Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4131
Revised: May 3, 2007
Accepted: May 12, 2007
Published online: August 14, 2007
AIM: To explore the molecular mechanisms of action of paclitaxel and NM-3 on human gastric cancer in severe combined immune deficiency (SCID) mice.
METHODS: Human gastric cancer cells SGC-7901 were implanted into SCID mice and mice were treated with paclitaxel and NM-3. The effects of paclitaxel and NM-3 on apoptosis of human gastric cancer cells were analyzed using flow cytometry, TUNEL assays, and DNA fragment analyses.
RESULTS: Apoptosis of SGC-7901 cells was successfully induced by paclitaxel, NM-3, and the combination of paclitaxel and NM-3 24 h after injection as shown by the presence of apoptotic hypodiploid peaks on the flow cytometer before G1-S and a characteristic apoptotic band pattern in the DNA electrophoresis. The apoptotic rate detected by TUNEL assay was found to be significantly higher in the paclitaxel/NM-3 compared to the control group (38.5% ± 5.14% vs 13.2% ± 1.75%, P < 0.01).
CONCLUSION: Paclitaxel in combination with NM-3 is able to induce apoptosis of the human gastric cancer cells in SCID mice effectively and synergistically.
- Citation: Zhu JS, Song MQ, Chen GQ, Li Q, Sun Q, Zhang Q. Molecular mechanisms of paclitaxel and NM-3 on human gastric cancer in a severe combined immune deficiency mice orthotopic implantation model. World J Gastroenterol 2007; 13(30): 4131-4135
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4131.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4131
Gastric cancer is the common leading cause of cancer death worldwide. Its clinical behavior depends on its capacity to establish metastases of the tumor, and prognosis of advanced gastric cancers is poor. To date, several molecules have been reported to play an important role in gastroenterological tumorigenesis and metastasis[1-3], but the molecular mechanisms remain to be elucidated[1-3].
In previous studies using LMD, P27-based RNA amplification, and cDNA microarray, we identified some differentially expressed genes between primary carcinoma cells and lymph node metastatic cells in two patients. Moreover, we further identified four differentially expressed genes in progression of gastric cancer in another group of 15 patients by means of semiquantitative reverse transcribed polymerase chain reaction (RT-PCR), and the expression patterns of these four genes were similar to tumor suppressor genes or oncogenes.
It is now widely accepted that many malignant tumors contain subpopulations of heterogeneous cells. This heterogeneity is exhibited in a wide range of genetic, biochemical and immunologic characteristics. It is likely that specific tumor cells or colonies within the larger heterogeneous tumor specimen are the forerunners of distant metastases[4]. Thus, many biologic differences might exist between tumor cells in primary and metastatic lesions. Furthermore, the interaction of tumor cells with their living environment may add more differences between these two groups of cells[2]. As a result, tumor metastasis related genes can be identified by comparing the gene expression profiles between them.
Apoptosis plays a crucial role in the proliferation and turnover of cells in various malignant tumors, and it is enhanced by many anticancer drugs as cytotoxic drugs, hormones, or some recombinant gene, medicine, etc. At present, there is no effective therapy for advanced gastric cancer, as the other malignant tumor, gastric cancer is not only a disease with abnormal cell proliferation and differentiation, but also a disease with abnormal apoptosis. Thus, the enhanced induction of apoptosis in human gastric cancer cells will be needed to explore. Paclitaxel enhanced the expression of smad3 and smad4 in SCID mice, smad3 and smad4 are kind of multifunction cyclin dependent kinase inhibitors. They play a negative role in cell cycle regulation by inhibiting transition from G0/G1 to S phase. Based on previous studies, we examined the apoptotic indices of human gastric cancer grafted into SCID mice. We investigated its apoptotic effects on human gastric cells, by which we explored its correlated anticancer mechanisms and its synergistic effect combined with NM-3 in order to look for a novel therapy for advanced gastric cancer.
RPMI1640 media and TRI201 total RNA isolation kit were purchased from Gibco BRL. The liposome, the trypsin, DMEM culture medium, Hepes and Csc1, 200 bp DNA ladder, dNTP, Taq enzyme and the restriction endonuclease were obtained from Sigma Co.
Paclitaxel was obtained from the Chinese Academy of Science. NM-3 was provided by Doctor Robert, Huston University, USA.
Male severe combined immune deficiency (SCID) mice were obtained from Shanghai Experimental Animal Center of Chinese Academy of Sciences. Animals used were 6-7 wk old and weighed 18-22 g. Human gastric cancer SGC-7901 (Shanghai Tumor Institution No: 01842), a poorly differentiated adenocarcinoma cell line, was originally derived from a primary tumor and maintained by passage in the subcutis of nude mice. Animal models were made using orthotopic implantation of histological intact tissue of human gastric carcinoma. Tumors were resected aseptically. Necrotic tissues were cut and the remaining healthy tumor tissues were scissor minced into pieces (about 5 mm × 7 mm in diameter) in Hank’s balanced salt solution. Each tumor piece was weighed and adjusted to 50mg. Mice were anesthetized with 4.3% trichloraldehyde hydrate. An incision was made through the left upper abdominal pararectal line. Then the peritoneal cavity was carefully exposed and a part of the serosal membrane in the middle of the greater curvature of the stomach was mechanically injured using scissors. A tumor piece of 150 mg was fixed on each injured site of the serosal surface. The stomach was returned to the peritoneal cavity, and the abdominal wall and skin were closed.
After 12 d, when the tumor reached the size of 0.8-1.0 cm3, mice were randomly separated into four groups, with ten mice per group. Body weight and tumor volume of these orthotopic grafted mice in every group had no obvious difference (P < 0.05). Via intraperitoneal injection animals received paclitaxel (5 mg/kg), NM-3 (10 mg/kg), paclitaxel (5 mg/kg) combined with NM-3 (10 mg/kg), or normal saline, respectively. 24 h later mice were sacrificed by cervical dislocation. Samples of the tumor were immediately frozen in liquid nitrogen for later use. Part of the fresh samples were immediately placed into Eppendorf tubes for flow cytometry analysis.
Ten mg of fresh tissue from every animal was minced with blades to millimeter sizes in tissue medium (RPMI 1640). The supernatant was separated and filtered through a 50-mm nylon mesh. The filtered cells were collected by centrifugation and washed twice with PBS.
Six cell suspensions (1 × 104 cells) of each group (NM-3, paclitaxel, paclitaxel/NM-3, and saline) were placed separately into 60-mm dishes containing cover glass slides (washed and high-pressure sterilized). Then the glass slides were taken out, washed twice with PBS, and fixed in methanol: freezing acetic acid (3:1) for 30 min. The following procedures were carried out according to the kit instruction. The average number of apoptotic cells was determined by counting 1000 cells on each glass slide and the apoptotic index (AI), i.e. the number of apoptotic cells per 100 cancer cells, was calculated.
1 × 106/L cells of four groups were fixed by 0.5% glutaral pentanediol for 15 min and washed thrice with PBS. X-gal staining solution (20:1) was added and cells were incubated at 37°C for 4-24 h in a humidified atmosphere containing 50 mL/L CO2. Blue-stained cells, i.e. those with LacZ gene expression, were counted under the microscope and the percentage of the positive cells was calculated.
5 mg fresh tissue specimens of every mouse in four groups were digested by 0.5 g/L trypsin. The cells were collected and washed twice with PBS. After cell lysis in 500 μL SDS-PAGE cell lysis solution and boiling for 5 min, supernatants were collected after centrifugation. Samples were subjected to western blot analysis.
Fresh tissue specimens of the four groups were minced with blades to millimeter sizes in tissue medium (RPMI 1640). Then supernatant was separated and filtered through a 50-mm nylon mesh. The filtered cells were collected by centrifugation, stained, washed twice with PBS, and cell suspension was adjusted to a density of 106/L. 100 μL of cell suspension was mixed with 200 μL of DNA-PREPTM LPR, followed by detection using Coulter Epics XL flow cytometer for 15 min. Cell cycle progression and cell apoptosis rate were analyzed.
SGC-7901 cells, after being collected and washed twice with PBS, were lysed in 500 μL cell lysis solution [1%Np40, 20 mm/L EDTA, 50 mmol/L Tris-HCl (pH 7.5)] in the presence of 10 μL of protease K. Samples were heated in a 56°C water bath for 1-2 h before extraction with phenol/chloroform. After the DNA precipitate had been washed once with 700 mL/L alcohol, 200 μL of TE was added followed by an overnight incubation with RNase (final concentration 50 μL/mL) at 37°C. The final DNA was separated by agarose gel electrophoresis (10 g/L) and visualized with the aid of an ultraviolet light lamp.
Data were analyzed by t test and a P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 11.5 for windows.
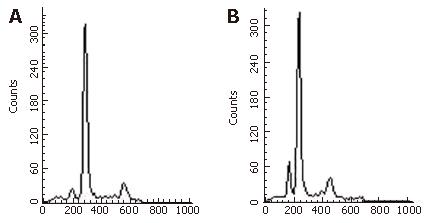
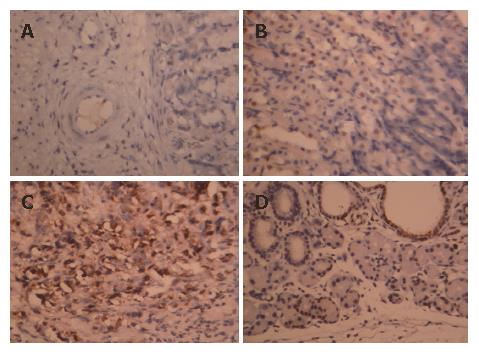
The nucleus of apoptotic cells was dark stained, dark brown and the cytoplasm was concentrated and the cell shrank. The AI of samples of the paclitaxel/NM-3 and the control group was 82.6% (± 3.12%) and 5.0% (± 0.35)%), respectively. This difference was statistically significant (P < 0.05) showing that paclitaxel/NM-3 could obviously induce apoptosis of gastric cancer cells (Figure 1 and Figure 2).
Cell cycle progression is shown in Table 1. Whereas the number of cells in G0/G1 phase is lower in the paclitaxel and in the control group compared to the others, the percentage of the S phase cells is higher, indicating that transition time of cell cycle was shortened and cell proliferation was active. However, the percentage of G0/G1 phase cells increased and the cell cycle was arrested in G0/G1 phase in the paclitaxel/NM-3 group, which was significantly different between the control and the paclitaxel group (Table 1 and Table 2).
The adenovirus mediated gene transfer rate was evaluated by X-gal staining. The results showed that the infection efficiency could reach 90%, indicating that recombinant adenovirus could effectively transfer genes in vitro.
The expression of p27 protein was evaluated after being injected into the cancer cells with paclitaxel in vitro: After the cells that could be used in experiment were injected by paclitaxel/NM-3 for 24 h, these cells were collected and lysed with 1 × SDS PAGE cell lysis solution. After boiling at 100°C for 5 min, the solution was centrifuged. The supernatant was collected and the protein was detected by TMB system western blot kit, KPL USA. Followed by X-gal chemical staining, there was high expression of a 27 kDa protein in the paclitaxel/NM-3 group while only slight traces were seen (endogenous expression) in the paclitaxel and in the control group. Thus, the human mutant p27 recombinant adenovirus constructed in the present study expresses the p27 gene properly in SGC-7901 cells and the protein product could be expressed at a high level in cells.
The pathological change of SGC-7901 cells and their culture fluid were collected and centrifuged. Five milliliters of the supernatant was mixed with 1 mg of protease K, 2 mL of 1% SDS, 10 mmoL/L EDTA and 20 mmol/L Tris-HCl to be digested for 2 h. After being precipitated by dehydrated alcohol, the DNA was collected. PCR reaction was carried out after adding the forward and reverse primers. Finally, a 275-bp target fragment was amplified, which showed that paclitaxel, paclitaxel/NM-3 had already played an inhibitory role on the p27mt target gene.
After the animals had been treated with NM-3, paclitaxel, paclitaxel/NM-3, or saline for 24 h, the detection of apoptotic cells was carried out by flow cytometry and repeated six times. The mean value of hypodiploid cells was: 8.23%, 4.67%, 41.0%, and 1.96%, respectively. Statistical analysis revealed a significant difference between the paclitaxel/NM-3 group and the paclitaxel group and the NM-3 group, (paclitaxel/NM-3 vs paclitaxel group, paclitaxel/NM-3 group, NM-3 group and paclitaxel group vs normal saline group, P < 0.01; paclitaxel group vs NM-3 group, P > 0.05).
The result of DNA electrophoresis showed intact genomic DNA in samples obtained from the paclitaxel and the normal control group while there was an obvious 180-200 bp diploid ‘trapezia’ pattern in samples derived from NM-3 or the paclitaxel/NM-3 injected group, which was in concordance with the characteristic changes of apoptosis.
Since paclitaxel was extracted from Taxul which has special anti-tumor effect, paclitaxel had cell toxic side-effects as Taxol either. We found that paclitaxel could inhibit the activation of CyclinE-CDK2 complex and the activation of CyclinD-CDK4 and CyclinA-C DK2 complex as well. In addition, it could also down-regulate the expression of CyclinB, because the arrest of G0/G1 was mainly caused by p27 whose accumulation could be induced by exogenous signal. Gastric tumorigenesis was closely correlated with translocation, deletion and mutation of p27 gene and its expression or activity changes[2,5]. At present, gastric cancer cells treated by paclitaxel/NM-3 in SCID mice were not reported. If the expression level of p27 mt in gastric cancer cell was down-regulated or inhibited by p27, thus the DNA damaged cell could not transit form form G1 phase to S phase directly, that would induced the apoptosis of human gastric cancer cells in SCID mice.
Paclitaxel was first found in 1998, the researchers revealed it effected on cell contact inhibition with TGF-β[4]. The degradation of the p27 protein is mainly caused by phosphorylation of the threonine residue at position 187 which is mediated by ubiquitin[6-8]. Kudo and his colleagues[9] found that if the 187th threonine of p27 which was mediated by p27 protein, could inhibit cell growth obviously, these inhibitory effects were more obvious on mutant p27 (T187A) than on wild-type p27. The target phosphorylation site of CDK would be protected from phosphorylaton. A replication-deficient recombinant adenovirus was constructed which carried p27mt to study apoptosis of the gastric cancer cell line, by which it expected to find a more effective p27 gene to treat gastric cancer. Koguchi K[11] reported that they prohibited the viability of the astrocyte when these cells were transfected with exogenous gene p27 carried by adenovirus. Zhang D[12] reported that the upregulation of the expression of the p27 by retinoic acid significantly inhibited the growth of the oophoroma cell. Koh TY[13] found high expression of p27 and raised the expression level of cyclinD1 and cyclinE in the cell lines SUN-1066, SUN-1041, SUN-1076 derived from cephalocervical squamous cell carcinoma by transfecting these cells with p27kip1 carried by reconstructed adenovirus, the cancer cells’ proliferation were significantly prohibited and the cell cycle analysis showed that the cancer cells were mainly stopped at the G1-S stage in their study. All this showed that p27 was a very important gene related to the development of carcinoma and had significant impact on the onset, development and prognosis of the tumor.
Nowadays, functional reconstruction of anti-oncogene has been a reasonable strategy of gene therapy for tumor. Sasaki T[14] found that p27mt had stronger suppressive effects than p27wt on apoptosis and cell proliferation when they applied adenovirus-induced p27mt and p27mt to transfect the cholangiocarcinoma cell lines TFK-1 and HuCCT-1. Park KH[15,16] got the same results when they used adenovirus mediated p27mt and p27wt to transfect lung cancer cell lines NCI H460, NCI H1264, NCI H358 and NCI H157, and a spongioblast line. In our study, mutant p27 was used to transfect gastric cancer cells and the high expression of this gene was proven by p27 polyclonal antibody. This result supported that adenovirus with reconstructed p27mt could transfect the target gene into a gastric cancer cell which derived from human gastric adenocarcinoma tissue, developed into tumor in SCID mice and expressed endogenous p27. By flow cytometry, rate of apoptosis up to 41.0% in paclitaxel/NM-3 group was proven which had significant difference compared to the control group, DNA analysis showed 180-200bp DNA ladder. By TUNEL technique, a value of apoptotic index up to 82.6% was detected which showed significant difference compared to the control group. The results showed that the gene p27 was an important gene related to the occurance of the large gastric carcinoma and the down-regulation of p27 may be the main cause of cell differentiation dysfunction and apoptosis dysfunction. Upregulating the expression of p27 by mutant p27, which promotes the apoptosis of the tumor cell, could serve as a new scheme in the treatment of advanced gastric carcinoma. Circle analysis showed that the cleavage of tumor cells was stopped at G1 stage via suppressing the activity of the cyclin/CDK kinase by p27mt. Winteringham LH[17] thought that the accumulation of p27 played an important role in apoptotic mechanisms of the gastric cell cycle arrest at the initiation of cell differentiation. Whether other apoptotic factors might affect this process should be explored further.
Recently, although the apoptosis of the human gastric cancer cell was successfully induced by the application of NM-3 and other gene therapy, but the apoptosis of human gastric cancer cells induced by paclitaxel/NM-3 had not been reported in the SCID mice model[18,19], it was very useful experimental support of tumor-suppressed function for human gastric cancer treated by paclitaxel/NM-3. The efficacy of this method and the mechanism of apoptosis strongly indicate that paclitaxel/NM-3 is synergistically effective against human gastric carcinomas.
S- Editor Liu Y L- Editor Mihm S E- Editor Wang HF
| 1. | Nan KJ, Jing Z, Gong L. Expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27Kip1 in hepatocellular carcinoma. World J Gastroenterol. 2004;10:1425-1430. [PubMed] |
| 2. | Bryja V, Pacherník J, Faldíková L, Krejcí P, Pogue R, Nevrivá I, Dvorák P, Hampl A. The role of p27(Kip1) in maintaining the levels of D-type cyclins in vivo. Biochim Biophys Acta. 2004;1691:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Chen J, Xu SY, Deng CS. Efficient generation of human mutant p27 recombinant adenovirus by homologous recombination in bacteria. J Fourth Mil Med Univ. 2004;5:406-409. |
| 4. | Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1442] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 5. | Liu E, Li X, Yan F, Zhao Q, Wu X. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J Biol Chem. 2004;279:17283-17288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3'-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Guo W, Shang F, Liu Q, Urim L, West-Mays J, Taylor A. Differential regulation of components of the ubiquitin-proteasome pathway during lens cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Kudo Y, Kitajima S, Sato S, Ogawa I, Miyauchi M, Takata T. Transfection of p27(Kip1) threonine residue 187 mutant type gene, which is not influenced by ubiquitin-mediated degradation, induces cell cycle arrest in oral squamous cell carcinoma cells. Oncology. 2002;63:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hurteau JA, Brutkiewicz SA, Wang Q, Allison BM, Goebl MG, Harrington MA. Overexpression of a stabilized mutant form of the cyclin-dependent kinase inhibitor p27(Kip1) inhibits cell growth. Gynecol Oncol. 2002;86:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Koguchi K, Nakatsuji Y, Nakayama K, Sakoda S. Modulation of astrocyte proliferation by cyclin-dependent kinase inhibitor p27(Kip1). Glia. 2002;37:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Holmes WF, Wu S, Soprano DR, Soprano KJ. Retinoids and ovarian cancer. J Cell Physiol. 2000;185:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, Lee CT, Heo DS, Kim KH, Sung MW. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003;25:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sasaki T, Katayose Y, Suzuki M, Yamamoto K, Shiraso S, Mizuma M, Unno M, Takeuchi H, Lee CT, Matsuno S. Adenovirus expressing mutant p27kip1 enhanced apoptosis against cholangiocarcinoma than adenovirus-p27kip1 wild type. Hepatogastroenterology. 2004;51:68-75. [PubMed] |
| 15. | Park KH, Seol JY, Kim TY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. An adenovirus expressing mutant p27 showed more potent antitumor effects than adenovirus-p27 wild type. Cancer Res. 2001;61:6163-6169. [PubMed] |
| 16. | Park KH, Lee J, Yoo CG, Kim YW, Han SK, Shim YS, Kim SK, Wang KC, Cho BK, Lee CT. Application of p27 gene therapy for human malignant glioma potentiated by using mutant p27. J Neurosurg. 2004;101:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Winteringham LN, Kobelke S, Williams JH, Ingley E, Klinken SP. Myeloid Leukemia Factor 1 inhibits erythropoietin-induced differentiation, cell cycle exit and p27Kip1 accumulation. Oncogene. 2004;23:5105-5109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kumagai H, Masuda T, Ohsono M, Hattori S, Naganawa H, Sawa T, Hamada M, Ishizuka M, Takeuchi T. Cytogenin, a novel antitumor substance. J Antibiot (Tokyo). 1990;43:1505-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Zhu JS, Shen B, Chen JL, Chen GQ, Yu XH, Yu HF, Zhu ZM. Molecule action mechanisms of NM-3 on human gastric cancer SGC-7901 cells in vivo or in vitro. World J Gastroenterol. 2003;9:2366-2369. [PubMed] |