, ↑ transferrin saturation | Hepatocyte iron loading, fibrosis, cirrhosis | No/inactive hepcidin, leading to maximal iron absorption and release of iron from reticuloendothelial cells |
| 3 | TransferrinReceptor 2(TfR2) | Autosomal recessive | As for HFE. | ↑ serum ferritin, ↑ transferrin saturation | Hepatocyte iron loading, fibrosis, cirrhosis | Impaired hepcidin regulation by iron, leading to increased intestinal iron absorption and release of iron from reticuloendothelial cells |
| 4 | Ferroportin(Fpn), SLC40A1, IREG1, MTP1 | Autosomal dominant | Typical presentation: as for HFE, except generally milder. May have mild anaemia and lower tolerance to venesection. | ↑↑ serum ferritin, normal transferrin saturation | Predominant Kupffer cell iron loading, fibrosis | Reduced ferroportin iron transport ability, leading to accumulation of iron in reticuloendothelial cells |
| Atypical: as for HFE | ↑ serum ferritin, ↑ transferrin saturation | Predominant hepatocyte iron loading, fibrosis, cirrhosis | Loss of ferroportin regulation by hepcidin, leading to increased intestinal iron absorption and release of iron from reticuloendothelial cells |
An early onset form of juvenile haemochromatosis (JH), distinct from the typical HFE-HH has been recognised for some time[10]. As with HFE-HH, JH or type 2 HH is an autosomal recessive disorder, and is characterised by elevated serum iron indices and iron deposition in parenchymal cells. JH usually presents before the age of 30 years and has a more rapid and severe course than HFE-HH. Unlike HFE-HH both sexes are affected equally[10]. Cardiomyopathy and hypogonadism are more prominent features of JH, hypogonadism being the most common symptom at presentation[11]. The rapid accumulation of iron in patients with JH can often be fatal, death usually resulting from heart failure[11].
When the HFE gene was identified in 1996, it became apparent that JH was indeed a disorder genetically distinct the typical HFE-HH. Linkage to the HFE gene region on chromosome 6 was ruled out, in a study utilising microsatellite markers in five Italian JH families[12]. This was followed by a genome wide search that identified linkage to the chromosome 1q21 region in nine JH families. Subsequently a subset of families who did not have linkage to 1q21 were found to have mutations in the hepcidin (HAMP) gene on chromosome 19[5]. This subset of JH has been termed type 2B HH and is described in more detail later. The chromosome 1 form of JH has been termed type 2A HH.
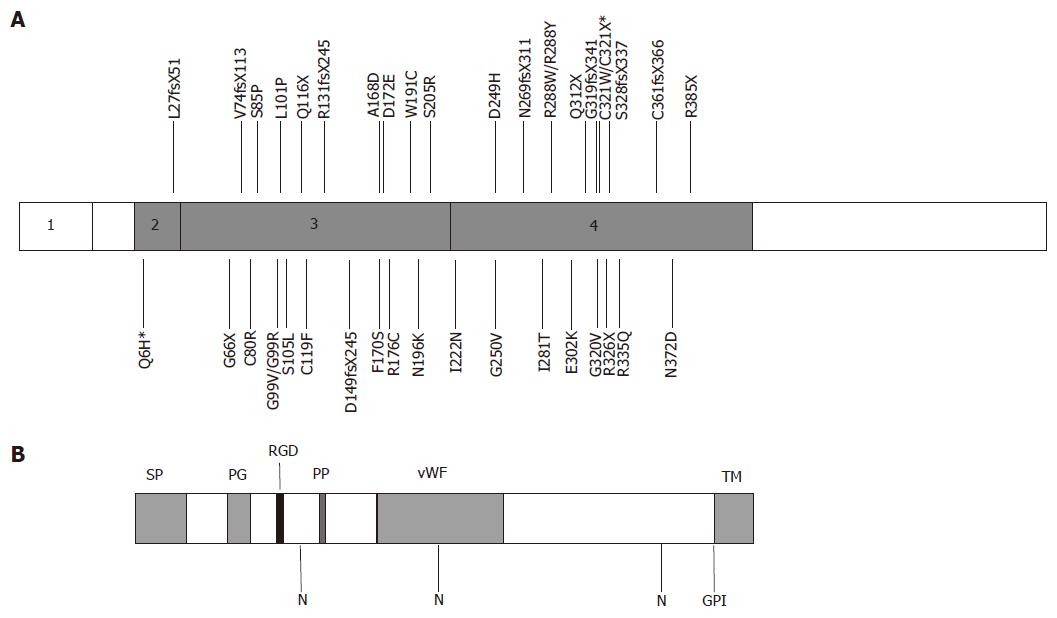
The gene responsible for the chromosome 1 form of JH was identified in 2004[6]. Fine mapping of the JH locus was performed in 12 JH families of Greek, Canadian and French origin. Sequencing of genes in this region revealed a novel gene that was mutated in all affected individuals[6]. The gene originally named HFE2 encodes hemojuvelin (HJV), a protein with homology to the repulsive guidance molecule (RGM) family of proteins. Six mutations were initially identified in the affected individuals either in the homozygous or compound heterozygous state. One mutation G320V was present in nine of the 12 families[6]. Since the identification of HJV, numerous mutations have been identified in JH families worldwide[6,13-24]. An Italian study identified 16 more mutations among 34 JH patients from various European backgrounds[13]. Figure 1 illustrates the structure of hemojuvelin and position of disease-causing mutations. Most mutations are private and were detected in single families. A few have been detected in more than one population. One mutation in particular (G320V) is significantly more frequent and has been reported in JH patients in many populations[6,13,16-20,24].
