Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4126
Revised: April 3, 2007
Accepted: April 11, 2007
Published online: August 14, 2007
AIM: To investigate the expression and significance of G3BP and RhoC proteins in esophageal squamous carcinoma (ESC).
METHODS: The expression of G3BP and Rhoc proteins in 80 cases of ESC was detected by immunohistochemistry. The relationship was studied between the expression of the two proteins and tumor size, differentiation degree, TNM stage, lymph node metastasis and prognosis of ESC.
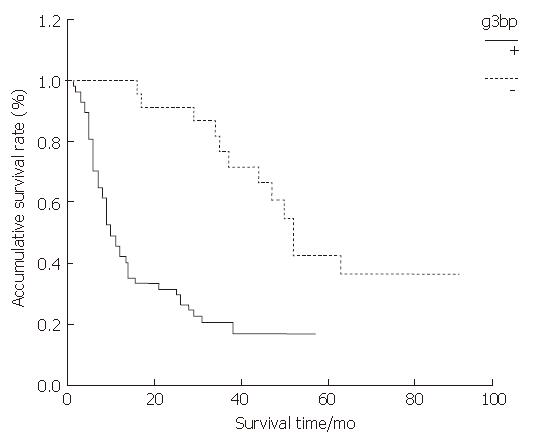
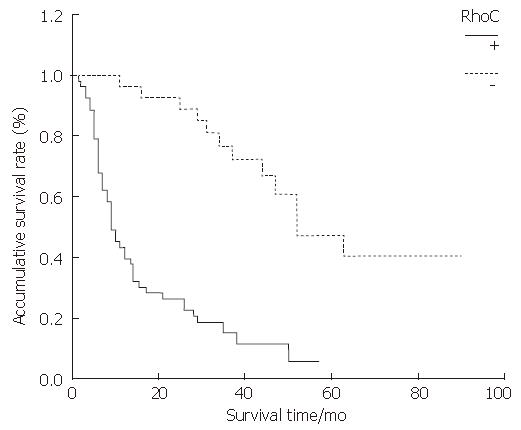
RESULTS: The positive expression rate of G3BP in ESC was 71.25%; and the rate in the lymph node metastasis group was significantly higher than that in the non-lymph node metastasis group (Z = -2.283, P = 0.022), but no relations were found between G3BP expression and tumor size, differentiation degree and TNM stage (P > 0.05). The group with G3BP positive expression had shorter survival time than the group with G3BP negative expression (P = 0.000). The positive expression rate of RhoC in ESC was 66.25%; and the rate in the lymph node metastasis group was significantly higher than that in the non-lymph node metastasis group (Z = -2.115, P < 0.05), but no relations were found between RhoC expression and tumor size, differentiation degree and TNM stage (P > 0.05). The RhoC positive expression group had a shorter survival time than the RhoC negative expression group (P < 0.001. The expression of G3BP protein correlated positively with the expression of RhoC in ESC tissues (rs = 0.656, P < 0.001).
CONCLUSION: The expression of G3BP and RhoC protein is closely related to the lymph node metastasis and survival in ESC patients. G3BP and RhoC proteins can be considered as predictors of prognosis in ESC patients.
- Citation: Zhang HZ, Liu JG, Wei YP, Wu C, Cao YK, Wang M. Expression of G3BP and RhoC in esophageal squamous carcinoma and their effect on prognosis. World J Gastroenterol 2007; 13(30): 4126-4130
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4126
Many patients with esophageal carcinoma are in intermediate or advanced stage when they see doctors. Because of this, early diagnosis, early treatment and energetic prevention are very important for esophageal carcinoma. The current routine examination methods are insufficient for the diagnosis of small cancer focus and metastatic focus when they are in subclinical stage. But the detection of tumor markers can retrieve the insufficiency of examination methods. The tumor markers not only can be used for the early diagnosis of cancer, but also for post-treatment follow-up, recurrence detection, therapeutic effect evaluation and prognosis monitoring.
G3BP (Ras-GTPase-activiting protein SH 3 domain binding protein) and RhoC (Ras homology C) were overexpressed in many kinds of malignant tumors, which was closely related to the invasion and metastasis of tumor cells. Up to date, there has been no study in the relationship between G3BP and esophageal carcinoma. For RhoC, researches have been available in esophageal carcinoma tissues, but without follow-up data[1]. In the current study, we investigated the expression of G3BP and RhoC proteins in esophageal squamous carcinoma (ESC) by immunohistochemistry, and explored the relationship between the expression of the two proteins and biological behaviour of ESC and post-operative survival time.
Eighty ESC patients (65 men and 15 women; mean age 59 years, range 38-84 years) who underwent operation in the Department of Cardiothoracic Surgery of Second Affiliated Hospital to Sun Yat-Sen University were studied. None of the patients had received any preoperative treatment. The follow-up times are more than 28 mo. Of the 80 patients, 58 were dead, and 22 still alive. The post-operative survival time of the dead patients were between 1.5 and 63 (mean 17.2) mo. Tissue specimens were fixed promptly with 100 g/L formaldehyde solution, embedded in paraffin and cut into 4 μm sections.
The two-step method was used to detect the expression of G3BP and RhoC. The mouse monoclonal antibody against human G3BP was purchased from Department of Pathology, Health Science Center, Peking University, Beijing, China. PowerVisionTM Two-Step Histostaining Reagent (PV-6002) and DAB Reagent were purchased from Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China. The goat polyclonal antibody against human RhoC was purchased from Santa Cruz Company. The HRP conjugated rabbit anti-goat IgG was purchased from Boster Biotechnology Company.
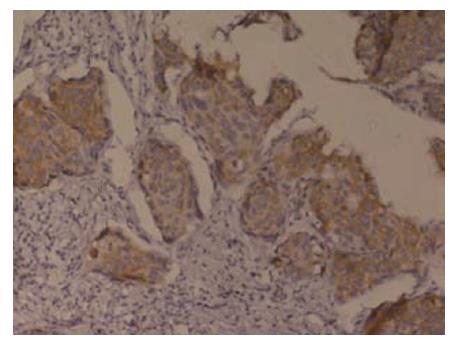
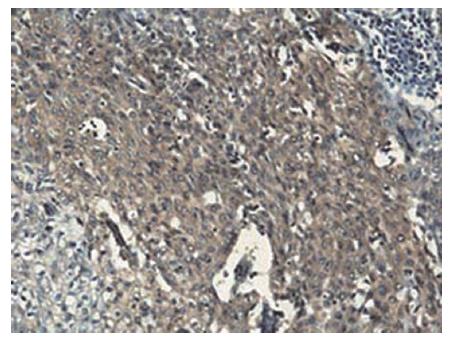
All sections were deparaffinnized and dehydrated with graded alcohol. Endogenous peroxidase was then blocked with 3 mL/L hydrogen peroxide methanol for 10 min at room temperature. Antigen retrieval was performed by treating the slides in citrate buffer in a microwave for 15 min. The sections were cooled for 20 min at room temperature, and then washed with distilled water for 3 × 5 min. The slides were incubated in a moist chamber with G3BP mouse monoclonal antibody (1:50) and RhoC goat polyclonal antibody (1:100) at 37°C for 2 h. After a complete wash in PBS, the slides were treated with the HRP conjugated goat anti-mouse IgG and the HRP conjugated rabbit anti-goat IgG (1:100) respectively for 25 min at 37°C. After a complete wash in PBS, the slides were developed in 0.05% freshly prepared diaminobenzedine solution (DAB) for 10 min, and then counterstained with hematoxylin, dehydrated, air-dried, and mounted. PBS was used to substitute the primary antibody as a negative control. Positive staining with G3BP and RhoC was defined as brown staining of cytoplasm. The determination was performed by two persons.
