Published online Sep 7, 2007. doi: 10.3748/wjg.v13.i33.4523
Revised: June 12, 2007
Accepted: June 23, 2007
Published online: September 7, 2007
Hepatocellular Carcinoma (HCC) is a common malignancy worldwide. While bleeding from the gastrointestinal tract (BGIT) has a well known association with HCC, such cases are mainly due to gastric and esophageal varices. BGIT as a result of invasion of the gastrointestinal tract by HCC is extremely rare and is reportedly associated with very poor prognosis. We describe a 67-year-old male who presented with BGIT. Endoscopy showed the site of bleeding to be from a gastric ulcer, but endoscopic therapy failed to control the bleeding and emergency surgery was required. At surgery, the ulcer was found to have arisen from direct invasion of the gastrointestinal tract by HCC of the left lobe. Control of the bleeding was achieved by surgical resection of the HCC en-bloc with the lesser curve of the stomach. The patient remains alive 33 mo after surgery. Direct invasion of the gastrointestinal tract by HCC giving rise to BGIT is very uncommon. Surgical resection may offer significantly better survival over non-surgical therapy, especially if the patient is a good surgical candidate and has adequate functional liver reserves. Prognosis is not uniformly grave.
- Citation: Ong JC, Chow PK, Chan WH, Chung AY, Thng CH, Wong WK. Hepatocellular carcinoma masquerading as a bleeding gastric ulcer: A case report and a review of the surgical management. World J Gastroenterol 2007; 13(33): 4523-4525
- URL: https://www.wjgnet.com/1007-9327/full/v13/i33/4523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i33.4523
Hepatocellular carcinoma (HCC) is an important malignancy and is responsible for more than 250 000 deaths worldwide each year[1]. Bleeding from the gastrointestinal tract (BGIT) is a common complication of HCC and a frequent cause of death from this disease. Most cases of BGIT are due to esophageal and gastric varices[2] and the rest are mainly due to peptic ulcers. However, invasion of the gastrointestinal tract by HCC, through metastasis or direct invasion, is found in only 4-12% of cases at autopsy[3-5,12]. These lesions tend to be asymptomatic and are thus mainly only discovered at post-mortem examination. Direct invasion of the stomach by HCC is extremely rare. A detailed literature review showed only 10 cases of HCC with direct or contiguous invasion of the stomach have been reported[2,6-12]. We describe the case of a 67-year-old Chinese man with this uncommon clinical entity, who presented with BGIT and was managed by surgical resection.
A 67-year-old Chinese man presented to the Accident and Emergency Unit with a 3 d history of non-specific epigastric pain associated with postural dizziness and exertional dyspnea. There was no history of significant alcohol consumption and his hepatitis B and C status were unknown. History was negative for malaena, hematemesis, and hematochezia. He had a history of one episode of mild hemoptysis subsequent to which he was diagnosed as having bronchietasis due to heavy smoking.
Physical examination revealed a cachectic patient who was alert but had marked pallor of the conjunctiva. He was mildly hypotensive with a supine blood pressure of 105/60 with no postural drop. His pulse rate was 78 beats per minute, and he was not tachypneic. Palpation of the abdomen revealed mild epigastric tenderness on deep palpation but no significant organomegaly. He did not exhibit any stigmata of chronic liver disease. Bowel sounds were normal as was the digital rectal examination. There was no clinical sign of cardiac failure.
Initial investigations showed a white cell count of 8.5 × 109 (normal range 4.0-10.0 × 109/L), hemoglobin of 4.2 g/dL (normal range 14.0-18.0 g/dL), platelet count of 274 × 109 (normal range 140-440 × 109) and amylase of 71 U/L (normal range 30-110 U/L), He was transfused with 3 units of packed red cells, and an urgent referral to the surgical service was made. Esophageal-gastroduodenoscopy and colonoscopy were suggested but the patient declined referral or admission and discharged himself from the hospital against medical advice.
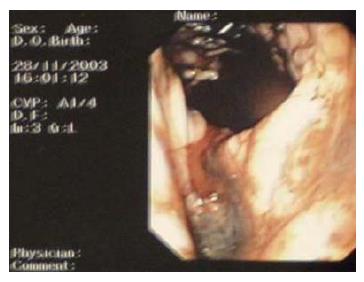
One month later, he presented himself at the outpatient specialist surgical clinic. Hemoglobin level on arrival was 9.4 g/dL. He finally consented to endoscopic examination as an outpatient procedure. Esophageal-gastroduodenoscopy was carried out on the same afternoon, during which an actively bleeding ulcer was seen in the lesser curve (Figure 1). Repeated injection with adrenaline failed to stop the bleeding and he was immediately transfused with packed cells and prepared for emergency surgery.
