INTRODUCTION
Cholangiocarcinoma (CC) accounts for 3% of all gastrointestinal cancers[1] and is the second commonest primary hepatic tumor[1,2]. It is characterized by the malignant proliferation of cholangiocytes that line intra-hepatic and extra-hepatic bile ducts and ductules. Biliary tumors are highly malignant and have a poor prognosis[3]. The incidence of intra-hepatic CC is rising in North America, Australia and Asia[2,4,5]. In addition to the well described risk factors, such as primary sclerosing cholangitis, liver fluke infestations or hepatolithiasis[6], recent studies suggest that chronic hepatitis B and C infections, HIV, non-alcoholic steatohepatitis, especially when combined with cirrhosis, also contribute to intra-hepatic CC risk[7]. On the other hand, the incidence of extra-hepatic CC is declining[2,4,5,8] most likely as a result of increasing rates of cholecystectomy over the past decades[2,5].
Treatment options for cholangiocarcinoma are limited. Unfortunately, the majority of patients suffer from advanced CC at presentation. Therefore, curative surgical resection or liver transplantation can only be offered to a minority of CC patients, leaving biliary drainage, radiotherapy or conventional chemotherapy as unsatisfactory palliative treatment options for advanced CC[6], with marginal effect on survival or quality of life[9].
Histone deacetylase (HDAC) inhibitors receive growing interest as cancer therapeutics due to their ability to induce cell differentiation, growth arrest and apoptosis[10]. Acetylation and deacetylation of histones play an important role in the regulation of gene transcription and in the modulation of chromatin structure[11,12]. The steady state of histone acetylation is tightly controlled by antagonistic effects of histone acetyltransferases (HAT) and HDAC. Aberrant gene expression resulting in functional inactivation of HAT activity or over-expression of HDAC can promote tumor cell proliferation and survival[13]. Moreover, deregulation of HDAC recruitment to transcriptional promoters is a mechanism by which these enzymes contribute to tumorigenesis[14].
HDAC inhibitor monotherapy can inhibit the growth of various tumors in vitro and in vivo[11,15,17]. Importantly, HDAC inhibitors are relatively non-toxic to non-transformed cells[18,19], leading to their evaluation in phaseI/II clinical cancer trials[14,15,20].
The synthetic orally available HDAC inhibitor, MS-275, potently inhibits histone deacetylases of several human tumor cells[21]. With a benzamide backbone, MS-275 is structurally unrelated to previous HDAC inhibitors, while showing a 30-fold stronger HDAC inhibitory activity than other natural HDAC inhibitors like sodium butyrate[22]. Recently, we and others demonstrated strong anti-proliferative activity of MS-275 towards several human cancer cells in vitro and in vivo[21,23,24]. MS-275 has now entered clinical trials both for single and combination therapy in solid and haematological malignancies. Since HDAC inhibition has not yet been evaluated for its anti-neoplastic effects on cholangiocarcinoma, we characterized the anti-neoplastic potency of the HDAC inhibitor MS-275 in human CC cells. We showed that MS-275 potently inhibited growth of CC cells, especially in combination with conventional cytostatic drugs or new, targeted anticancer agents, such as sorafenib (NexavarTM) or bortezomib (VelcadeTM). Furthermore, we provided an insight into major underlying mechanisms of MS-275-induced growth inhibition of CC cells.
MATERIALS AND METHODS
Cell lines and drugs
The poorly differentiated human bile duct adenocarcinoma cell line EGI-1[25] (DSMZ # ACC385) and the human papillary bile duct adenocarcinoma cell line TFK-1[26] (DSMZ # ACC344) were derived from patient cells prior to any exposure to chemotherapy or radiotherapy. Both cell lines were cultured in RPMI 1640 medium supplemented with 100 mL/L fetal calf serum (FCS), 100 kU/L penicillin and 100 mg/L streptomycin (Biochrom, Berlin, Germany) and kept at 37°C in a humidified atmosphere containing 50 mL/L CO2 in air.
MS-275 (N-(2-Aminophenyl)-4-[N-(3-pyridineyl-methoxycarbonyl)aminomethyl]-benzamide) was purchased from ALEXIS Biochemicals (Lausen, Switzerland). The 26S proteasome inhibitor bortezomib (VelcadeTM) was bought from Millennium Pharmaceuticals, Inc. (Cambridge, MA, USA). The multi-kinase inhibitor sorafenib tosylate (NexavarTM) was a kind gift from Bayer Health Care (West Haven, CT, USA). Stock solutions were prepared in dimethyl sulfoxide (DMSO) and stored at -20°C until use. Gemcitabine hydrochloride (GemzarTM) was bought from Lilly Pharma (Gieβen, Germany). Doxorubicin hydrochloride was from Sigma (Deisenhofen, Germany) and also prepared in DMSO and stored at -20°C. All drugs were diluted in fresh medium before each experiment. In all experiments, the final DMSO concentration was ≤ 5 g/L, not affecting cell growth. To evaluate the effects of the drugs, cells were incubated with either control medium or medium containing rising concentrations of the respective drug(s). Cell culture material was from Biochrom (Berlin, Germany). All other chemicals were from Sigma if not stated otherwise.
Measurement of growth inhibition
Drug-induced changes in cell numbers of EGI-1 and TFK-1 cells were evaluated by crystal violet staining as previously described[27]. In brief, cells in 96-well microtiter plates were fixed with 10 g/L glutaraldehyde. Then cells were stained with 1 g/L crystal violet in phosphate buffer solution (PBS). The unbound dye was removed by washing with water. Bound crystal violet was solubilized with 2 mL/L Triton-X-100 in PBS. Light extinction which increases linearly with the cell number was analyzed at 570 nm using an ELISA-Reader. To check for possible overadditive anti-proliferative effects, combination treatments of MS-275 plus conventional cytostatics (gemcitabine or doxorubicin) or plus sorafenib or plus bortezomib were performed. Increasing concentrations of the respective drug were combined with 0.25 and/or 0.5 μmol/L MS-275. The anti-neoplastic effects of the combination therapies were compared to those of each drug alone. Concentration range and effectiveness of the respective drugs have been determined in prior experiments.
Determination of cytotoxicity
Cells were seeded at a density of 8000 cells/well into 96-well microtiter plates and incubated with rising concentrations of MS-275 for 1, 2.5, 5 or 24 h. Release of the cytoplasmic enzyme lactate dehydrogenase (LDH), indicating cytotoxicity, was measured by using a colorimetric kit from Roche Diagnostics (Mannheim, Germany) as described elsewhere[28].
Detection of apoptosis
Preparation of cell lysates and determination of caspase-3 activity were performed as described previously[27]. The activity of caspase-3 was calculated from cleavage of the fluorogenic substrate Ac-DEVD-AMC (Calbiochem, Bad Soden, Germany). Cell lysates were incubated with substrate solution (caspase-3 substrate Ac-DEVD-AMC 20 mg/L, HEPES 20 mmol/L, glycerol 100 mL/L, DTT 2 mmol/L, pH 7.5) for 1 h at 37°C, and substrate cleavage was measured with a VersaFluor fluorometer (excitation: 360 nm emission: 460 nm) from Biorad (Munich, Germany).
Cell cycle analysis
Cell cycle analysis was performed by the method of Vindelov and Christensen as described previously[29]. Cells were trypsinized, washed and the nuclei were isolated using the CycleTest PLUS DNA Reagent Kit (Becton Dickinson, Heidelberg, Germany). DNA was stained with propidium iodide according to the manufacturers’ instructions. The DNA content of the nuclei was measured by flow cytometry and analyzed using ModFit software (Becton Dickinson, Heidelberg, Germany).
Western blot analysis
Western blotting was performed as described previously[30]. In brief, whole-cell extracts were prepared by lysing cells. Lysates containing 30 μg protein were subjected to gel electrophoresis. Proteins were then transferred onto PVDF membranes by electroblotting for 90 min. Blots were blocked with 50 g/L non-fat dry milk in TBS-Tween-solution for 1 h at room temperature, and then incubated at 4°C overnight with antibodies directed against anti-human Bax (1:1000), p27Kip1 (1:200) (both from Santa Cruz Biotechnology, CA, USA), Bcl-2 (1:1000), or p21Waf-1/CIP1 (1:1000) (both from Cell Signaling, MA USA). Anti-β-actin (1:5000) from Sigma (Deisenhofen, Germany) served as loading control. After incubation with horseradish peroxidase-coupled anti-IgG antibodies (1:10 000, GE Healthcare, Uppsala, Sweden) at room temperature for at least 1 h, the blot was developed using enhanced chemiluminescent detection (GE Healthcare) and subsequently exposed to Hyperfilm ECL film (GE Healthcare, Uppsala, Sweden).
Statistical analysis
If not stated otherwise, data were expressed as means of at least three independent experiments ± SEM. Significance between controls and treated samples was calculated by Student’s two-sided t-test. Caspase-3 measurements were evaluated using the two-sided Welch t-test. P < 0.05 was considered statistically significant.
RESULTS
Growth inhibitory effects of the HDAC inhibitor MS-275
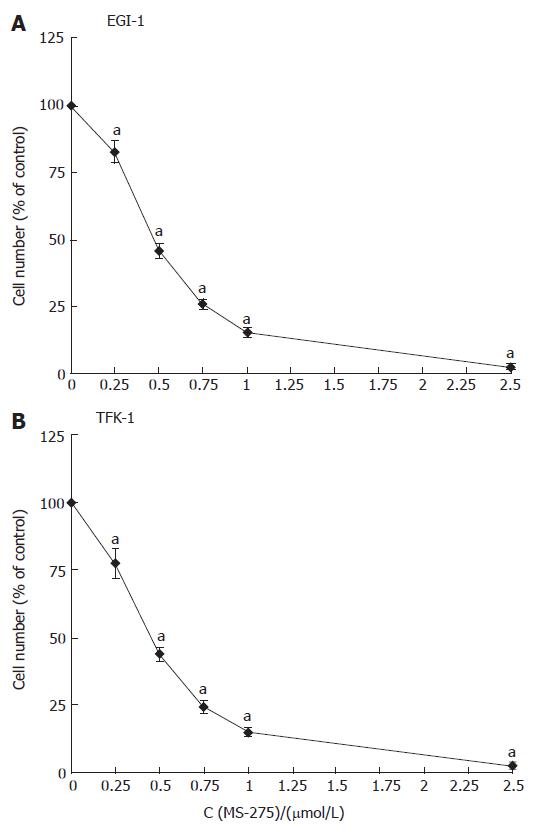
The growth inhibitory effects of MS-275 on CC cells were studied by crystal violet staining. Treating EGI-1 and TFK-1 cells with 0.25-2.5 μmol/L MS-275 for 3 d reduced the growth of both cell lines in a dose-dependent manner by up to 100% (Figure 1). The IC50 value of MS-275, determined after 72 h of incubation, was 0.48 ± 0.02 μmol/L for EGI-1 cells and 0.46 ± 0.02 μmol/L for TFK-1 cells. Determination of growth inhibition after 24 h and 48 h of incubation revealed that MS-275 inhibited cell proliferation in a time-dependent manner (data not shown).
Figure 1 Anti-proliferative effects of MS-275 on human EGI-1 and TFK-1 cholangiocarcinoma cells.
mean ± SEM, n > 5, aP < 0.05 vs controls.
LDH release after MS-275 treatment
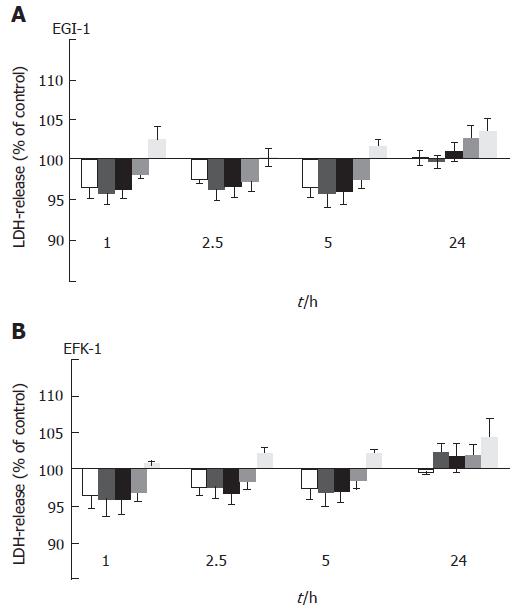
Cytotoxicity was evaluated by LDH release of the cells into the culture medium. Incubating EGI-1 and TFK-1 cells with 0.1-1 μmol/L MS-275 for up to 24 h did not result in a significant increase of LDH release (Figure 2), indicating that MS-275 does not directly affect cell membrane integrity and does not have immediate toxic effects even at higher concentrations.
Figure 2 MS-275-induced LDH release into the supernatant of EGI-1 and TFK-1 cells.
mean ± SEM, n > 3 independent experiments; No significant increase in LDH release was observed.
Induction of apoptosis by MS-275
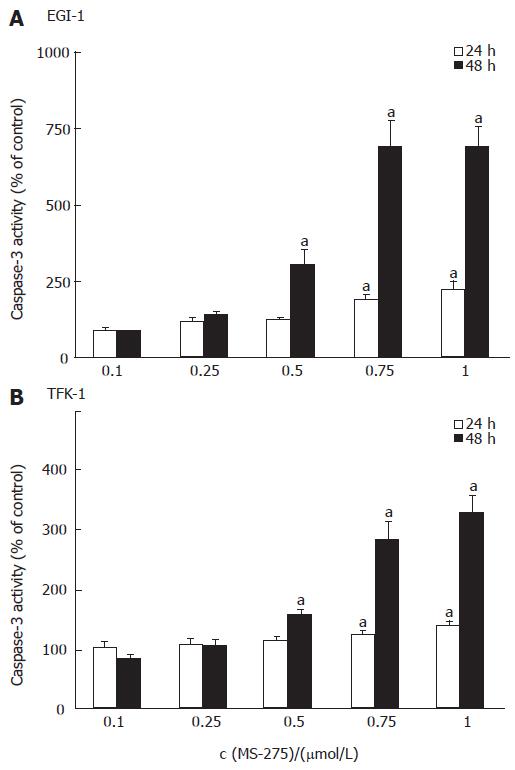
To determine the contribution of apoptosis to the observed growth inhibitory effect of MS-275, the activation of caspase-3, one of the key enzymes in the apoptotic signaling cascade, was determined after 24 h and 48 h of incubation. In both cell lines, HDAC inhibition by MS-275 resulted in a time- and dose-dependent increase of caspase-3 enzyme activity (Figure 3). After 48 h, 1 μmol/L MS-275 led to a 3-fold increase of caspase-3 activity in TFK-1 cells and even 7-fold increase in EGI-1 cells.