Patients with HJV-HH were shown to have low levels of urinary hepcidin[6]. This suggested that HJV may be involved in regulating hepcidin expression in response to iron. The generation of mouse knockouts confirmed that HJV was critical for the regulation of iron homeostasis and the induction of hepcidin[25,26]. Recent studies have suggested that HJV regulates hepcidin expression through signalling pathways involving bone morphogenic proteins (BMPs)[27].
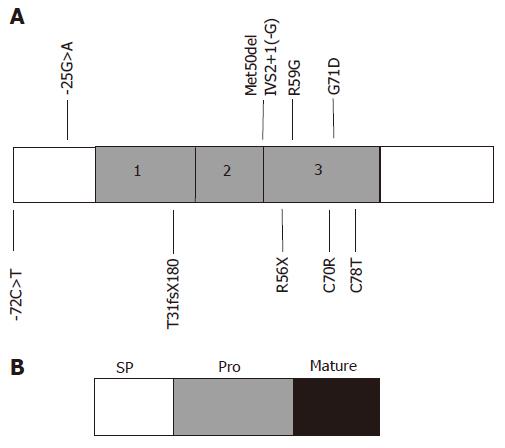
While most cases of juvenile haemochromatosis have been linked to a locus on the long arm of chromosome 1 (1q21) and mutations in HJV, a small number of families have been described with a JH-like disorder unlinked to 1q21[28]. This subset of patients was found to have mutations in the hepcidin (HAMP) gene. Two consanguineous families of Italian and Greek origin were originally reported with linkage to a region on chromosome 19, encompassing the region containing the hepcidin gene[5]. Homozygosity for mutations in the hepcidin coding sequence was detected in both families. One family harboured a one base pair deletion (93delG), causing a frameshift and an abnormal extended protein (T31fsX180). The other family carried a single base pair substitution (166C>T) causing the replacement of an arginine with a stop codon (R56X), in the predicted cleavage site for prohormone convertases. Both of these mutations severely affect the protein sequence and would result in the absence of any mature hepcidin peptide[5].
Hepcidin mutations remain a rare cause of JH. However, since the first report, other cases and mutations have been described[5,29-37]. Mutations described in the hepcidin gene are shown in Figure 2. These include mutations affecting two of the eight highly conserved cysteine residues (C70R and C78T), important for the complex disulphide bonded structure of mature hepcidin[33-35]. A mutation in the 5′UTR of the hepcidin mRNA (-25G>A) has been described in two Portuguese families[36,37]. This mutation creates a new initiation codon upstream from the original ATG. Measurement of urinary hepcidin in a patient homozygous for this mutation, suggests that steady state transcription of hepcidin from the original ATG codon does take place. But there is loss of upregulation of hepcidin transcription in response to iron[37]. In another study it was shown in vitro that the out of frame upstream initiation codon was functional and prevented normal transcription from the original ATG[38].
The remaining mutations in hepcidin have been detected in the heterozygous state in patients carrying HFE mutations. Merryweather-Clarke et al[29,30] described patients with haemochromatosis who carried mutations in both HFE and hepcidin. One patient carried a four base pair deletion in HAMP (Met50del IVS2+1(-G)) and had a JH-like phenotype. Another family carried the G71D mutation in combination with either heterozygous or homozygous C282Y, and adult-onset iron overload. This was the first description of iron overload due to digenic inheritance of mutations in two separate genes. Two studies have detected hepcidin mutations in large cohorts of patients with HFE-HH. Jacolot et al detected HAMP mutations in five individuals from a cohort of 392 C282Y homozygotes and found that these were among the more iron-loaded. In addition, four of 31 subjects with iron overload, but at least one chromosome lacking an HFE mutation also carried a HAMP mutation. This supports the concept that digenic inheritance of HFE and HAMP mutations can lead to iron overload. Biasiotto et al[30] also screened for hepcidin mutations in iron overload patients carrying the C282Y allele and detected sequence variations in some. They concluded that a novel substitution in the hepcidin promoter (-72C>T) may aggravate iron loading in patients with HFE mutations.
HJV mutations have also been detected in patients with HFE-HH. Two studies suggested that heterozygosity for HJV mutations may aggravate the phenotype in HFE-HH[30,39]. Le Gac et al[39] reported nine of 310 C282Y homozygous patients with additional HJV mutations. Eight of the nine patients appeared to have a more severe phenotype, suggesting that heterozygosity for mutations in HJV were having a modifying effect. A similar effect of HJV mutations was reported by Biasiotto et al[30], the N196K mutation being associated with abnormally high iron indices in a C282Y/H63D compound heterozygote. The effect of HJV mutations on phenotypic expression of HFE-HH is small, and has not been detected in all studies. Lee et al[24] did not detect any HJV mutations in a group of 49 C282Y homozygotes. Wallace et al[17] reported a G320V heterozygous relative of a JH patient, who was also a C282Y/H63D compound heterozygote, but with normal iron indices.
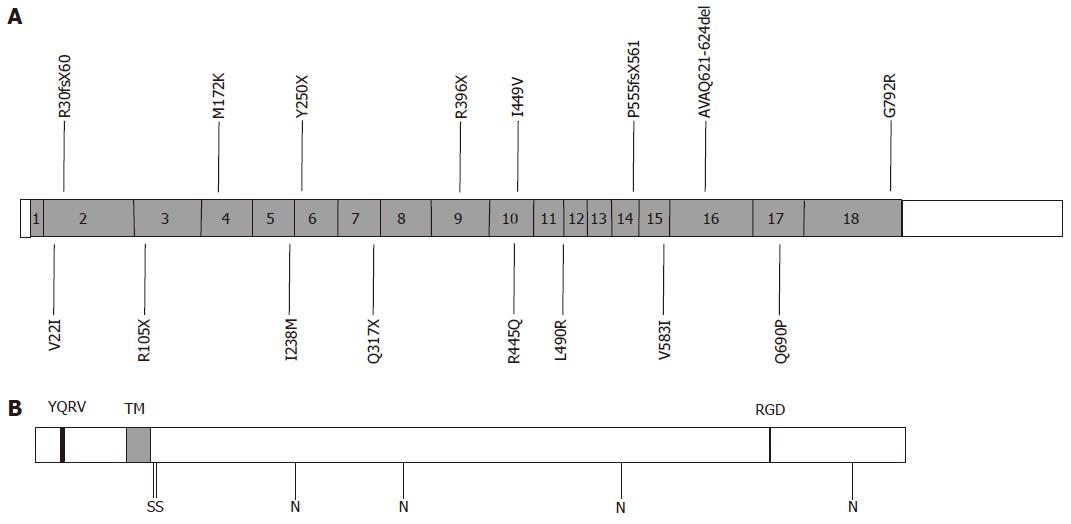
Transferrin Receptor 2 (TfR2)-HH was first described in 2000[7]. This was the first HH syndrome to be attributed to non-HFE mutations. Mutations in TfR2 were first detected in six members of two Sicilian families. The defect was linked to a region on the long arm of chromosome 7 (7q22), and affected individuals were found to be homozygous for a nonsense mutation (Y250X) in TfR2[7]. Affected individuals had iron overload with a similar phenotype to HFE-HH. TfR2-HH is a rare condition; however, several mutations have been reported worldwide associated with haemochromatosis[7,30,40-52]. Mutations reported in TfR2 are illustrated in Figure 3.
TfR2 is a homologue of the classical transferrin receptor (TfR1)[53], the molecule responsible for the uptake of transferrin-bound iron into cells. Unlike the ubiquitous expression of TfR1, TfR2 expression and activity is restricted almost exclusively to the liver[54]. Rather than being involved in the uptake of transferrin bound iron, it appears that the main function of TfR2 is as a sensor of iron levels and regulator of hepcidin. In both patients and animal models with TfR2-HH hepcidin levels are low in relation to iron stores[55-57].
The clinical features of TfR2-HH resemble that found in HFE-HH. Onset is usually in adulthood and is associated with increased serum iron indices and iron accumulation in parenchymal cells. Clinical features reported in patients with TfR2-HH include abnormal liver function, liver fibrosis, cirrhosis, arthritis, diabetes, hypogonadism, cardiomyopathy and skin pigmentation[41,44]. All of these features are typical of HFE-HH. A direct comparison of phenotype between HFE-HH and TfR2-HH is difficult, due to the low prevalence of TfR2 mutations and small number of reported cases. It appears, however, that TfR2-HH may have a more severe phenotype. Early onset of disease has been reported in a number of cases. Two adolescent siblings homozygous for the R105X mutation were reported with elevated transferrin saturation[51]. However, the serum ferritin in both cases was normal and liver biopsy was not performed. Two unrelated cases, presenting at ages 3 and 16 years were reported, homozygous for the Y250X mutation[40]. Both had raised serum iron, transferrin saturation and hepatic iron. The 16-year-old, who presented with fatigue, also had raised serum ferritin. Other cases suggestive of an earlier onset and more severe phenotype than HFE-HH have also been reported[42,44].
Pietrangelo et al[52] recently reported an unusual family with both adult and juvenile onset haemochromatosis. Two siblings presented in their early twenties with features typical of JH. These included hypogonadotropic hypogonadism, cardiomyopathy and cirrhosis. Both were found to be homozygous for a mutation Q317X in TfR2. In addition compound heterozygosity for HFE-C282Y/H63D was also present. A brother who had a less severe adult onset phenotype was homozygous for the Q317X TfR2 mutation, but had wild type HFE sequence. This is the first and only report of juvenile haemochromatosis due to mutations in two genes normally associated with adult-onset haemochromatosis[52]. This observation suggests that defects in either HFE or TfR2 can be compensated to some extent by the other. Homozygosity for mutations in either one can lead to appreciable iron overload with onset normally in adulthood. The combination of mutations in both genes has an additive effect on iron loading, leading to an earlier onset and JH-like phenotype. Both HFE and TfR2 are thought to regulate hepcidin expression in the liver through as yet unidentified signalling pathways. It is possible that HFE and TfR2 work through either parallel or converging signalling pathways resulting in the induction of hepcidin. This would explain why the loss of one can be compensated to some extent by the other. The loss of both, however, would lead to complete loss of regulation of hepcidin by iron, as occurs in JH. Further studies will be needed to clarify the relationship between HFE and TfR2, and the signalling pathways they are involved in.
Ferroportin disease differs from other genetic iron overload disorders in that it is inherited in an autosomal dominant pattern. An autosomal dominant form of haemochromatosis was first reported in 1990, in a Melanesian pedigree from the Solomon Islands[58]. In a large 96 member pedigree, 31 of 81 members tested were affected in at least three generations. All affected individuals had raised transferrin saturation and serum ferritin levels. Liver biopsies in 19 individuals showed a pattern of iron staining consistent with HFE-HH, with iron present in hepatocytes and Kupffer cells. Some degree of fibrosis or cirrhosis was present in the majority of cases. This study was performed before the identification of the HFE gene; hence, analysis of HFE mutations could not be performed. However, linkage to the HFE locus on chromosome 6 was excluded, by HLA typing of affected and non-affected family members[58]. Another large pedigree with multiple affected individuals, but without pathogenic mutations in the HFE gene was described in 1999[59]. This Italian family consisted of 53 living members, with 15 affected across three generations. Linkage to the HFE region on chromosome 6 was excluded by typing of microsatellite markers.
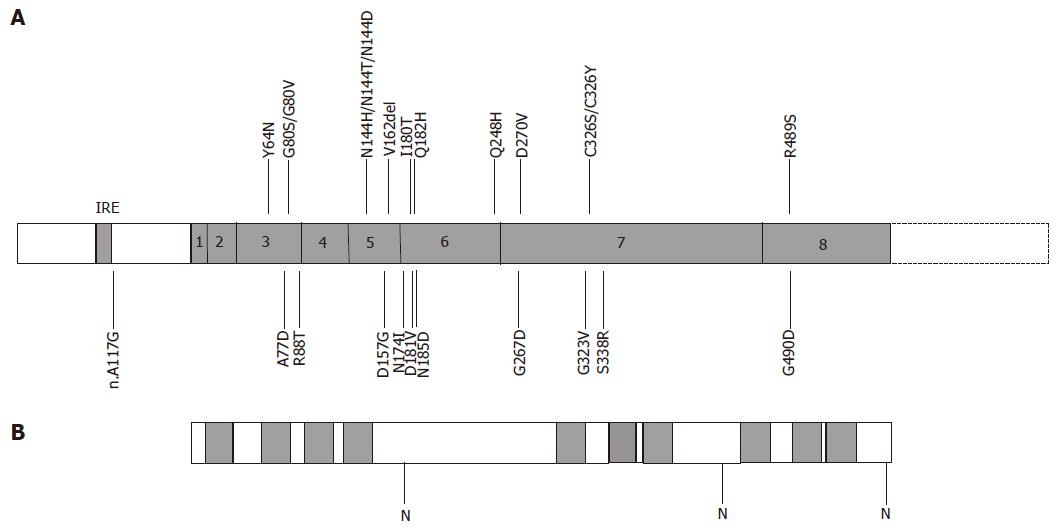
Mutations in ferroportin, associated with autosomal dominant haemochromatosis were first described in 2001. An asparagine to histidine mutation (N144H) was identified in a large multi-generation family from the Netherlands[9]. At the same time an alanine to aspartate mutation (A77D) was reported in the large Italian pedigree described previously[8]. Since these first reports, many more ferroportin mutations have been described in association with autosomal dominant haemochromatosis[60-85]. Figure 4 shows the mutations reported in the literature to date. Ferroportin mutations have been reported in populations throughout the world. Most of the reported ferroportin mutations are private and restricted to single families. Some mutations, however, are more common and have been reported in diverse populations. The most prevalent is the deletion of one of a group of three valine residues (V162del). This mutation has been reported in families from Australia, the UK, Italy, Greece, Sri Lanka and Austria[61-66]. It has been proposed that this mutation has occurred independently, several times, due to slippage mispairing in a repeat sequence. Other mutations reported in multiple populations include A77D (Italy and Australia)[8,60] and Q248H[75-79]. It is unclear whether Q248H is a mutation or polymorphism. It has been reported at high frequencies in African and African-American populations, in both controls and individuals with iron overload, where it can occur in the heterozygous or homozygous state. There is suggestive evidence that it may contribute to slightly higher serum ferritin levels[76-79], but this effect is very small compared to other ferroportin mutants. There are three mutations affecting asparagine 144, suggesting that this residue is important for the functioning of ferroportin. In the original Dutch family reported by Njajou et al[9], it was mutated to a histidine (N144H)[9], in an Australian family to an aspartate (N144D)[71] and in a Solomon Island patient to a threonine (N144T)[67]. Whether this mutation is responsible for the autosomal dominant Solomon Island iron overload syndrome reported by Eason et al[58], remains to be determined.
The phenotypic features of most cases of ferroportin disease differ significantly from that of HFE-HH. The typical features are an early elevation in serum ferritin, with normal transferrin saturation, and iron accumulation preferentially in the Kupffer cells of the liver. With increasing age, iron stores increase, and iron is seen in hepatocytes as well as Kupffer cells, and the transferrin saturation can be elevated. In these cases liver damage is minimal, with fibrosis occurring in some individuals. Venesection therapy is not always tolerated, with anaemia developing, especially in early cases when the transferrin saturation is low. It is now apparent that some cases of ferroportin disease differ from this typical pattern. A second atypical phenotype has been proposed, with features that more closely resemble HFE-HH. Atypical features include an early rise in transferrin saturation, and iron accumulation preferentially in hepatocytes, with some Kupffer cell iron apparent in some cases. Venesection therapy in these cases is usually tolerated well, but liver damage would appear to be more prevalent, with two reports of cirrhosis[71,80].
The heterogeneity of ferroportin disease has led to the suggestion that mutational differences account for the phenotypic variation observed in patients. In general each mutation can be classified as leading to either the typical or atypical ferroportin disease phenotype. It has been proposed that particular mutations affect the function of ferroportin in different ways. The ferroportin gene, also referred to as IREG1, MTP1, SLC11A3 and SLC40A1, encodes a multiple transmembrane domain iron transporter, highly expressed in duodenum, liver and reticuloendothelial cells. It is responsible for iron transport across the basolateral surface of enterocytes into the blood and recycling of iron in the reticuloendothelial system. Mutations such as A77D and V162del lead to the typical phenotype of reticuloendothelial iron storage, with relatively low transferrin saturation. A non-functional ferroportin molecule would be predicted to lead to this phenotype. Heterozygosity for a non-functional mutant would be predicted to lead to haploinsufficiency for ferroportin, with only half the amount of functional ferroportin present on the surface of cells at any one time. In the reticuloendothelial system, where the vast majority of daily iron turnover occurs, this would be predicted to cause a blockage in the release of iron back into the circulation. Hence, iron would accumulate in the reticuloendothelial macrophages, and serum iron concentrations would be relatively low. These low levels of circulating iron would in turn lead to an increase in iron absorption in the duodenum, possibly involving sensors in the liver, such as HFE and TfR2 and signalling via the hepcidin pathway. The turnover of iron in the reticuloendothelial system far outweighs that in the duodenum. Hence, the ferroportin required to transport iron across the basolateral surface of enterocytes would probably be sufficient to transport more iron into the body, even if a non-functional mutant was present. Over a long period of time body iron stores would increase to a point where the capacity of the reticuloendothelial cells to store iron would be reached, and iron would accumulate in parenchymal cells such as hepatocytes. This is seen in advanced typical ferroportin disease and is usually accompanied by an increase in the transferrin saturation.
It was recently reported that ferroportin expression on the cell surface can be regulated by hepcidin[86]. It was shown that hepcidin could bind to ferroportin on the cell surface and induce its internalisation and degradation. In this way hepcidin could rapidly reduce iron absorption in the intestine and release of iron from the reticuloendothelial system, resulting in a reduction in serum iron. It has been proposed that the mutations which cause the atypical ferroportin disease phenotype affect the ability of hepcidin to internalise ferroportin. Failure to internalise would lead to a permanently “switched on” ferroportin molecule. This would lead to increased iron absorption in the duodenum and release from reticuloendothelial cells, resulting in high serum iron levels and storage of iron in parenchymal cells. Having a permanently “switched on” ferroportin molecule would effectively be the same as having hepcidin deficiency, as they both result in the same end point. This explains why the atypical form of ferroportin disease phenotypically resembles other forms of haemochromatosis, which all result from hepcidin deficiency.
There are four main genes implicated in non-HFE haemochromatosis. Mutations in these genes occur in populations world wide and account for the majority of HH cases not linked to HFE. The study of these disorders has led to a greater understanding of how the body regulates iron homeostasis. All the genes implicated in the different forms of haemochromatosis are involved in the regulation and maintenance of iron homeostasis. Hepcidin is at the centre of the iron regulatory pathway. Its expression in the liver can be regulated by the activities of HFE, TfR2 and HJV. Hepcidin itself can regulate the activity of the iron exporter ferroportin. Mutations in any one of these genes can disrupt the regulation of iron homeostasis and lead to iron overload. Further study of these molecules, their relationships to each other, and signalling pathways they are involved in will further illuminate our understanding of iron metabolism and its regulation.
S- Editor Liu Y L-Editor Alpini GD E- Editor Li JL
| 1. | Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2676] [Cited by in RCA: 2551] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 2. | Merryweather-Clarke AT, Pointon JJ, Shearman JD, Robson KJ. Global prevalence of putative haemochromatosis mutations. J Med Genet. 1997;34:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 539] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Piperno A, Sampietro M, Pietrangelo A, Arosio C, Lupica L, Montosi G, Vergani A, Fraquelli M, Girelli D, Pasquero P. Heterogeneity of hemochromatosis in Italy. Gastroenterology. 1998;114:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Hayashi H, Wakusawa S, Motonishi S, Miyamoto K, Okada H, Inagaki Y, Ikeda T. Genetic background of primary iron overload syndromes in Japan. Intern Med. 2006;45:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 6. | Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dubé MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 663] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 7. | Camaschella C, Roetto A, Calì A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 548] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 8. | Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, Trenor CC, Gasparini P, Andrews NC, Pietrangelo A. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Njajou OT, Vaessen N, Joosse M, Berghuis B, van Dongen JW, Breuning MH, Snijders PJ, Rutten WP, Sandkuijl LA, Oostra BA. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 312] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Lamon JM, Marynick SP, Roseblatt R, Donnelly S. Idiopathic hemochromatosis in a young female. A case study and review of the syndrome in young people. Gastroenterology. 1979;76:178-183. [PubMed] |
| 11. | De Gobbi M, Roetto A, Piperno A, Mariani R, Alberti F, Papanikolaou G, Politou M, Lockitch G, Girelli D, Fargion S. Natural history of juvenile haemochromatosis. Br J Haematol. 2002;117:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Camaschella C, Roetto A, Cicilano M, Pasquero P, Bosio S, Gubetta L, Di Vito F, Girelli D, Totaro A, Carella M. Juvenile and adult hemochromatosis are distinct genetic disorders. Eur J Hum Genet. 1997;5:371-375. [PubMed] |
| 13. | Lanzara C, Roetto A, Daraio F, Rivard S, Ficarella R, Simard H, Cox TM, Cazzola M, Piperno A, Gimenez-Roqueplo AP. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood. 2004;103:4317-4321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Lee P, Promrat K, Mallette C, Flynn M, Beutler E. A juvenile hemochromatosis patient homozygous for a novel deletion of cDNA nucleotide 81 of hemojuvelin. Acta Haematol. 2006;115:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Jánosi A, Andrikovics H, Vas K, Bors A, Hubay M, Sápi Z, Tordai A. Homozygosity for a novel nonsense mutation (G66X) of the HJV gene causes severe juvenile hemochromatosis with fatal cardiomyopathy. Blood. 2005;105:432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Lee PL, Beutler E, Rao SV, Barton JC. Genetic abnormalities and juvenile hemochromatosis: mutations of the HJV gene encoding hemojuvelin. Blood. 2004;103:4669-4671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Wallace DF, Dixon JL, Ramm GA, Anderson GJ, Powell LW, Subramaniam N. Hemojuvelin (HJV)-associated hemochromatosis: analysis of HJV and HFE mutations and iron overload in three families. Haematologica. 2005;90:254-255. [PubMed] |
| 18. | Daraio F, Ryan E, Gleeson F, Roetto A, Crowe J, Camaschella C. Juvenile hemochromatosis due to G320V/Q116X compound heterozygosity of hemojuvelin in an Irish patient. Blood Cells Mol Dis. 2005;35:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Gehrke SG, Pietrangelo A, Kascák M, Braner A, Eisold M, Kulaksiz H, Herrmann T, Hebling U, Bents K, Gugler R. HJV gene mutations in European patients with juvenile hemochromatosis. Clin Genet. 2005;67:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Aguilar-Martinez P, Lok CY, Cunat S, Cadet E, Robson K, Rochette J. Juvenile hemochromatosis caused by a novel combination of hemojuvelin G320V/R176C mutations in a 5-year old girl. Haematologica. 2007;92:421-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Koyama C, Hayashi H, Wakusawa S, Ueno T, Yano M, Katano Y, Goto H, Kidokoro R. Three patients with middle-age-onset hemochromatosis caused by novel mutations in the hemojuvelin gene. J Hepatol. 2005;43:740-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Huang FW, Rubio-Aliaga I, Kushner JP, Andrews NC, Fleming MD. Identification of a novel mutation (C321X) in HJV. Blood. 2004;104:2176-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Filali M, Le Jeunne C, Durand E, Grinda JM, Roetto A, Daraio F, Bruneval P, Jeunemaitre X, Gimenez-Roqueplo AP. Juvenile hemochromatosis HJV-related revealed by cardiogenic shock. Blood Cells Mol Dis. 2004;33:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lee PL, Barton JC, Brandhagen D, Beutler E. Hemojuvelin (HJV) mutations in persons of European, African-American and Asian ancestry with adult onset haemochromatosis. Br J Haematol. 2004;127:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 277] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA. 2006;103:10289-10293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Papanikolaou G, Papaioannou M, Politou M, Vavatsi N, Kioumi A, Tsiatsiou P, Marinaki P, Loukopoulos D, Christakis JI. Genetic heterogeneity underlies juvenile hemochromatosis phenotype: analysis of three families of northern Greek origin. Blood Cells Mol Dis. 2002;29:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Jacolot S, Le Gac G, Scotet V, Quere I, Mura C, Ferec C. HAMP as a modifier gene that increases the phenotypic expression of the HFE pC282Y homozygous genotype. Blood. 2004;103:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Biasiotto G, Roetto A, Daraio F, Polotti A, Gerardi GM, Girelli D, Cremonesi L, Arosio P, Camaschella C. Identification of new mutations of hepcidin and hemojuvelin in patients with HFE C282Y allele. Blood Cells Mol Dis. 2004;33:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Merryweather-Clarke AT, Cadet E, Bomford A, Capron D, Viprakasit V, Miller A, McHugh PJ, Chapman RW, Pointon JJ, Wimhurst VL. Digenic inheritance of mutations in HAMP and HFE results in different types of haemochromatosis. Hum Mol Genet. 2003;12:2241-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Biasiotto G, Belloli S, Ruggeri G, Zanella I, Gerardi G, Corrado M, Gobbi E, Albertini A, Arosio P. Identification of new mutations of the HFE, hepcidin, and transferrin receptor 2 genes by denaturing HPLC analysis of individuals with biochemical indications of iron overload. Clin Chem. 2003;49:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Delatycki MB, Allen KJ, Gow P, MacFarlane J, Radomski C, Thompson J, Hayden MR, Goldberg YP, Samuels ME. A homozygous HAMP mutation in a multiply consanguineous family with pseudo-dominant juvenile hemochromatosis. Clin Genet. 2004;65:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Majore S, Binni F, Pennese A, De Santis A, Crisi A, Grammatico P. HAMP gene mutation c.208T& gt; C (p.C70R) identified in an Italian patient with severe hereditary hemochromatosis. Hum Mutat. 2004;23:400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Roetto A, Daraio F, Porporato P, Caruso R, Cox TM, Cazzola M, Gasparini P, Piperno A, Camaschella C. Screening hepcidin for mutations in juvenile hemochromatosis: identification of a new mutation (C70R). Blood. 2004;103:2407-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Matthes T, Aguilar-Martinez P, Pizzi-Bosman L, Darbellay R, Rubbia-Brandt L, Giostra E, Michel M, Ganz T, Beris P. Severe hemochromatosis in a Portuguese family associated with a new mutation in the 5'-UTR of the HAMP gene. Blood. 2004;104:2181-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Porto G, Roetto A, Daraio F, Pinto JP, Almeida S, Bacelar C, Nemeth E, Ganz T, Camaschella C. A Portuguese patient homozygous for the -25G& gt; A mutation of the HAMP promoter shows evidence of steady-state transcription but fails to up-regulate hepcidin levels by iron. Blood. 2005;106:2922-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Rideau A, Mangeat B, Matthes T, Trono D, Beris P. Molecular mechanism of hepcidin deficiency in a patient with juvenile hemochromatosis. Haematologica. 2007;92:127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Le Gac G, Scotet V, Ka C, Gourlaouen I, Bryckaert L, Jacolot S, Mura C, Férec C. The recently identified type 2A juvenile haemochromatosis gene (HJV), a second candidate modifier of the C282Y homozygous phenotype. Hum Mol Genet. 2004;13:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Piperno A, Roetto A, Mariani R, Pelucchi S, Corengia C, Daraio F, Piga A, Garozzo G, Camaschella C. Homozygosity for transferrin receptor-2 Y250X mutation induces early iron overload. Haematologica. 2004;89:359-360. [PubMed] |
| 41. | Roetto A, Totaro A, Piperno A, Piga A, Longo F, Garozzo G, Calì A, De Gobbi M, Gasparini P, Camaschella C. New mutations inactivating transferrin receptor 2 in hemochromatosis type 3. Blood. 2001;97:2555-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Majore S, Milano F, Binni F, Stuppia L, Cerrone A, Tafuri A, De Bernardo C, Palka G, Grammatico P. Homozygous p.M172K mutation of the TFR2 gene in an Italian family with type 3 hereditary hemochromatosis and early onset iron overload. Haematologica. 2006;91:ECR33. [PubMed] |
| 43. | Riva A, Mariani R, Bovo G, Pelucchi S, Arosio C, Salvioni A, Vergani A, Piperno A. Type 3 hemochromatosis and beta-thalassemia trait. Eur J Haematol. 2004;72:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Girelli D, Bozzini C, Roetto A, Alberti F, Daraio F, Colombari R, Olivieri O, Corrocher R, Camaschella C. Clinical and pathologic findings in hemochromatosis type 3 due to a novel mutation in transferrin receptor 2 gene. Gastroenterology. 2002;122:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Hattori A, Wakusawa S, Hayashi H, Harashima A, Sanae F, Kawanaka M, Yamada G, Yano M, Yoshioka K. AVAQ 594-597 deletion of the TfR2 gene in a Japanese family with hemochromatosis. Hepatol Res. 2003;26:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Mattman A, Huntsman D, Lockitch G, Langlois S, Buskard N, Ralston D, Butterfield Y, Rodrigues P, Jones S, Porto G. Transferrin receptor 2 (TfR2) and HFE mutational analysis in non-C282Y iron overload: identification of a novel TfR2 mutation. Blood. 2002;100:1075-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Barton JC, Lee PL, West C, Bottomley SS. Iron overload and prolonged ingestion of iron supplements: clinical features and mutation analysis of hemochromatosis-associated genes in four cases. Am J Hematol. 2006;81:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Barton JC, Lee PL. Disparate phenotypic expression of ALAS2 R452H (nt 1407 G --& gt; A) in two brothers, one with severe sideroblastic anemia and iron overload, hepatic cirrhosis, and hepatocellular carcinoma. Blood Cells Mol Dis. 2006;36:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Lee PL, Barton JC. Hemochromatosis and severe iron overload associated with compound heterozygosity for TFR2 R455Q and two novel mutations TFR2 R396X and G792R. Acta Haematol. 2006;115:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Koyama C, Wakusawa S, Hayashi H, Suzuki R, Yano M, Yoshioka K, Kozuru M, Takayamam Y, Okada T, Mabuchi H. Two novel mutations, L490R and V561X, of the transferrin receptor 2 gene in Japanese patients with hemochromatosis. Haematologica. 2005;90:302-307. [PubMed] |
| 51. | Le Gac G, Mons F, Jacolot S, Scotet V, Férec C, Frébourg T. Early onset hereditary hemochromatosis resulting from a novel TFR2 gene nonsense mutation (R105X) in two siblings of north French descent. Br J Haematol. 2004;125:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Pietrangelo A, Caleffi A, Henrion J, Ferrara F, Corradini E, Kulaksiz H, Stremmel W, Andreone P, Garuti C. Juvenile hemochromatosis associated with pathogenic mutations of adult hemochromatosis genes. Gastroenterology. 2005;128:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826-20832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 54. | Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 56. | Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O'Kelly J, Umehara Y, Wano Y, Said JW, Koeffler HP. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Eason RJ, Adams PC, Aston CE, Searle J. Familial iron overload with possible autosomal dominant inheritance. Aust N Z J Med. 1990;20:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Pietrangelo A, Montosi G, Totaro A, Garuti C, Conte D, Cassanelli S, Fraquelli M, Sardini C, Vasta F, Gasparini P. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. N Engl J Med. 1999;341:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Subramaniam VN, Wallace DF, Dixon JL, Fletcher LM, Crawford DH. Ferroportin disease due to the A77D mutation in Australia. Gut. 2005;54:1048-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Wallace DF, Pedersen P, Dixon JL, Stephenson P, Searle JW, Powell LW, Subramaniam VN. Novel mutation in ferroportin1 is associated with autosomal dominant hemochromatosis. Blood. 2002;100:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Devalia V, Carter K, Walker AP, Perkins SJ, Worwood M, May A, Dooley JS. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3). Blood. 