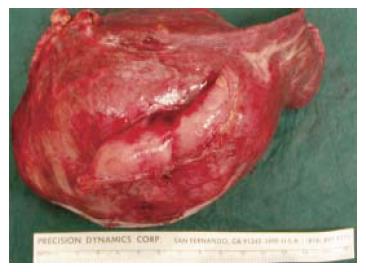
At celiotomy, a 10 cm tumor arising from segments II and III of the liver was found; the tumor involved the lesser curve of the stomach and had eroded into the lumen. On-table referral to the hepato-biliary surgical service was made. Resection of segments II and III of the liver with en-bloc wedge resection of the lesser curve of the stomach was carried out both to control the hemorrhage and as a definitive therapy for the tumor (Figure 2).
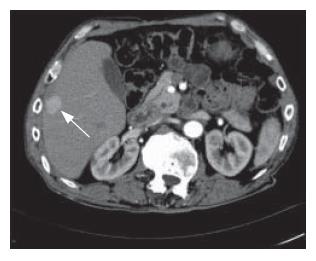
The patient’s post-operative recovery was uneventful. He was discharged from the surgical intensive care unit on the 2nd post-operative day. He tested positive for hepatitis B surface antigen and his serum hepatitis B antibody was found to be less than 10 IU/ml. The resected liver segments showed cirrhotic changes and a large necrotic tumor measuring 10 cm × 9 cm × 9 cm. Surgical margins were free of tumor. Histology revealed a trabecular hepatocellular carcinoma that was grade 2 by Edmondson’s grading. He was discharged from the hospital in good health on the 9th post-operative day. An early post-operative CT scan showed no residual tumor, but a repeat CT scan performed 9 mo later showed multiple new lesions of HCC in the liver (Figure 3). He opted for conservative management and was still alive 2 years and nine months after surgery.
HCC is a highly malignant type of tumor. Extrahepatic metastases occur in 30%-75% of patients, commonly affecting the lungs, regional lymph nodes, bones and adrenal glands[3]. Direct gastrointestinal tract involvement is rarely seen. In a clinical study, Chen et al[12] reported 8 out of 396 patients (2%) with HCC who developed gastrointestinal involvement during the course of the disease. Lin et al[2,6-12] similarly reported gastrointestinal metastases in 11 out of 2237 patients with HCC (0.5%). Only 10 cases of HCC invading directly into the stomach could be found in the literature. Eight of these presented with BGIT, and only one of the 10 subsequently underwent surgical resection of the HCC.
Of these 10 cases reported[2,6-12], 6 had received some form of regional therapy, such as trans-arterial chemoembolization (TACE), intra-arterial chemotherapy or radiotherapy, either alone or in combination, prior to the HCC invading the gastro-intestinal tract. This includes 4 of the 5 patients with direct invasion of the gastrointestinal tract by contiguous HCC reported by Chen et al[12]. Chen et al[12] postulated a relationship between regional therapy and the development of direct invasion of the gastrointestinal tract by HCC. It was proposed that when a large, subcapsular, massive-type HCC adjacent to the gastrointestinal tract is treated with TACE, the wall of the gastrointestinal tract could be affected by the inflammatory response secondary to TACE and become adherent to the tumor capsule. Viable tumor tissue could then invade the GI tract.
Our patient presented with non-specific symptoms and only a very low hemoglobin level, suggesting gastrointestinal bleeding. He was not known to be a hepatitis B carrier or a patient with HCC at initial presentation and, thus had no history of regional therapy for HCC. However the resected tumor was large, measuring 10 cm × 9 cm × 9 cm. In the published literature, of the 19 cases of HCC with gastrointestinal tract involvement (both direct invasion and distant metastasis) reported by Lin et al[7] and Chen et al[12], 14 tumors were considered to be large and 17 were larger than 6 cm, with the tumor sizes of the remaining 2 cases not being described. A casual relationship between tumor size and the probability of direct invasion to the surrounding viscera should therefore be considered.