We used the t test for the comparison of tumor size. The comparison of rate was performed by the rank sum test (Kruskal Wallis and Wilcoxon’s test). Postoperative prognosis was evaluated by the Kaplan-Meier method and compared between groups by the log-rank test. The relationship of the expression of G3BP and RhoC was analysed by Chi-square test. Differences were considered as significant when the P value was less than 0.05. All statistical analyses were performed using SPSS Win program package 11.5.
Immunohistochemical staining showed that G3BP was expressed in 57 (71.25%) of 80 cases and RhoC was expressed in 53 (66.25%) of 80 cases (Figure 1 and Figure 2).
The expression rates of G3BP and RhoC protein in the lymph node metastasis group were significantly higher than those in non-lymph node metastasis group (Z = -2.283, P < 0.05; Z = 2.115, P < 0.05, respectively), but no relation was found between the two proteins expression and tumor size, differentiation degree and TNM stage (P > 0.05) (Table 1).
| G3BP | RhoC | ||||||
| Variables | n | - | + | P | - | + | P |
| Tumor size (cm) | 5.0 ± 2.1 | 5.7 ± 2.9 | 0.265 | 5.1 ± 2.0 | 5.7 ± 3.0 | 0.396 | |
| Differentiation | |||||||
| Well | 43 | 14 | 29 | 0.717 | 17 | 26 | 0.469 |
| Mediate | 24 | 6 | 18 | 7 | 17 | ||
| Low | 13 | 3 | 10 | 3 | 10 | ||
| TNM stage | |||||||
| Stage 1 | 2 | 2 | 0 | 0.083 | 2 | 0 | 0.182 |
| Stage 2 | 47 | 15 | 32 | 17 | 30 | ||
| Stage 3 | 23 | 5 | 18 | 6 | 17 | ||
| Stage 4 | 8 | 1 | 7 | 2 | 6 | ||
| Lymph node metastasis | |||||||
| No | 43 | 17 | 26 | 0.022 | 19 | 24 | 0.034 |
| Yes | 37 | 6 | 31 | 8 | 29 | ||
As shown by the Kaplan-Meier curve, both G3BP and RhoC, the overall survival rates of the positive expression group were significantly lower than those of the negative expression group (P < 0.001) (Figure 3 and Figure 4).
Among the 80 cases, 49 cases had positive expression of both G3BP and RhoC, 19 cases had negative expression of G3BP and RhoC, 8 cases had positive expression of G3BP, and 4 cases had positive expression of RhoC.There was positive correlation between the two proteins expression (rs = 0.656, P < 0.001).
G3BP was first isolated by Parker from the Chinese hamster lung fibroblasts in 1996[2]. It is comprised of 466 amino acids, and located in the cytoplasm. Experiment confirmed that G3BP promotes S phase entry in cultured fibroblasts[3]. According to recent researches, G3BP is the unique protein that could bind with Ras-GTPase-activiting protein SH3 domain. The effects of Ras signal transduction pathway in the tumorigenesis and metastasis have been confirmed[4]. In the Ras signal transduction pathway, Ras-GTPase-activiting protein (GAP) attenuated the function of Ras by promoting the hydrolysis from GTP that conjugated with activated Ras to GDP, and then acted as a moderator of Ras signal transduction pathway. The SH3 domain of Ras-GAP was indispensable, for it played an important role in the Ras signal transduction pathway[5]. Up to now, the proteins that could bind with Ras-GAP have been discovered, such as p62, p190, but they have no relations with SH3 domain[6,7].
G3BP expresses in many kinds of malignant tumors in different genetic locums and stages, so it probably participates in the process of tumorigenesis and metastasis. French et al[8] discovered that G3BP expression was specific in human breast cancer tissues and was overexpressed in 88% of tumors examined. Ning et al[9] demonstrated that G3BP was overexpressed in formalin-fixed and paraffin-embedded tissues of some human tumors, such as lung cancer, colon cancer, gastric cancer and breast cancer through immunohistochemical staining. In breast cancer specimens, the degree of G3BP expression correlated positively with the presence of lymph node metastasis. Our study demonstrated that the expression of G3BP had a close relationship with lymph node metastasis, and negative expression group had longer survival time than positive expression group. G3BP may play an important role in the invasion and lymph node metastasis of ESC. The expression of G3BP can be considered as an independant predictor of prognosis of ESC patients. The subtle mechanisms of G3BP inducing tumorigenesis and metastasis remains unknown. Further researches are needed to develop new anti-tumor drugs.
RhoC is a member of the Ras-superfamily, and it is an important molecule of the signal transduction pathway. Madaule et al[10] identified a new family of ras genes, the rho genes. It participates in many steps of tumorigenesis and metastasis, such as regulating the cystoskeleton and cell cycle, modulating cell adhesion and movement, impacting cell transformation, facilitating tumor cell genesis, invasion and metastasis, etc. RhoC enhances cellular migrating ability by regulating actin-based cystoskeleton, and plays a prominent role in the invasion and metastasis of tumor. Clark et al[11] discovered that the metastatic capacity of melanoma cells was enhanced when the RhoC gene was overexpressed. He proposed for the first time the view that the RhoC gene might be the switch for tumor invasion and metastasis. RhoC expressed in many kinds of malignant tumors. Kleer et al[12] demonstrated that RhoC protein level in tumor tissue was strongly associated with biologically aggressive invasive carcinomas of the breast. Yao et al[13] indicated that RhoC GTPase was required for PC-3 prostate cancer cell invasion in vitro. Wang et al[14] demonstrated a positive association between RhoC gene overexpression and tumor invasion and lymphatic metastasis in gastric carcinoma.
Our study demonstrated that the expression of RhoC had a close relationship with lymph node metastasis, and the negative expression group had longer survival time than the positive expression group. RhoC may play an important role in the invasion and lymph node metastasis of ESC. The expression of RhoC can be considered as an predictor of predictoof prognosis of ESC patients. Faried et al[1] found that RhoA and RhoC proteins promoted both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo, and in the two proteins, RhoC was more prominent in enhancing cellular migration. This is coincident with our research. It is significant to further study the role of RhoC in the invasion and metastasis of malignant tumors. RhoC may be considered as an independant predictor of the invasive and metastatic capacity of malignant tumors, and provide powerful assistance for judging prognosis of malignant tumor patients.
Like other members of the Ras-superfamily, RhoC protein cycles between the active, GTP-bound form and the inactive GDP-bound form. Ras is regulated by GTPase activating proteins (GAPs), which attenuate signaling. The SH3 domain of GAPs is indispensable, for it plays an important role in the Ras signal transduction pathway. G3BP is a unique protein that could bind with Ras-GTPase-activiting protein SH3 domain. There was positive correlation between the two proteins expression in ESC. So it is reasonable to presume that G3BP and RhoC proteins promote the invasion and metastasis of tumors through the same pathway and different effective sites.
Many patients with esophageal carcinoma are in intermediate or advanced stage when they see the doctors. Because of this, early diagnosis, early treatment and energetic prevention are very important for esophageal carcinoma. The current routine examination methods are insufficient for the diagnosis of small cancer focus and metastatic focus when they are in subclinical stage. However, the detection of tumor markers can retrieve the insufficiency of the examination methods. The tumor markers not only can be used for the early diagnosis of cancer, but also for post-treatment follow-up, recurrence detection, therapeutic effect evaluation and prognosis monitoring.