Figure 3 MS-275-induced apoptosis-specific caspase-3 activity increase in EGI-1 and TFK-1 cells.
mean ± SEM, n > 3 independent experiments, aP < 0.05 vs controls.
Cell cycle regulation
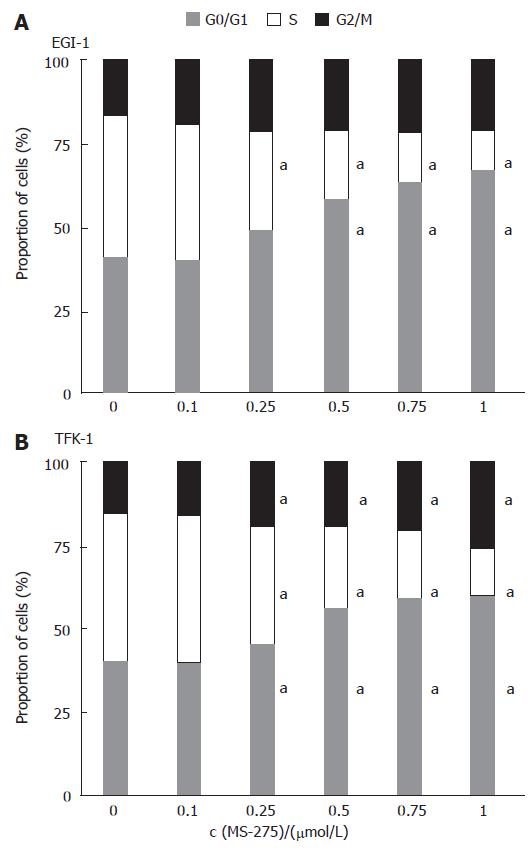
To test whether an induction of cell cycle arrest contributed to the antiproliferative potency of MS-275 in cholangiocarcinoma cells, we performed flow cytometric cell cycle analyses. Incubating EGI-1 and TFK-1 cells with 0.1-1 μmol/L MS-275 for 24 h resulted in a dose-dependent arrest in the G0/G1 phase of the cell cycle, whereas the proportion of cells in the S phase significantly decreased (Figure 4). The G0/G1-phase arrest by MS-275 was significant above concentrations of 0.1 µmol/L in TFK-1 and 0.25 μmol/L in EGI-1 cells. Moreover, the proportion of cells in the G2/M phase also increased, indicating an additional block in the G2/M phase. The G2/M-phase arrest was more pronounced in TFK-1 cells.
Figure 4 Induction of cell cycle arrest by MS-275 on EGI-1 and TFK-1 cells.
means of > 3 independent experiments, aP < 0.05 vs controls.
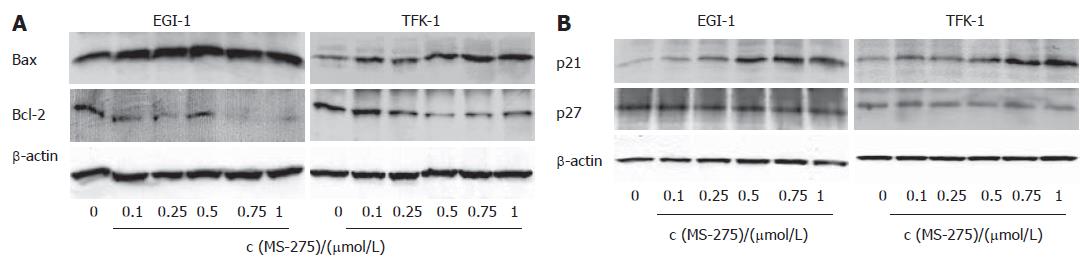
Bax, Bcl-2, p21Waf-1/CIP1 and p27Kip1 expression
To further characterize the apoptotic and cell cycle arresting effects of the HDAC inhibition, we performed Western blotting. Treating EGI-1 and TFK-1 cells for 48 h with MS-275 (0.1-1 μmol/L) resulted in a dose-dependent decrease of anti-apoptotic Bcl-2, whereas the expression of the pro-apoptotic Bax was increased in both cell lines (Figure 5A). Moreover, upon MS-275 treatment, a dose-dependent increase in the expression of p21Waf-1/CIP1 was observed. By contrast, no regulation of the cyclin-dependent kinase inhibitor (CDKI) p27Kip1 was detected (Figure 5B).
Figure 5 MS-275-induced modulation of apoptosis- and cell cycle-relevant proteins (n = 3 independent experiments).
Antineoplastic potency of MS-275 in combination with other drugs
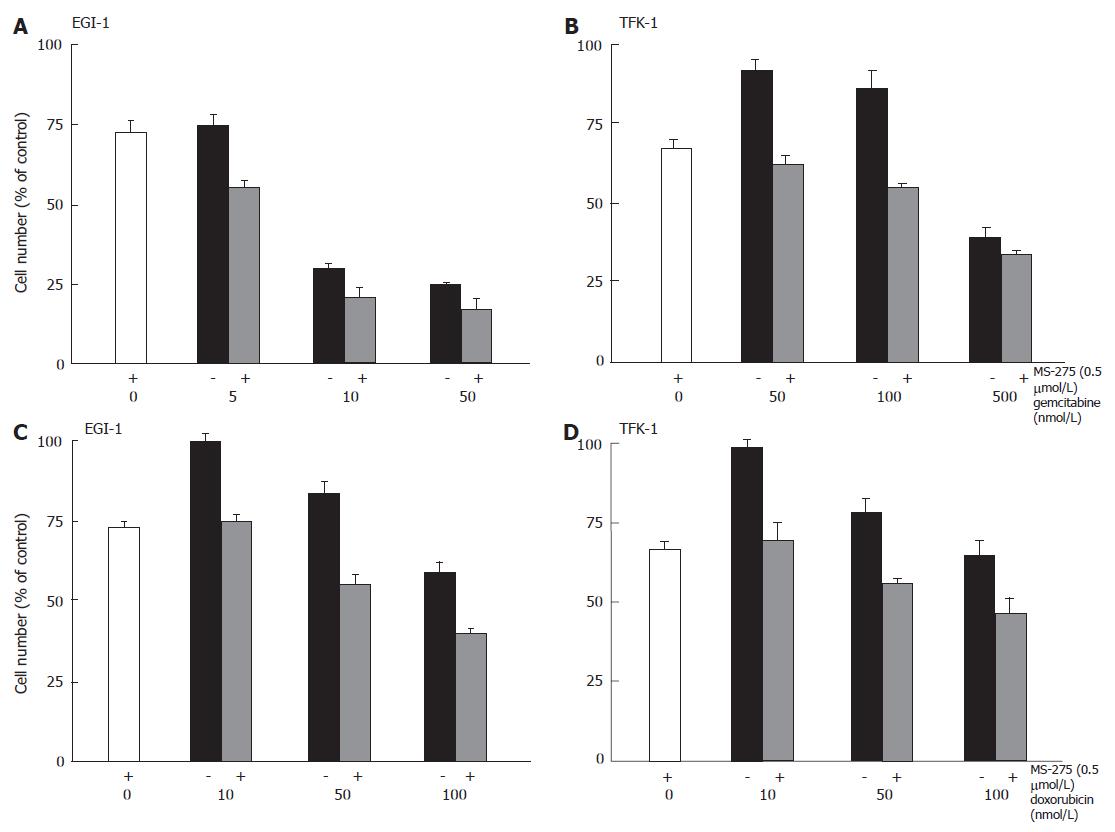
To test potential (over-) additive anti-proliferative effects of MS-275-based combination treatment, EGI-1 or TFK-1 cells were treated with combinations of 0.5 μmol/L MS-275 plus either gemcitabine (0-500 nmol/L) or doxorubicin (0-100 nmol/L) for 2 d. In both combinations, an augmentation of the anti-proliferative effect was observed (Figure 6). Although the anti-proliferative efficacy of gemcitabine monotherapy differed in EGI-1 and TFK-1 cells, the addition of MS-275 caused an enhanced anti-proliferative efficacy in either cell line (Figure 6A and B). Similar results were obtained when co-administering MS-275 and doxorubicin, with additive growth inhibitory effects on both cell lines (Figure 6C and D).
Figure 6 Anti-proliferative effects of MS-275 plus cytostatics on EGI-1 and TFK-1 cells.
mean ± SEM, n = 3-4 independent experiments.
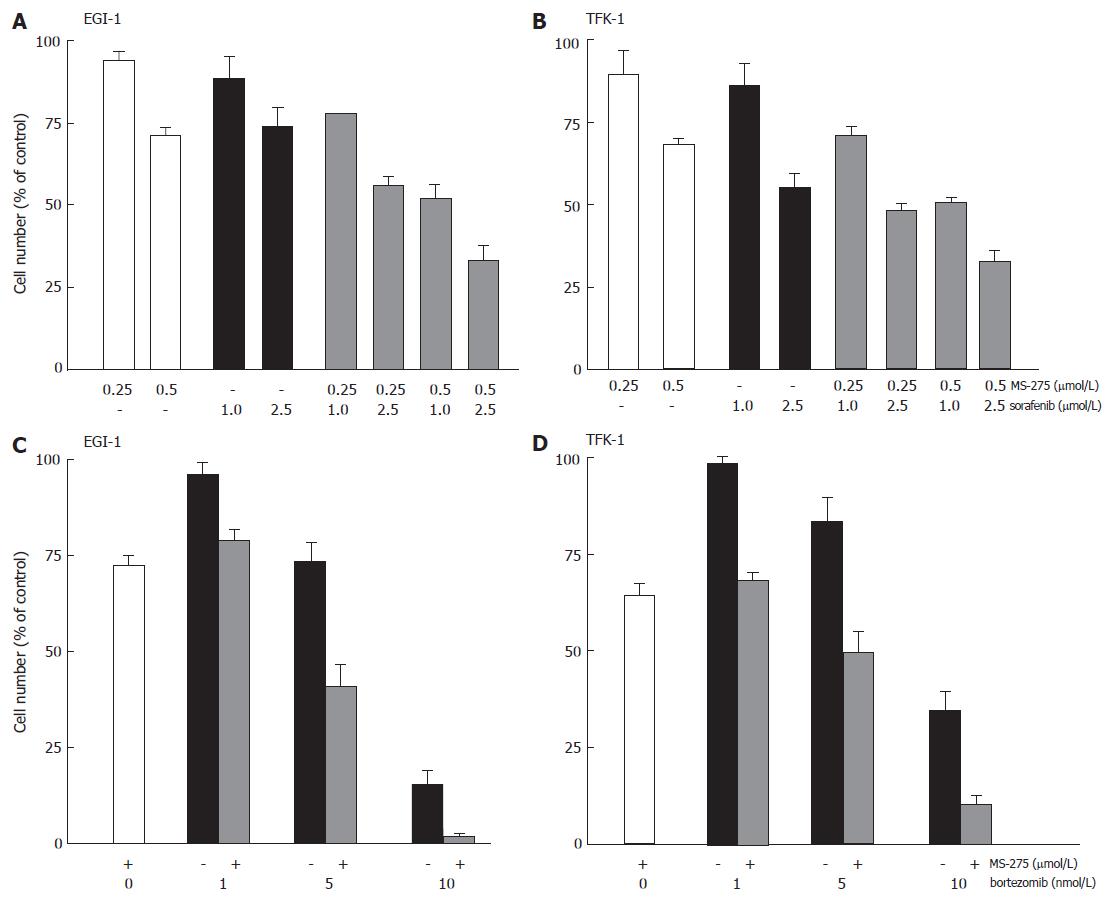
Recently, we could demonstrate that the multi-kinase inhibitor sorafenib potently inhibited the proliferation of cholangiocarcinoma cells[31]. To check out the potency of inhibiting multiple targets of mitogenic signaling pathways for enhanced inhibition of cholangiocarcinoma cell growth, we treated EGI-1 and TFK-1 cells with MS-275 and sorafenib. After two days of incubation, a pronounced (over-)additive growth inhibitory effect of the combination was observed on both cell lines (Figure 7A and B).
Figure 7 Anti-proliferative effects of MS-275 plus sorafenib or bortezomib on EGI-1 and TFK-1.
mean ± SEM, n = 3-4 independent experiments.
Applying the proteasome inhibitor bortezomib alone (1-10 nmol/L) for two days reduced the growth of TFK-1 and EGI-1 cells by up to 65% and 84%, respectively. Again, additive and overadditive growth inhibitory effects were observed, when bortezomib was combined with MS-275 (Figure 7C and D).
DISCUSSION
Treatment options of advanced cholangiocarcinoma (CC) are unsatisfactory, and the prognosis of patients suffering from advanced CC is poor. New, effective and well-tolerated therapy strategies are urgently needed.
Aberrant gene expressions resulting in functional inactivation of histone acetyltransferase (HAT) activity or over-expression of histone deacetylases (HDAC) have been shown to contribute to tumor cell proliferation[13]. For this reason, HDAC inhibition has been regarded as a promising anticancer strategy to inhibit the multiple cellular processes that are dysregulated in various neoplastic cells[14].
The novel synthetic benzamide derivative MS-275 is an orally available HDAC inhibitor which has been shown to exert strong anti-proliferative effects on a variety of human cancers[21], but its potential suitability for the treatment of cholangiocarcinomas have not been tested so far. Here, we provide evidence that inhibition of HDAC activity by MS-275, alone or in combination with conventional chemotherapeutics or new, targeted anticancer drugs, may be a promising approach for the treatment of cholangiocarcinoma.
MS-275 potently inhibited cell growth of the cholangiocarcinoma cells EGI-1 and TFK-1 in a time- and dose-dependent manner. Submicromolar concentrations of MS-275 were already sufficient to significantly inhibit the proliferation of EGI-1 and TFK-1 cells, and the concentration needed to induce halfmaximal anti-neoplastic effects (IC50 value) was approximately 0.5 μmol/L in both cell lines. This is comparable to our previous findings on the effects of MS-275 on the growth of gastrointestinal neuroendocrine tumor cells[24].
Recent studies have shown that inhibition of HDAC activity induces apoptosis in a variety of cancers, including breast and prostate cancer, neuroblastoma, hepatoma, gastrointestinal neuroendocrine tumor cells and some types of hematologic malignancies[24,32-34].
The mechanisms involved in the HDAC inhibitor-induced apoptosis are complex and differ among cell types[35]. They can involve both intrinsic pathways and extrinsic pathways of apoptosis. MS-275 can induce mitochondrial permeability transition with a subsequent release of pro-apoptotic cytochrome c into the cytosol, resulting in the activation of caspase-9 and caspase-3, thus triggering the intrinsic apoptotic pathway[36-38]. Additionally, other HDAC inhibitors have been shown to upregulate pro-apoptotic Fas, a member of the tumor necrosis factor receptor superfamily and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL-) receptors/death receptors DR4 and DR5, thereby triggering the extrinsic pathway, paralleled by down-regulation of the caspase-8 inhibitor c-FLIP, leading to caspase-8 and subsequently to caspase-3 activation[39,40]. Moreover, an altered expression of several pro- and anti-apoptotic intracellular genes by HDAC inhibitors has been reported. Up-regulation of pro-apoptotic Bak and induction of conformational changes of the pro-apoptotic protein Bax are some of the HDAC inhibitor-induced upstream events that trigger the mitochondrial pathway of apoptosis[33,41,42]. HDAC inhibitors can also down-regulate anti-apoptotic proteins, such as Bcl-2, Bcl-XL, XIAP, Mcl-1, and survivin[37,43], further tilting the balance in favor of apoptosis. Here, we demonstrated that the pro-apoptotic effect of HDAC inhibition by MS-275 in CC cells is mediated by the activation of caspase-3 and a shift in the balance of pro-apoptotic Bax over anti-apoptotic Bcl-2.
HDAC-inhibition-induced changes in the expression pattern of apoptosis-related proteins seem to be cell type-specific. For example, in hepatoma and breast cancer cells, HDAC inhibition decreased the expression of Bcl-2, while expression of pro-apoptotic Bax was increased[33,44], in line with the present study. On the other hand, no changes in the expression pattern of Bax and Bcl-2 were found in HDAC-inhibitor-treated glioma cells[45], and in gastrointestinal neuroendocrine tumor cells, Bcl-2 expression was down-regulated upon MS-275 treatment, while Bax remained unchanged[24]. Especially for an estimation of suitable combination treatment regimens being based on the enhanced induction of apoptosis, it is necessary to check out cell type-specific changes in the expression pattern of HDAC-induced pro- and anti-apoptotic proteins.
To further characterize the growth inhibitory activity of MS-275, we performed cell cycle analyses. Upon treatment with MS-275 we found that cell cycle progression was blocked both in the G0/G1 and the G2/M phase. The induction of cell cycle arrest was associated with an increase in the expression of the cyclin-dependent kinase inhibitor (CDKI) p21Waf-1/Cip1. p21Waf-1/CIP1 is a key component of cell cycle checkpoints, i.e., the G1/S[46] and the G2/M checkpoints[47,48]. Accordingly, HDAC inhibitors were found to inhibit both the G1/S and G2/M transition[49-51].
The combination of HDAC inhibitors with existing chemotherapeutic agents appears to be a promising approach to reduce the dose of other anti-neoplastic drugs and to overcome drug resistance. For non-cholangiocarcinoma tumors (e.g. glioblastoma and breast cancer cells), a potentiation of anti-neoplastic effects has already been described for HDAC inhibitors combined with cytostatic drugs[52]. However, except for the interaction of HDAC and topoisomerase inhibitors, in which HDAC-inhibitor-mediated increases in topoisomerase II levels appear to contribute to lethality[53], little is known about the mechanisms by which HDAC inhibitors increase the anti-neoplastic efficacy of cytostatic agents. Maggio et al[37] reported that combination of MS-275 with nucleoside analogues (e.g. Fludarabine) produced high synergy in triggering mitochondrial dysfunction and caspase activation in human leukemia cells. In our study, the nucleoside analogue gemcitabine and the topoisomerase-II-inhibitor doxorubicin were chosen for the evaluation of synergy with MS-275 in CC cells. While both combinations resulted in additive anti-proliferative effects, further studies are needed to clarify the exact mechanisms underlying the observed potentiation by MS-275. Nevertheless, the fact that MS-275-based combination therapies with cytostatics lead to an enhanced growth inhibition of CC cells gives rise to the hope that a HDAC-inhibitor-based combination therapy may hold a promise for more effective treatment of CC.
The same holds true for the combination of HDAC inhibition and multi-kinase inhibition by sorafenib, which mainly targets raf and ras kinases that are members of the MAPK (mitogen-activated protein kinase) pathway. The MAPK pathway is centrally involved in the regulation of multiple cellular functions, such as cell growth, apoptosis and cell cycle progression[54]. Recently, we could demonstrate a potent growth inhibition of CC cells by sorafenib monotherapy[31]. HDAC inhibitors can also down-regulate the MAPK-signaling pathway, as exemplified by HDAC-induced growth inhibition of transformed hepatocytes due to the suppression of oncogenic ras and ERK1/2 (extracellular regulated kinase)[55]. Here we found that coapplication of MS-275 and sorafenib resulted in an additive to overadditive growth inhibition of CC cells, indicating that HDAC-based dual-targeting of MAPK and pro-apoptotic pathways may have promising clinical implications for the future treatment of CC.
Finally, we also studied how far combination treatment of MS-275 together with the proteasome inhibitor bortezomib could enhance the anti-neoplastic effects of the monotherapies. Bortezomib has recently been shown to enhance the anti-proliferative effects of HDAC inhibitors on several non-CC cancer cells[56-58]. The sensitizing effect of bortezomib has been attributed to a blockade of the cytoprotective nuclear factor kappa B (NFκB)[59,60]. There is accumulating evidence that NFκB activation status plays an important role in regulating the response of cells to HDAC inhibitors[61]. Thus, in non-small cell lung cancer, HDAC inhibitors are only modestly effective due to high steady state levels of NFκB[11,57], but addition of bortezomib dramatically increased the pro-apoptotic effect of the HDAC inhibitor sodium butyrate[57]. Our findings are in line with these data in clearly demonstrating that bortezomib overadditively enhanced the anti-proliferative effect of MS-275 on CC cells. Thus, the combined treatment with HDAC inhibitors and bortezomib (and possibly sorafenib as a third agent) appears to be a promising approach for the treatment of cholangiocarcinomas.
In conclusion, our study provides first evidence that the HDAC inhibitor MS-275 potently inhibits the growth of human CC cells by inducing both cell cycle arrest and apoptosis. Importantly, we could demonstrate that combination treatment of MS-275 with gemcitabine, doxorubicin, and especially sorafenib or bortezomib leads to (over-)additive growth inhibitory effects. Our study may provide a rationale for future in vivo evaluations of MS-275 in combination therapies for growth control of advanced cholangiocarcinomas.
COMMENTS
Background
Cholangiocarcinomas (CC) are highly malignant and have a poor prognosis. Since there is no satisfactory therapy for advanced CC, we studied the effects of the histone deacetylase inhibitor MS-275 alone or in combination with bortezomib or sorafenib on the proliferation of cholangiocarcinoma cells.
Research frontiers
Histone modifications have been identified to play prominent roles in the epigenetic regulation of gene transcription. Accumulating evidence indicates that dysregulation of epigenetic processes causes transcriptional repression of a subset of genes, contributing to the pathogenesis of many human diseases, including cancer. In the past decade, substantial progress has been made in understanding the relationship between aberrant epigenetic changes and tumorigenesis. Enzymes involved in these epigenetic events include histone acetyltransferases (HAT) and histone deacetylases (HDAC), which tightly control the steady state of histone acetylation. Recently, histone deacetylase inhibitors receive growing interest as cancer therapeutics, due to their ability to induce growth arrest, differentiation and/or apoptosis.
Innovations and breakthroughs
Previous studies already demonstrated that targeting the histone deacetylase (HDAC) activity by specific HDAC inhibitors is an effective treatment option for cell growth control of haematological malignancies and of several solid tumors. Especially the novel, orally available HDAC inhibitor MS-275 has been shown to exert strong anti-proliferative effects on a variety of cancers.
Applications
In this paper, we studied the anti-proliferative effect of the potent histone deacetylase inhibitor MS-275 on cholangiocarcinoma cells for the first time. MS-275 significantly inhibits the proliferation of cholangiocarcinoma cells by inducing apoptosis and cell cycle arrest. Importantly, we could further demonstrate that combination treatment using MS-275 together with doxorubicin, gemcitabine, sorafenib or bortezomib, respectively, leads to (over-)additive growth inhibitory effects, thus providing a rationale for future in vivo evaluations.
Terminology
Histone acetyltransferases (HAT) and histone deacetylases (HDAC): HAT and HDAC are two sets of histone-modifying enzymes, which control the steady state of histone acetylation. The equilibrium of acetylation (HAT) and deacetylation (HDAC) of histones plays an important role in the modulation of chromatin structure and in the regulation of gene transcription.
Peer review
This manuscript is a good suggestion to be considered and it is true that the treatment options for advanced cholangiocarcinomas are still unsatisfactory. New therapeutic approaches are urgently needed.