In previously reported cases of HCC with gastro-intestinal tract involvement, treatments have included surgery, TACE and local injection with ethanol. Results of treatment have been poor, with almost all patients dying within 5 mo except for 1 case described by Nicoll et al[11]. In this reported case, the HCC invaded the stomach and the patient was treated with surgical resection. He was reported to be still alive 7 mo after resection. Our patient similarly had surgical resection with clear margins and remains alive 33 mo after surgery. Thus, such contiguous invasion of the stomach does not always represent terminal disease. Surgery should be considered wherever feasible as other modalities of treatment for HCC, including chemotherapy, are poorly efficacious[13].
Large HCCs may have a predisposition towards contiguous involvement of the gastro-intestinal tract, including the stomach. Diagnosis is difficult as most patients are asymptomatic and diagnosis by endoscopy is rarely achieved as these lesions have no special or characteristic endoscopic features. In our patient, diagnosis was made during emergency surgery to control bleeding from a supposed gastric ulcer. Based on limited case reports, the accepted paradigm is that an HCC directly invading the gastrointestinal tract is associated with very poor prognosis and that it may not be worthwhile to pursue an aggressive treatment policy. However, the scientific basis of this belief is limited, and this case report shows that aggressive resection can both resolve the acute clinical problem (gastrointestinal bleeding) as well as result in a relatively long-term survival. Surgery remains a viable option in patients with HCC and presenting with bleeding from the upper gastrointestinal tract as a result of direct invasion by a tumor.
Invasion of the upper gastrointestinal tract by HCC is extremely uncommon. In our case, the patient did not present with any signs and symptoms of chronic liver disease or hepatomegaly suggestive of hepatocellular carcinoma. The discovery of hepatocellular carcinoma invading directly into the stomach was an incidental finding from laparotomy performed for a bleeding peptic ulcer. Resection of segments II and III of the liver with en-bloc wedge resection of the lesser curve of the stomach was performed as a definitive procedure. This report demonstrates that the prognosis is not necessarily dismal in such cases if there are no distant metastases. Surgical resection may offer a significantly better survival over non-surgical therapy, especially if the patient is a good surgical candidate and has adequate functional liver reserves.
S- Editor Liu Y L- Editor McGowan D E- Editor Yin DH
| 1. | Ramsey WH, Wu GY. Hepatocellular carcinoma: update on diagnosis and treatment. Dig Dis. 1995;13:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Yeo W, Sung JY, Ward SC, Chung SC, Lee WY, Li AK, Johnson PJ. A prospective study of upper gastrointestinal hemorrhage in patients with hepatocellular carcinoma. Dig Dis Sci. 1995;40:2516-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Anthony PP. Primary carcinoma of the liver: a study of 282 cases in Ugandan Africans. J Pathol. 1973;110:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 126] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Primary liver cancer in Japan. The Liver Cancer Study Group of Japan. Cancer. 1984;54:1747-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Maruyama A, Murabayashi K, Hayashi M, Nakano H, Isaji S, Uehara S, Kusuda T, Miyahara S, Kondo A, Yabana T. Hepatocellular carcinoma complicated by gastrointestinal hemorrhage caused by direct tumor invasion of stomach. J Hepatobiliary Pancreat Surg. 1999;6:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lin CP, Cheng JS, Lai KH, Lo GH, Hsu PI, Chan HH, Hsu JH, Wang YY, Pan HB, Tseng HH. Gastrointestinal metastasis in hepatocellular carcinoma: radiological and endoscopic studies of 11 cases. J Gastroenterol Hepatol. 2000;15:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Srivastava DN, Gandhi D, Julka PK, Tandon RK. Gastrointestinal hemorrhage in hepatocellular carcinoma: management with transheptic arterioembolization. Abdom Imaging. 2000;25:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Shiota T, Watanabe A, Mitani K, Ito T, Tobe K, Nagashima H. Long-term survival in a case of hepatocellular carcinoma. Acta Med Okayama. 1983;37:73-78. [PubMed] |
| 10. | Takahashi M, Beppu T, Doi K, Ishiko T, Kai K, Doi Y, Okabe H, Egami H, Ashihara H, Fujiyama S. Multidisciplinary treatment for hepatocellular carcinoma invading the stomach. Gan To Kagaku Ryoho. 2003;30:1741-1744. [PubMed] |
| 11. | Nicoll AJ, Ireton HJ, Crotty B. Gastrointestinal bleeding from hepatocellular carcinoma invading the stomach. J Gastroenterol Hepatol. 1994;9:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Chen LT, Chen CY, Jan CM, Wang WM, Lan TS, Hsieh MY, Liu GC. Gastrointestinal tract involvement in hepatocellular carcinoma: clinical, radiological and endoscopic studies. Endoscopy. 1990;22:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40:1474-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |