Published online Sep 7, 2007. doi: 10.3748/wjg.v13.i33.4452
Revised: May 23, 2007
Accepted: May 28, 2007
Published online: September 7, 2007
AIM: To establish the therapeutic potential of proteasome inhibition, we examined the therapeutic effects of MG132 (Z-Leu-Leu-Leu-aldehyde) in an experimental model of acute pancreatitis.
METHODS: Pancreatitis was induced in rats by two hourly intraperitoneal (ip) injections of cholecystokinin octapeptide (CCK; 2 × 100 μg/kg) and the proteasome inhibitor MG132 (10 mg/kg ip) was administered 30 min after the second CCK injection. Animals were sacrificed 4 h after the first injection of CCK.
RESULTS: Administering the proteasome inhibitor MG132 (at a dose of 10 mg/kg, ip) 90 min after the onset of pancreatic inflammation induced the expression of cell-protective 72 kDa heat shock protein (HSP72) and decreased DNA-binding of nuclear factor-κB (NF-κB).
Furthermore MG132 treatment resulted in milder inflammatory response and cellular damage, as revealed by improved laboratory and histological parameters of pancreatitis and associated oxidative stress.
CONCLUSION: Our findings suggest that proteasome inhibition might be beneficial not only for the prevention, but also for the therapy of acute pancreatitis.
- Citation: Letoha T, Fehér LZ, Pecze L, Somlai C, Varga I, Kaszaki J, Tóth G, Vizler C, Tiszlavicz L, Takács T. Therapeutic proteasome inhibition in experimental acute pancreatitis. World J Gastroenterol 2007; 13(33): 4452-4457
- URL: https://www.wjgnet.com/1007-9327/full/v13/i33/4452.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i33.4452
Proteasome inhibition is an emerging strategy to attenuate the inflammatory response[1]. Inhibiting the proteasome blocks nuclear factor-κB (NF-κB) activation by detaining proteolysis of its inhibitory subunit, the IκB. Preventing NF-κB activation then decreases NF-κB dependent proinflammatory gene expression, resulting in reduced inflammatory response. However studies also reveal that NF-κB, one of the major initiators of pro-inflammatory pathways, has anti-inflammatory roles in the resolution of inflammation. Thus inhibiting NF-κB during the resolution of inflammation has been shown to protract the inflammatory response in vivo[2].
Acute pancreatitis is a severe inflammatory disease characterized by intrapancreatic activation of digestive enzyme zymogens that leads to acinar cell injury and subsequent inflammatory response[3-5]. The inflammatory response is first localized only to the pancreas, but due to the release of inflammatory mediators, later overspreads and becomes systematic affecting other organs including the lung and kidney. This exacerbation of pancreatitis results in multiple organ failure and systemic inflammatory response syndrome that is responsible for the mortality of acute pancreatitis. There have been many experimental attempts for the treatment of acute pancreatitis, however most failed to succeed in the clinics[6,7]. This might stem from the fact that many studies aim to examine only the prophylactic effects of compounds. One thing is clear however, the therapeutic potential of a compound in acute pancreatitis can only be established if it is given after onset of the disease[8,9]. In our previous study the peptide aldehyde proteasome inhibitor MG132 prevented the development of pancreatic inflammation when administered before the induction of the disease[10]. In order to estimate the clinical potential of proteasome inhibition, we also had to examine the therapeutic effects of the compound administered after the onset of pancreatitis. Given the NF-κB inhibitory effects of MG132, it was also crucial to determine whether NF-κB inhibition with MG132 after the onset of pancreatitic inflammation might worsen or ameliorate pancreatitis.
The following paper will summarize the observed effects of therapeutic administration of MG132 in this experimental model of acute pancreatitis and suggest that proteasome inhibition might be beneficial for the therapy of the disease.
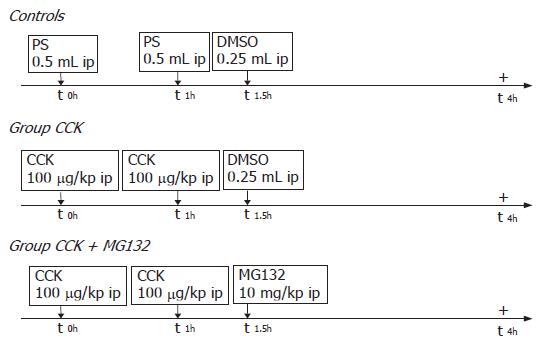
For the in vivo studies male Wistar rats (provided by the Animal Center of the University of Szeged) weighing 250-300 g were used. The animals were kept at constant room temperature with a 12-h light-dark cycle, and were allowed free access to water and standard laboratory chow (Biofarm, Zagyvaszántó, Hungary). Animal experiments performed in this study were approved by the Animal Care Committee of the University and complied with the European Communities Council Directive of 24 November 1986 (86/609/EEC). In each experimental group eight rats were used (n = 8). Acute pancreatitis was induced by injecting 100 μg/kg of CCK (synthesized in the Department of Medical Chemistry, Szeged, Hungary as described by Penke et al[11]; dissolved in physiological saline) twice with an interval of 1 h (Figure 1). Ninety minutes after the first CCK injection, the animals were injected intraperitoneally (ip) either with 10 mg/kg of MG132 [Z-Leu-Leu-Leu-aldehyde; Sigma; dissolved in 0.25 mL dimethyl sulfoxide (DMSO)] or with an equal volume of DMSO (Sigma) alone. Controls received physiological saline (PS) and DMSO in the same manner. Four hours after the first CCK or saline injections, the animals were anesthetized (with pentobarbital sodium 50 mg/kg, ip) and killed by exsanguination through the abdominal aorta. Pancreases and lungs were quickly removed, the former were cleaned of fat and lymph nodes, weighed, frozen in liquid nitrogen and stored at -80°C until use.
Nuclear protein extraction: Nuclear protein extracts were prepared as described previously[12].
Electrophoretic mobility shift assay (EMSA) of NF-kB: EMSA of NF-κB was carried out as described previously[12,13].
Western blotting: Western blot analysis of pancreatic heat shock protein 72 (HSP72) and IκBa was performed as described by Rakonczay et al[12,14]. α-tubulin was used as a loading control.
Serum amylase activity assay: The pancreatic weight/body weight ratio was utilized to evaluate the degree of pancreatic edema. To measure the serum amylase activities, all blood samples were centrifuged at 2500 ×g for 20 min. The serum levels of amylase were determined by a colorimetric kinetic method (Dialab, Vienna, Austria).
Pancreatic tumor necrosis factor-a and interleukin-6 levels: Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) concentrations were measured in the pancreatic cytosolic fractions with ELISA kits (Bender Medsystems, Vienna, Austria) according to the manufacturers’ instructions.
Pancreatic and lung myeloperoxidase activity: Pancreatic and lung myeloperoxidase (MPO) activity, as a marker of tissue leukocyte infiltration, was assessed by the method of Kuebler et al[15].
Real time quantitative polymerase chain reaction (RT-qPCR): RT-qPCR was performed on a RotorGene 3000 instrument (Corbett Research, Australia) with gene-specific primers (designed with the software PrimerExpress, Applied Biosystems, USA) and SYBRGreen I protocol as described previously[10]. Relative expression ratios were normalized to cyclophilin and calculated with the Pfaffl method[16]. The PCR primers used were as follows: cyclophilin, forward primer, 5'-TCTCTTCAAGGGACAAGGCTG-3', reverse primer, 5'-TGGCAAATCGGCTGACG-3'; pancreatitis-associated protein (PAP), forward primer, 5'-CCTCTGCACGCATTAGTTGC-3', reverse primer, 5'-TGAAACAGGGCATAGCAGTAGG-3'.
Lipid peroxidation, reduced glutathione levels and activities of superoxide dismutase and catalase: Lipid peroxides may undergo metal- or enzyme-catalyzed decomposition to form multiple products, including malondialdehyde (MDA). Pancreatic MDA levels were measured according to the MDA/TBA-high performance liquid chromatographic (HPLC) method of Wong et al[17] and were corrected for the protein content of the pancreas. Reduced glutathione (GSH) levels were determined spectrophotometrically with Ellman’s reagent[18]. Pancreatic total superoxide dismutase (SOD) activity was determined on the basis of the inhibition of epinephrine-adrenochrome autoxidation[19].
Ferric reducing ability of plasma (FRAP): The total antioxidant activity of the plasma was determined with the method of Benzie and Strain[20]. Ferric to ferrous ion reduction-in a complex with tripyridyl-triazine - at low pH causes the development of an intense blue color, which has an absorption maximum at 593 nm. FRAP values are obtained by preparing a calibration curve with a solution of known Fe (II) concentration.
A portion of the pancreas was fixed in 8% neutral formaldehyde solution and subsequently embedded in paraffin. Sections were cut at 4 μm thicknesses and stained with hematoxylin and eosin (HE). The slides were coded and read for the traditional histological markers of pancreatic tissue injury by two independent observers who were blind to the experimental protocol. Semiquantitative grading of intestinal edema, inflammation, hemorrhage, vacuolization and acinar cell necrosis was performed on a scale of 0 to 3 (0-absent, 1-mild, 2-moderate, 3-severe).
Results were expressed as mean ± SD. Differences between experimental groups were evaluated by using analysis of variance (ANOVA). Values of P < 0.05 were accepted as significant.
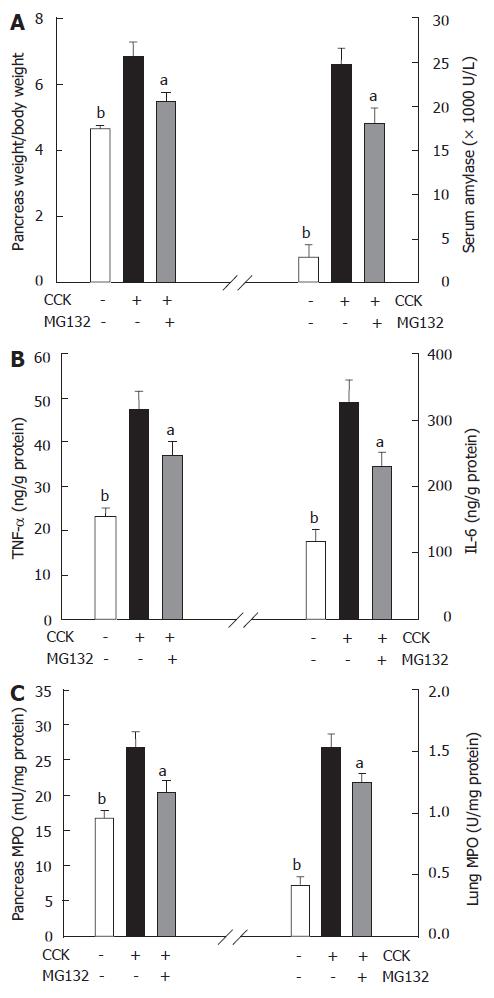
Injecting 2 × 100 μg/kg body weight of CCK resulted in elevated serum amylase levels and pancreatic weight/body weight ratio, signs of acinar injury and pancreatic inflammation[21,22]. These actions of CCK were interfered by MG132 treatment (Figure 2A).
Inflammatory mediators, like TNF and IL-6 couple the local pancreatic inflammation with systemic complications such as pancreatitis-associated lung and renal-injury[23,24]. In our study CCK significantly increased the expression of TNF and IL-6 in the pancreas compared to controls. MG132 treatment reduced intrapancreatic TNF and IL-6 levels (although, compared to Group CCK, the effect of MG132 on pancreatic TNF levels were not statistically significant, as shown in Figure 2B).
Neutrophils produce an enzyme called myeloperoxidase that can be used to identify the amount of neutrophils infiltrating a tissue after inflammation[25]. CCK hyperstimulation increased MPO activity in both the pancreas and lung, reflecting the elevated levels of neutrophil infiltration within these organs. Proteasome inhibition with MG132 decreased MPO activity in the lung and pancreas (Figure 2C).
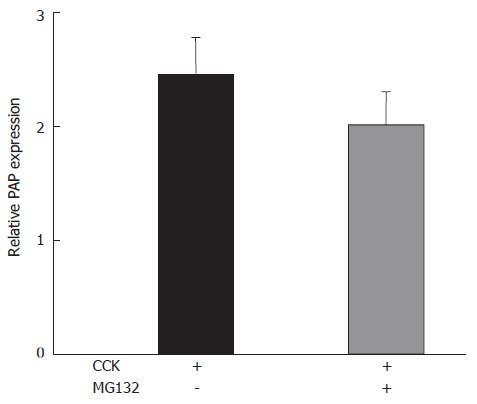
Pancreatitis-associated protein (PAP), the acute-phase protein of the pancreas, is overexpressed in acute pancreatitis[26]. Supramaximal CCK doses significantly increased the expression of PAP mRNA. MG132 treatment could interfere markedly with this effect of CCK (Figure 3).
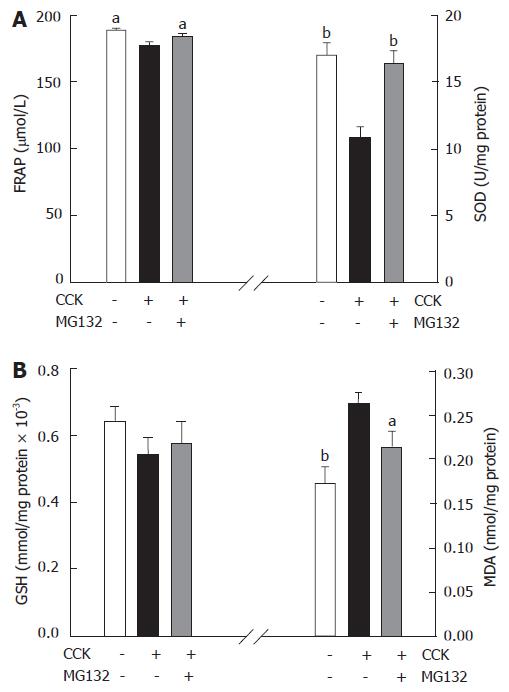
Two hourly injections of CCK induced pancreatic inflammation and underlying oxidative stress. Thus, the ferric reducing ability of plasma (FRAP), as an index of total antioxidant capacity was reduced four hours after the induction of pancreatitis. Moreover CCK stimulation depleted SOD activity and GSH, the two important antioxidant defense systems and increased malondialdehyde content (the marker of lipid peroxidation) in the pancreas. MG132 treatment inhibited the production of reactive oxygen species due to CCK hyperstimulation, as judged by the improvements of above mentioned laboratory parameters of antioxidant power and oxidative stress (Figure 4A and B).
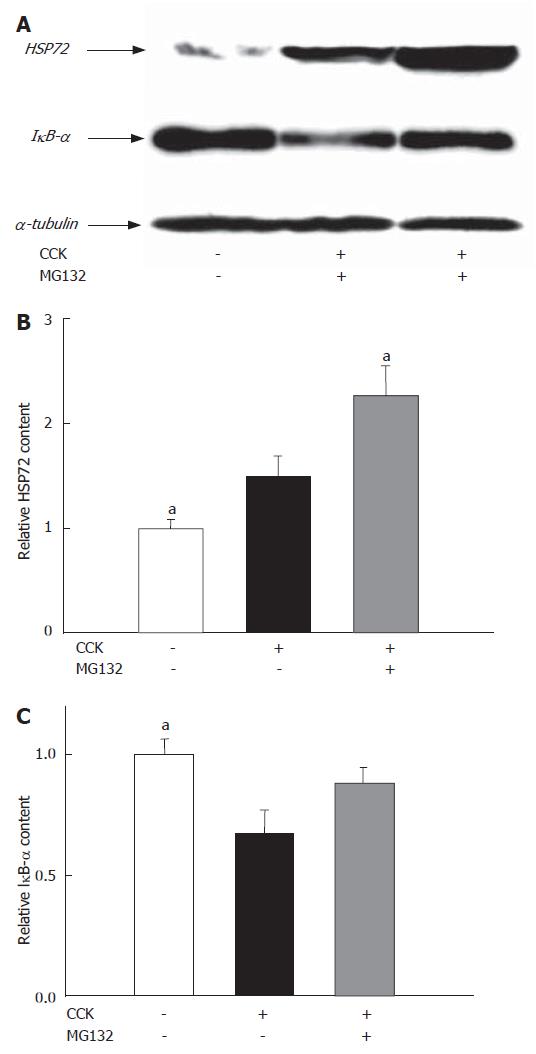
Induction of heat-shock proteins is a useful tool to increase cellular tolerance against stress[27,28]. Injections of CCK elevated the levels of pancreatic HSP72 four hours after the first CCK injection. MG132, the well-known inducer of heat-shock proteins, further increased the expression of HSP72 in the pancreas (Figure 5A and B).
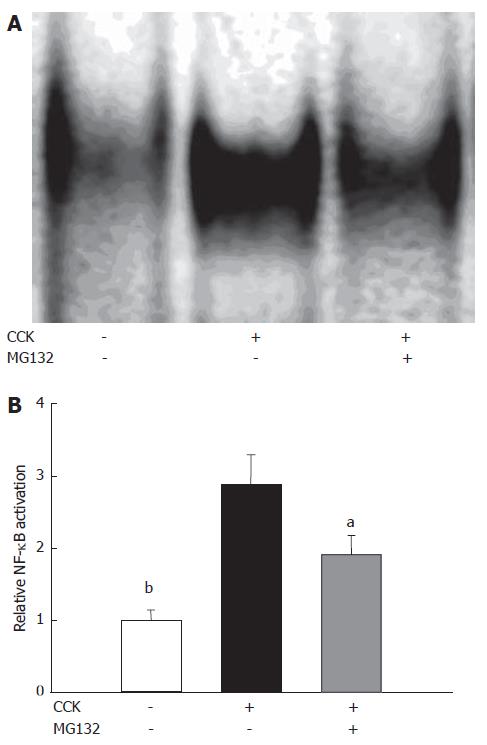
In the pancreas, supramaximal doses of CCK triggered the degradation of IκBα and subsequent activation of NF-κB, based on Western blots and EMSAs carried out on pancreatic samples of animals involved in our study. Inhibiting the proteasome decreased IκBα degradation (Figure 5A and C) and DNA-binding of NF-κB (Figure 6A and B) (The effects of MG132 on IκBα degradation were not significant statistically).
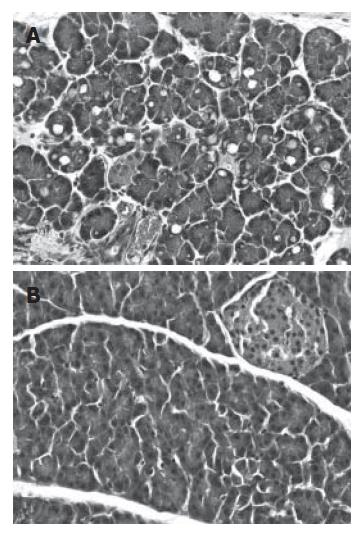
CCK hyperstimulation resulted in cytoplasmic vacuolization and death of acinar cells, edema formation, and infiltration of inflammatory cells in the pancreas samples of CCK-treated animals (Figure 7A). Treating the animals with the proteasome inhibitor MG132 inhibited the cellular damage and inflammatory response due to CCK, as reflected by milder histopathological changes in the pancreas (Figure 7B).
Acute pancreatitis is a severe inflammatory disease triggered by abnormal activation of intrapancreatic proteases and enhanced transcriptional activity of stress-responsive transcriptional factors like NF-κB[3-5]. Intrapancreatic activation of digestive enzyme zymogens can be prevented by the inhibition of lysosomal hydrolases like cathepsin B[29-31]. NF-κB activation can also be prevented by inhibiting the proteasome and other proteases (like calpains) that degrade the inhibitory IκB subunit[32-35]. MG132 is a peptide aldehyde proteasome inhibitor with a broad inhibitory range, showing selectivity towards both serine and cysteine proteases including cathepsins and calpains[1,36]. To make it more complex, MG132 has the ability to induce heat shock proteins (including HSP72), which increases cellular tolerance to stress[37,38].
In our earlier study we have shown that pretreatment of rats with MG132 protected against acute pancreatitis by preventing NF-κB activation and inducing the expression of HSP72[10]. However the therapeutic value of prophylactic treatment in acute pancreatitis is indeed very doubtful. In order to validate the therapeutic potential of proteasome inhibition in pancreatitis, we also tested the effects of therapeutic administration of MG132 in an experimental model of the disease. Pancreatitis was induced by two hourly injections of the cholecystokinin octapeptide (CCK). In this model of the disease, CCK hyperstimulation resulted in pancreatitic inflammation characterized by intracellular activation of digestive enzymes and elevation of their serum levels, cytoplasmic vacuolization and death of acinar cells, edema formation, infiltration of inflammatory cells and oxidative stress. Thus severity of pancreatitis could be very accurately detected by monitoring the laboratory parameters of the disease.
Administering MG132 90 min after the onset of pancreatitic inflammation could still ameliorate the severity of the disease. So MG132 treatment could decrease cellular damage, inflammation and subsequent oxidative stress associated with pancreatitis. These beneficial effects of MG132 can be explained by its ability to induce the expression of HSP72 that protects cells against stressful conditions. MG132 also decreased the transcriptional activity of NF-κB. NF-κB, however, has a dual role in inflammatory diseases, because besides triggering proinflammatory cellular events during first phase of the inflammatory response, it has also anti-inflammatory role during the resolution of inflammation[2]. In CCK-induced pancreatitis, NF-κB activation peaks in the first phase of the disease[39]. Since in MG132 treatment had more pronounced effects on HSP72 than on NF-κB, thus it is likely that in our case the induction of heat shock proteins made larger contribution to the observed beneficial effects of MG132 in acute pancreatitis and than NF-κB inhibition.