2002;100:695-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Roetto A, Merryweather-Clarke AT, Daraio F, Livesey K, Pointon JJ, Barbabietola G, Piga A, Mackie PH, Robson KJ, Camaschella C. A valine deletion of ferroportin 1: a common mutation in hemochromastosis type 4. Blood. 2002;100:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Cazzola M, Cremonesi L, Papaioannou M, Soriani N, Kioumi A, Charalambidou A, Paroni R, Romtsou K, Levi S, Ferrari M. Genetic hyperferritinaemia and reticuloendothelial iron overload associated with a three base pair deletion in the coding region of the ferroportin gene (SLC11A3). Br J Haematol. 2002;119:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Wallace DF, Browett P, Wong P, Kua H, Ameratunga R, Subramaniam VN. Identification of ferroportin disease in the Indian subcontinent. Gut. 2005;54:567-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Zoller H, McFarlane I, Theurl I, Stadlmann S, Nemeth E, Oxley D, Ganz T, Halsall DJ, Cox TM, Vogel W. Primary iron overload with inappropriate hepcidin expression in V162del ferroportin disease. Hepatology. 2005;42:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Arden KE, Wallace DF, Dixon JL, Summerville L, Searle JW, Anderson GJ, Ramm GA, Powell LW, Subramaniam VN. A novel mutation in ferroportin1 is associated with haemochromatosis in a Solomon Islands patient. Gut. 2003;52:1215-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Jouanolle AM, Douabin-Gicquel V, Halimi C, Loréal O, Fergelot P, Delacour T, de Lajarte-Thirouard AS, Turlin B, Le Gall JY, Cadet E. Novel mutation in ferroportin 1 gene is associated with autosomal dominant iron overload. J Hepatol. 2003;39:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Rivard SR, Lanzara C, Grimard D, Carella M, Simard H, Ficarella R, Simard R, D'Adamo AP, De Braekeleer M, Gasparini P. Autosomal dominant reticuloendothelial iron overload (HFE type 4) due to a new missense mutation in the FERROPORTIN 1 gene (SLC11A3) in a large French-Canadian family. Haematologica. 2003;88:824-826. [PubMed] |
| 70. | Hetet G, Devaux I, Soufir N, Grandchamp B, Beaumont C. Molecular analyses of patients with hyperferritinemia and normal serum iron values reveal both L ferritin IRE and 3 new ferroportin (slc11A3) mutations. Blood. 2003;102:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Wallace DF, Clark RM, Harley HA, Subramaniam VN. Autosomal dominant iron overload due to a novel mutation of ferroportin1 associated with parenchymal iron loading and cirrhosis. J Hepatol. 2004;40:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Robson KJ, Merryweather-Clarke AT, Cadet E, Viprakasit V, Zaahl MG, Pointon JJ, Weatherall DJ, Rochette J. Recent advances in understanding haemochromatosis: a transition state. J Med Genet. 2004;41:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Zaahl MG, Merryweather-Clarke AT, Kotze MJ, van der Merwe S, Warnich L, Robson KJ. Analysis of genes implicated in iron regulation in individuals presenting with primary iron overload. Hum Genet. 2004;115:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Corradini E, Montosi G, Ferrara F, Caleffi A, Pignatti E, Barelli S, Garuti C, Pietrangelo A. Lack of enterocyte iron accumulation in the ferroportin disease. Blood Cells Mol Dis. 2005;35:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Barton JC, Acton RT, Rivers CA, Bertoli LF, Gelbart T, West C, Beutler E. Genotypic and phenotypic heterogeneity of African Americans with primary iron overload. Blood Cells Mol Dis. 2003;31:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Gordeuk VR, Caleffi A, Corradini E, Ferrara F, Jones RA, Castro O, Onyekwere O, Kittles R, Pignatti E, Montosi G. Iron overload in Africans and African-Americans and a common mutation in the SCL40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Beutler E, Barton JC, Felitti VJ, Gelbart T, West C, Lee PL, Waalen J, Vulpe C. Ferroportin 1 (SCL40A1) variant associated with iron overload in African-Americans. Blood Cells Mol Dis. 2003;31:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | McNamara L, Gordeuk VR, MacPhail AP. Ferroportin (Q248H) mutations in African families with dietary iron overload. J Gastroenterol Hepatol. 2005;20:1855-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Kasvosve I, Gomo ZA, Nathoo KJ, Matibe P, Mudenge B, Loyevsky M, Gordeuk VR. Effect of ferroportin Q248H polymorphism on iron status in African children. Am J Clin Nutr. 2005;82:1102-1106. [PubMed] |
| 80. | Sham RL, Phatak PD, West C, Lee P, Andrews C, Beutler E. Autosomal dominant hereditary hemochromatosis associated with a novel ferroportin mutation and unique clinical features. Blood Cells Mol Dis. 2005;34:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Liu W, Shimomura S, Imanishi H, Iwamoto Y, Ikeda N, Saito M, Ohno M, Hara N, Yamamoto T, Nakamura H. Hemochromatosis with mutation of the ferroportin 1 (IREG1) gene. Intern Med. 2005;44:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Cremonesi L, Forni GL, Soriani N, Lamagna M, Fermo I, Daraio F, Galli A, Pietra D, Malcovati L, Ferrari M. Genetic and clinical heterogeneity of ferroportin disease. Br J Haematol. 2005;131:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Koyama C, Wakusawa S, Hayashi H, Ueno T, Suzuki R, Yano M, Saito H, Okazaki T. A Japanese family with ferroportin disease caused by a novel mutation of SLC40A1 gene: hyperferritinemia associated with a relatively low transferrin saturation of iron. Intern Med. 2005;44:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Bach V, Remacha A, Altés A, Barceló MJ, Molina MA, Baiget M. Autosomal dominant hereditary hemochromatosis associated with two novel Ferroportin 1 mutations in Spain. Blood Cells Mol Dis. 2006;36:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Morris TJ, Litvinova MM, Ralston D, Mattman A, Holmes D, Lockitch G. A novel ferroportin mutation in a Canadian family with autosomal dominant hemochromatosis. Blood Cells Mol Dis. 2005;35:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3538] [Article Influence: 168.5] [Reference Citation Analysis (0)] |