The effects of Ras signal transduction pathway in the tumorigenesis and metastasis have been confirmed. SH3 domain of Ras-GAP was indispensable, for it plays an important role in the Ras signal transduction pathway. G3BP is a unique protein that could bind with Ras-GTPase-activiting protein SH3 domain. G3BP expresses in many kinds of malignant tumors at different genetic locums and different stages. Up to now, no study is available in the relationship between G3BP and esophageal carcinoma. RhoC is a member of the Ras-superfamily, and it is an important molecule of signal transduction pathway. It participates in many steps of tumorigenesis and metastasis, such as regulating cystoskeleton and cell cycle, modulating cell adhesion and movement, impacting cell transformation, facilitating tumor cell genesis, invasion and metastasis, etc.
The authors investigated the expression of G3BP in esophageal squamous carcinoma for the first time, and confirmed that it is overexpressed in esophageal squamous carcinoma. They discovered that the expressions of G3BP and Rhoc proteins have a close relationship with lymph node metastasis and survival in ESC patients.
G3BP and RhoC proteins can be considered as predictors of prognosis of ESC patients. Further researches about the subtle mechanisms of G3BP and RhoC can provide extensive perspective for the development of new antitumor drugs.
G3BP: Ras-GTPase-activiting protein SH 3 domain binding protein. It is a unique protein that could bind with Ras-GTPase-activiting protein SH3 domain specifically. It expresses in many kinds of malignant tumors. RhoC: Ras homology C. It is a member of the Ras-superfamily, and it is an important molecule of signal transduction pathway. It participates in many steps of tumorigenesis and metastasis, such as regulating cystoskeleton and cell cycle, modulating cell adhesion and movement, impacting cell transformation, facilitating tumor cell genesis, invasion and metastasis, etc.
This is an interesting manuscript reporting two new immunohistochemical markers (G3BP and RhoC) which appear to serve as prognosticators for esophageal squamous carcinoma. This new information is certainly worthy of publication.
S- Editor Zhu LH L- Editor Ma JY E- Editor Lu W
| 1. | Faried A, Faried LS, Kimura H, Nakajima M, Sohda M, Miyazaki T, Kato H, Usman N, Kuwano H. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, Debussche L, Dugue A, Schweighoffer F, Tocque B. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell Biol. 1996;16:2561-2569. [PubMed] |
| 3. | Guitard E, Parker F, Millon R, Abecassis J, Tocqué B. G3BP is overexpressed in human tumors and promotes S phase entry. Cancer Lett. 2001;162:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Waldmann V, Rabes HM. What's new in ras genes? Physiological role of ras genes in signal transduction and significance of ras gene activation in tumorigenesis. Pathol Res Pract. 1996;192:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Duchesne M, Schweighoffer F, Parker F, Clerc F, Frobert Y, Thang MN, Tocqué B. Identification of the SH3 domain of GAP as an essential sequence for Ras-GAP-mediated signaling. Science. 1993;259:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Tang J, Feng GS, Li W. Induced direct binding of the adapter protein Nck to the GTPase-activating protein-associated protein p62 by epidermal growth factor. Oncogene. 1997;15:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Leblanc V, Tocque B, Delumeau I. Ras-GAP controls Rho-mediated cytoskeletal reorganization through its SH3 domain. Mol Cell Biol. 1998;18:5567-5578. [PubMed] |
| 8. | French J, Stirling R, Walsh M, Kennedy HD. The expression of Ras-GTPase activating protein SH3 domain-binding proteins, G3BPs, in human breast cancers. Histochem J. 2002;34:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ning JY, You JF, Pei F, Wang JL, Cui XL, Zheng J. Monoclonal antibody against G3BP: preparation, characterization and its application in analysis of human tumors. Zhonghua BingLiXue ZaZhi. 2005;34:215-219. [PubMed] |
| 10. | Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 348] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1081] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 12. | Kleer CG, Griffith KA, Sabel MS, Gallagher G, van Golen KL, Wu ZF, Merajver SD. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat. 2005;93:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Wang ZN, Xu HM, Jiang L, Zhou X, Lu C, Zhang X. Positive association of RhoC gene overexpression with tumour invasion and lymphatic metastasis in gastric carcinoma. Chin Med J (Engl). 2005;118:502-504. [PubMed] |