Our observation that MG132 could ameliorate the severity of acute pancreatitis when administered 90 min after the induction of the disease is indeed very promising. Considering this, we have to note that although supramaximally stimulating doses of CCK cause the inflammatory response that underlies many of the features of human pancreatitis, still CCK-induced pancreatitis is a mild model of the disease[40]. Thus MG132 and other proteasome inhibitors should be further tested in other, more severe models of pancreatitis in order to accurately determine the clinical potential of proteasome inhibition for the treatment of acute pancreatitis.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Li JL
| 1. | Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med (Berl). 2003;81:235-245. [PubMed] |
| 2. | Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 600] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Algül H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatology. 2002;2:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 388] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Weber CK, Adler G. Acute pancreatitis. Curr Opin Gastroenterol. 2003;19:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Adler G. Has the biology and treatment of pancreatic diseases evolved? Best Pract Res Clin Gastroenterol. 2004;18 Suppl:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs. 2001;2:496-501. [PubMed] |
| 9. | Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scand J Surg. 2005;94:97-102. [PubMed] |
| 10. | Letoha T, Somlai C, Takács T, Szabolcs A, Rakonczay Z, Jármay K, Szalontai T, Varga I, Kaszaki J, Boros I. The proteasome inhibitor MG132 protects against acute pancreatitis. Free Radic Biol Med. 2005;39:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Penke B, Hajnal F, Lonovics J, Holzinger G, Kadar T, Telegdy G, Rivier J. Synthesis of potent heptapeptide analogues of cholecystokinin. J Med Chem. 1984;27:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Rakonczay Z, Duda E, Kaszaki J, Iványi B, Boros I, Lonovics J, Takács T. The anti-inflammatory effect of methylprednisolone occurs down-stream of nuclear factor-kappaB DNA binding in acute pancreatitis. Eur J Pharmacol. 2003;464:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Letoha T, Somlai C, Takacs T, Szabolcs A, Jarmay K, Rakonczay Z, Hegyi P, Varga I, Kaszaki J, Krizbai I. A nuclear import inhibitory peptide ameliorates the severity of cholecystokinin-induced acute pancreatitis. World J Gastroenterol. 2005;11:990-999. [PubMed] |
| 14. | Rakonczay Z, Iványi B, Varga I, Boros I, Jednákovits A, Németh I, Lonovics J, Takács T. Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. Free Radic Biol Med. 2002;32:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Kuebler WM, Abels C, Schuerer L, Goetz AE. Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int J Microcirc Clin Exp. 1996;16:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Nagy ZB, Kelemen JZ, Fehér LZ, Zvara A, Juhász K, Puskás LG. Real-time polymerase chain reaction-based exponential sample amplification for microarray gene expression profiling. Anal Biochem. 2005;337:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Wong SH, Knight JA, Hopfer SM, Zaharia O, Leach CN, Sunderman FW. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem. 1987;33:214-220. [PubMed] |
| 18. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5089] [Cited by in RCA: 5390] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 19. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 20. | Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12606] [Cited by in RCA: 12161] [Article Influence: 419.3] [Reference Citation Analysis (0)] |
| 21. | Ranson JH. Diagnostic standards for acute pancreatitis. World J Surg. 1997;21:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 24. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1544] [Cited by in RCA: 1625] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 26. | Savković V, Gaiser S, Iovanna JL, Bödeker H. The stress response of the exocrine pancreas. Dig Dis. 2004;22:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Rakonczay Z, Takács T, Boros I, Lonovics J. Heat shock proteins and the pancreas. J Cell Physiol. 2003;195:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Schäfer C, Williams JA. Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease. J Gastroenterol. 2000;35:1-9. [PubMed] |
| 29. | Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 414] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 30. | Van Acker GJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol. 2002;283:G794-G800. [PubMed] |
| 31. | Halangk W, Lerch MM. Early events in acute pancreatitis. Clin Lab Med. 2005;25:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Gao Y, Lecker S, Post MJ, Hietaranta AJ, Li J, Volk R, Li M, Sato K, Saluja AK, Steer ML. Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha degradation by a naturally occurring antibacterial peptide. J Clin Invest. 2000;106:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666-G676. [PubMed] |
| 34. | Weber H, Jonas L, Hühns S, Schuff-Werner P. Dysregulation of the calpain-calpastatin system plays a role in the development of cerulein-induced acute pancreatitis in the rat. Am J Physiol Gastrointest Liver Physiol. 2004;286:G932-G941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Virlos I, Mazzon E, Serraino I, Genovese T, Di Paola R, Thiemerman C, Siriwardena A, Cuzzocrea S. Calpain I inhibitor ameliorates the indices of disease severity in a murine model of cerulein-induced acute pancreatitis. Intensive Care Med. 2004;30:1645-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397-403 DOI : 10.1016/S0962-8924(98)01346-4. |
| 37. | Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086-9092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 353] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:30-38. [PubMed] |
| 39. | Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402-G1414. [PubMed] |
| 40. | Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159-170. [PubMed] |