Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4046
Revised: January 25, 2007
Accepted: March 8, 2007
Published online: August 14, 2007
Hepatorenal syndrome (HRS) is a “functional” and reversible form of renal failure that occurs in patients with advanced chronic liver disease. The distinctive hallmark feature of HRS is the intense renal vasoconstriction caused by interactions between systemic and portal hemodynamics. This results in activation of vasoconstrictors and suppression of vasodilators in the renal circulation. Epidemiology, pathophysiology, as well as current and emerging therapies of HRS are discussed in this review.
- Citation: Turban S, Thuluvath PJ, Atta MG. Hepatorenal syndrome. World J Gastroenterol 2007; 13(30): 4046-4055
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4046.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4046
The association between liver disease and renal dysfunction was reported more than a century ago when patients with chronic liver disease and normal renal histology were found to develop oliguric renal failure (Flint A, Am J Med Sci 1863). This led to proposed links between renal dysfunction and the derangement in systemic circulation secondary to the liver failure[1].
Renal failure in patients with liver disease may be caused by several factors, including shock, sepsis, nephrotoxic medications, intrinsic renal diseases, or volume depletion secondary to diuresis or large-volume paracentesis. However, renal failure may also occur in patients with liver disease in the absence of the above factors and in the absence of major renal histological changes. This is referred to as hepatorenal syndrome (HRS). HRS is considered a “functional” and reversible form of renal failure[2-6]. The International Ascites Club defined HRS as: “a syndrome that occurs in patients with advanced chronic liver disease and advanced hepatic failure and portal hypertension characterized by impaired renal function and marked abnormalities in the arterial circulation and activity of the endogenous vasoactive systems. In the kidney, there is marked renal vasoconstriction that results in low glomerular filtration rate (GFR). In the extrarenal circulation, there is a predominance of arterial vasodilation, that results in reduction of total systemic vascular resistance and arterial hypotension". The incidence of HRS in patients with chronic liver disease is not well studied. In one study of 234 non-azotemic patients with liver disease who had ascites and cirrhosis, 18% developed HRS at 1 year, and 39% by 5 years[7]. Although HRS usually occurs in patients with advanced cirrhosis, it has also been described in patients without ascites in the setting of acute fulminant hepatic failure[8].
Approximately 80% of hospitalized patients with cirrhosis and ascites have decreased renal perfusion due to moderate vasoconstriction in the renal circulation, which predisposes them to develop HRS[7-9]. In 10%-17% of these patients, renal vasoconstriction becomes intense enough to cause significant renal hypoperfusion, resulting in HRS[7,10]. This intense renal vasoconstriction is the distinctive hallmark feature of HRS[11,12]. The mechanisms of renal vasoconstriction are complex and multifactorial, and are incompletely understood. There appear to be interactions between changes in systemic hemodynamics, portal hypertension, activation of vasoconstrictors, and suppression of vasodilators in the renal circulation[13,14]. In contrast, significant vasodilation occurs in the splanchnic arterial bed secondary to increased production of local vasodilators, predominantly nitric oxide[15]. Other vasodilators hypothesized to play a role in splanchnic arterial vasodilation include prostacyclin, prostaglandin E2, atrial natriuretic peptide, kallikreins, and kinins[10,16,17]. This splanchnic vasodilation is believed to lead to compensatory responses by activating vasoconstrictors including the renin-angiotensin-aldosterone system (RAAS), neuropeptide Y, endothelin-1, norepinephrine, thromboxane A2, adenosine, and antinatriuretic agents such as arginine vasopressin (AVP). This leads to retention of sodium and water in addition to renal vasoconstriction[15,18,19]. Other factors such as the absence of or decrease in glomerulopressin or other liver-borne diuretic factors (factors that are released by the liver and target the kidney) could also contribute to renal failure[20]. In recent years, the potential role of cirrhotic cardiomyopathy has been postulated in the pathogenesis of HRS. Ruiz-del-Arbol et al[21] have demonstrated that HRS is due to decreased cardiac output in the setting of a severe arterial vasodilation. Similar circulatory events were also shown in cirrhotic patients who developed spontaneous bacterial peritonitis[22].
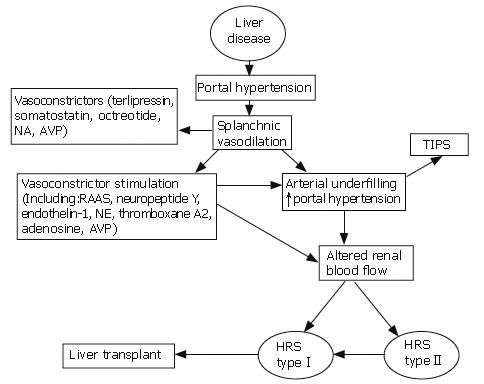
In the early stages of cirrhosis, the activation of local vasodilators may overcome the renal vascular effects of systemic vasoconstrictors, maintaining adequate renal perfusion[23]. As liver disease progresses, the renal vasodilators are no longer able to antagonize the circulating vasoconstrictors, and this results in severe renal vasoconstriction and impaired renal blood flow. In addition, the hypoperfusion itself may lead to further intrarenal vasoconstriction. Figure 1 summarizes the complex pathways involved in the development of HRS and potential therapeutic interventions.
There are two types of HRS (Table 1). Type 1 is characterized by a rapid elevation in blood urea nitrogen (BUN) and creatinine, often defined as a 100% increase in serum creatinine, reaching a level higher than 2.5 mg/dL in less than two weeks[2]. The mortality of patients with type 1 HRS has been reported to be 80% at two weeks[7]. Type 2 HRS, on the other hand, generally follows a slower course and has a better prognosis. It is usually characterized by recurrent, diuretic-resistant ascites[10,14], and is thought to be due to significant activation of anti-natriuretic systems[2].
| Type 1 | |
| (1) 100% increase in serum creatinine to a level higher than 2.5 mg/dL or a 50% reduction of the initial 24-h creatinine clearance to a level | |
| (2) Very poor short-term outcome | |
| Type 2 | |
| (1) Serum creatinine > 1.5 mg/dL, without meeting the criteria for type 1 HRS | |
| (2) Refractory ascites is usually present | |
| (3) Prognosis is not as poor as with type 1 | |
HRS may develop spontaneously without known precipitating factors, but there are known triggers[14,24]. Spontaneous bacterial peritonitis (SBP) has been associated with type 1 HRS in approximately 20% of cases[21,25], even with treatment and resolution of the infection. These patients have a very poor outcome. HRS may also occur after therapeutic paracentesis without plasma expansion[26,27]. Gastrointestinal bleeding has also been identified as a precipitant of HRS, but this usually occurs in patients with hypovolemic shock. In this setting, acute renal tubular ischemic injury or necrosis is more likely to be the cause of acute renal failure than HRS[28]. There is no clear evidence to support diuretic-induced volume depletion as a precipitating factor of HRS[14]. Other factors that have been associated with an increased risk of developing HRS in patients with ascites and cirrhosis include severe urinary sodium retention, spontaneous dilutional hyponatremia, and a mean arterial blood pressure less than 80 mmHg. There is not a direct linear association between the severity of liver failure and the incidence of HRS, but HRS is usually seen in patients with advanced liver disease and portal hypertension[14,23].
There are no specific clinical or laboratory findings for the diagnosis of HRS. The diagnosis is established based on predefined criteria in the appropriate clinical setting (Table 2). Patients with advanced liver disease may develop renal failure from a number of causes other than HRS, and these causes must be excluded before making a diagnosis of HRS. Common causes of renal failure in patients with cirrhosis include volume depletion (which could be secondary to over-diuresis, diarrhea, or poor fluid intake), nephrotoxic medications (commonly non-steroidal anti-inflammatory agents and aminoglycosides), allergic interstitial nephritis, acute tubular necrosis (from various factors including shock), contrast nephropathy, and intrinsic renal diseases such as glomerulonephritis. A renal biopsy may rarely be necessary if the diagnosis is unclear, mainly to exclude other treatable renal diseases. It is also important to note that there are significant limitations in using serum creatinine as a marker of renal function in patients with liver disease. Patients with advanced liver disease usually have reduced muscle mass and hence low endogenous production of creatinine. When creatinine clearances in cirrhotic patients were compared with inulin clearances, the glomerular filtration rates were significantly overestimated[29]. Alternative diagnostic approaches have been applied in order to overcome the limitation of serum creatinine values in this population. Platt et al[9] examined the utility of Doppler ultrasonography to assess the resistive indices of the renal vasculature. In their study of 180 patients with liver disease without azotemia, 42% of the patients were found to have an increase in renal vascular resistive indices. Of those patients, 55% subsequently developed renal failure as compared to 6% of those with normal resistive indices. The sensitivity and specificity of the resistive index in detecting renal failure were estimated at 71% and 80% respectively in a group of cirrhotic patients[30]. However, this technique is operator-dependent and is still under investigation, and therefore is not currently recommended as a standard method to diagnose HRS.
| Major criteria (all must be present for the diagnosis of HRS) |
| (1) Advanced hepatic failure (acute or chronic liver disease) and portal hypertension |
| (2) Low GFR defined as serum creatinine > 1.5 mg/dL or creatinine clearance < 40 mL/min |
| (3) Absence of shock, significant volume losses, ongoing infection, or treatment with nephrotoxic medications |
| (4) Absence of a sustained improvement in renal function after cessation of diuretics and expansion of plasma volume with 1.5 L of isotonic fluids |
| (5) Urine protein excretion < 500 mg/dL with no ultrasonographic evidence of obstruction or parenchymal renal disease |
| Additional criteria (not necessary for the diagnosis, but provide supportive evidence) |
| (1) Urine volume < 500 mL/d |
| (2) Urine sodium < 10 mEq/L |
| (3) Urine osmolality greater than plasma osmolality |
| (4) Urine red blood cells < 50 per high-power field |
| (5) Serum sodium concentration < 130 mEq/L |
The prognosis of HRS is extremely poor. The median survival time of patients with type 1 HRS is less than 2 wk, with less than 10% surviving their hospital stay[7]. The survival time of patients with type 2 HRS, although still short, is significantly longer, with a median survival time of approximately 6 mo[14].
Prevention of HRS is potentially possible in some high-risk patients. In patients with SBP, administering intravenous albumin (1.5 g/kg upon diagnosis, and then 1 g/kg after 48 h) in addition to antibiotics has been shown to decrease the incidence of HRS and to decrease hospital mortality as compared with treatment with antibiotics alone[26]. The authors of that study postulated that the administration of albumin prevented circulatory dysfunction by maintaining effective arterial blood volume and therefore prevented vasoconstrictor activation. However, albumin is expensive, and more studies are needed to determine if lower doses of albumin or less expensive artificial plasma expanders are as effective. In one study, administration of pentoxifylline (400 mg orally three times a day) to patients with severe acute alcoholic hepatitis decreased the incidence of HRS as well as the short-term mortality rate compared to placebo[31]. This benefit may be related to the inhibition of tumor necrosis factor production. Although both of the above studies support the idea of preventing renal failure in the setting of liver failure, there are no data evaluating the long-term survival benefit in this population. Moreover, there have been no further confirmatory studies.
In patients with type 1 HRS, diagnostic paracentesis is generally recommended to evaluate for SBP. In addition, diuretics should be discontinued as they may potentially worsen renal function. In the absence of contraindications, patients with type 1 HRS should also be evaluated for expedited liver transplantation.
Several systemic vasoconstrictors have been utilized in the treatment of type 1 HRS as summarized in Table 3. Renal vasodilators such as dopamine and prostaglandin analogues are no longer recommended due to their side effect profile and the lack of clinical evidence to support their use. Other potential forms of therapy that have not been extensively tested include endothelin blockers[32] and N-acetylcysteine[33].
| Authorand Year | n (# Type 1,# Type 2) | Study design | Intervention | Outcomemeasures | MeanbaselineSCr | Meanfollow-upSCr | Other results | Comments |
| Moreau et al[38] 2002 | 99 (99/0) | Multicenter, retrospective | Terlipressin (75% received albumin) | Reduction of SCr to < 130 μmol/L or a decrease of at least 20% at end of treatment) | 272 ± 114 μmol/L | Responders: 138 ± 59 μmol/L Nonresponders: 382 ± 210 μmol/L | Renal function improved in 58% of patients. | Twenty-three patients had adverse events that may have been terlipressin-related. Three patients required RRT 40% survival at 1 mo. |
| Kiser et al[44] 2005 | 43 (32/11) | Observational (retrospective cohort) | Vasopressin (AVP) vs octreotide vs combination | Clinical response; SCr 1.5 mg/dL or less | 3.9 ± 3.3 mg/dL | Responders: SCr decreased by 62% ± 9% Nonresponders: SCr increased by 46% ± 119% | 42% complete response with AVP vs 38% with AVP and octreotide vs 0% with octreotide alone. | No adverse effects related to AVP. RRT rates: 50% in AVP group, 58% in combination group, and 31% in octreotide alone group. |
| Solanki et al[43] 2003 | 24 (24/0) | Randomized placebo- controlled single-blind | Terlipressin vs placebo (all patients received albumin) for 4-15 d | Reversal of HRS and survival at 15 d | Terlipressin: 2.9 ± 0.1 mg/dL Placebo: 2.2 ± 0.2 mg/dL | Terlipressin: 1.2 ± 0.2 mg/dL at d 15 Placebo: no survival at d 15 (SCr 3.9 ± 0.2 mg/dL on d 8) | In terlipressin group, 5 of 12 patients survived. None survived by d 15 in placebo group. | |
| Ortega et al[39] 2002 | 21 (16/5) | Prospective, nonrandomized | Terlipressin with albumin vs without albumin for 4-14 d | SCr 1.5 mg/dL or lower | Terlipressin with albumin: 3.6 ± 1.5 mg/dL Terlipressin without albumin: 3.4 ± 0.3 mg/dL | Terlipressin with albumin: 1.5 ± 0.2 mg/dL Terlipressin without albumin: 3.4 ± 0.7 mg/dL | 10 of 13 patients who received terlipressin and albumin responded. Of 8 patients who received terlipressin alone, 2 responded. | One patient had ischemic side effects (finger ischemia). At 1 mo, there was a 5% recurrence of HRS after complete response. |
| Pomier- Layrargues et al[61] 2003 | 19 (NS) | Randomized, double-blind, placebo- controlled, crossover | Placebo, then octreotide (Group 1) vs octreotide, then placebo (Group 2) (all patients received albumin) | 20% decrease in SCr after 4 d | Group 1: 215 ± 32 μmol/ Group 2: 208 ± 16 μmol/L | Group 1: 222 ± 41 μmol/L after placebo; 270 ± 54 μmol/L after octreotide Group 2: 194 ± 34 μmol/L after octreotide; 204 ± 47 μmol/L after placebo | Treatment with octreotide was not effective. | The study included types 1 and 2 HRS patients (numbers in each group not specified). No side effects reported. |
| Colle et al[42] 2002 | 18 (18/0) | Chart review (retrospective analysis) | Terlipressin (some patients received albumin) | Decrease in SCr to < 130 μmol/L or decrease of at least 20% leading to a stable value; evaluation of predictive factors | Patients with improved SCr: 276 ± 47 μmol/L1 Patients without improved SCr: 295 ± 891μmol/L | Patients with improved SCr: 130 ± 13 μmol/L Patients with improved SCr: 411 ± 89 μmol/L | 11 patients had improved renal function | Some of these patients were included in the Moreau study. Patients with improved renal function had less severe cirrhosis than patients without. Patients without a precipitating factor for HRS or who responded to terlipressin were more likely to survive. |
| Halimi et al[41] 2002 | 18 (16/2) | Multicenter pilot (retrospective) | Terlipressin for 2-16 d | > 30% decrease in baseline SCr | 298 ± 124 μmol/L | 145 ± 85 μmol/L | 13 of 18 had improved renal function; 8 had a normal SCr at d 5 | Three patients had ischemic side effects. One had severe bronchospasms after terlipressin administration, and subsequently died. |
| Guevara et al[49] 1998 | 16 (Type NS) | Open pilot study | Ornipressin and albumin for 3 vs 15 d | Efficacy | 3-d arm: 2.9 ± 0.5 mg/dL 15-d arm: 3.0 ± 0.5 mg/dL | 3-d arm: 2.2 ± 0.4 mg/dL 15-d arm: 0.7 ± 0.1 mg/dL | 75% of patients had improved renal function. | Treatment was stopped in 4 patients on the 15-d protocol because of ischemic complications. |
| Angeli et al[62] 1999 | 13 (13/0) | Nonrandomized | Dopamine and albumin (Group A) vs midodrine, octreotride, and IV albumin (Group B) | Efficacy | Group A: 3.6 ± 0.6 mg/dL Group B: 5.0 ± 0.9 mg/dL | Group A: 5.1 ± 1.5 mg/dL at 15 d (only 1 patient survived to d 20) Group B: 3.3 ± 0.7 mg/dL at 20 d | All Group B patients had improved GFR. 7 of 8 patients in Group A had worsening renal function and died. | No significant side effects. |
| Holt et al[33] 1999 | 12 (NS) | Open label | N-acetylcysteine for 5 d | Efficacy | 222 ± 27 μmol/L | 169 ± 7 μmol/L | 67% survival at 1 mo, and 58% at 3 mo (2 patients received liver transplants). | |
| Mulkay et al[40] 2001 | 12 (12/0) | Pilot | Terlipressin for 1 wk to 2 mo | Safety and efficacy | 3.4 mg/dL | 1.8 mg/dL | Three patients received liver transplants, and had near-normal renal function. The other 9 died during follow-up. No ischemic complications. | |
| Duvoux et al[48] 2002 | 12 (12/0) | Pilot | Noradrenalin (NA), albumin, and furosemide for at least 5 d | Safety and efficacy | 2.6 ± 1.1 mg/dL pre- furosemide/ albumin; 3.9 ± 1.8 mg/dL after infusion (pre-NA) | 1.6 ± 0.8 mg/dL | Reversal of HRS in 10 of 12 patients | Two patients had previously received terlipressin (underwent 48-h washout before starting NA). Transient myocardial ischemia was observed in 1 patient. |
| Hadengue et al[63] 1998 | 9 (9/0) | Double-blind, short-term, controlled crossover study | Terlipressin and placebo for 2 d in randomized order | Efficacy | Baseline CrCl: 15 ± 2 mL/min | CrCl after terlipressin (includes both groups): 27 ± 4 mL/min CrCl after placebo (includes both groups): 16 ± 3 mL/min | No side effects reported. | |
| Uriz et al[37] 2000 | 9 (6/3) | Pilot | Terlipressin with albumin for 5-15 d | Reduction of serum creatinine to < 1.5 mg/dL | 3.9 ± 0.7 mg/dL | 1.5 ± 0.2 mg/dL | Reversal of HRS in 7 of 9 patients. | One patient did not complete the study due to pancreatitis. No ischemic complications. |
| Angeli et al[45] 1998 | 8 (0/8) + 17 cirrhotic patients without HRS | Open label | Midodrine (one dose) | Renal function and renal hemo- dynamics (acute effects) | GFR: 39.0 ± 6.4 mL/min | GFR: 45.1 ± 7.6 mL/min | No significant acute effect on renal hemodynamics or renal function. | This study looked at the acute effect of one dose of midodrine. The results include cirrhotic patients without HRS. |
| Gulberg et al[64] 1999 | 7 (7/0) | Nonrandomized | Orinpressin, dopamine, and albumin for 5-27 d | 2× increase in Crcl (to > 40 mL/min) | Treatment success group: 4.6 ± 0.9 mg/dL, and improved to 1.3 ± 0.2 mg/dL | Treatment success group: 1.3 ± 0.2 mg/dL | HRS was reversed in 4 of 7 patients | Two responders had a relapse. One of them responded to retreatment, but treatment was stopped in the other because of a ventricular tachyarrhythmia. Treatment was stopped in another patient because of intestinal ischemia. |
| Kaffy et al[65] 1999 | 5 (NS) | Pilot | Octreotide for 5 d | Efficacy | 194 μmol/L in 4 patients | 96 μmol/L in 4 patients | Improvement of SCr in 4 of 5 patients, | but 4 of 5 patients eventually died. HRS rapidly recurred when octreotide was stopped, and did not respond to further octreotide infusion. |
The rationale behind the use of vasoconstrictors along with plasma expansion is that they will counteract the splanchnic arterial vasodilation, which is hypothesized to be the initial event in the pathogenesis of HRS. Unopposed splanchnic arterial vasodilation may cause a decrease in effective arterial volume which in turn triggers the activation of vasoconstrictors[23,34]. Vasoconstrictors that have been widely used for type 1 HRS include vasopressin analogues (ornipressin and terlipressin), a somatostatin analogue (octreotide), and alpha-adrenergic analogues (midodrine and noradrenalin). In most studies, albumin was administered concurrently.
The vasopressin analogues are effective in causing marked splanchnic vasoconstriction. Ornipressin, although effective in treating HRS, may cause significant ischemic side effects and is not currently recommended for the management of HRS[35]. Studies using terlipressin, the long-acting analogue of vasopressin, have shown significant improvement in renal function in approximately 60%-75% of patients, with a lower than 5% incidence of ischemic side effects[36-43]. In these studies, patients with Child-Pugh scores less than or equal to 13 and/or those who received albumin infusions had a more favorable outcome. However, it is important to note that GFR was not normalized in most patients who responded[37,39]. Approximately 15% of patients had recurrence of HRS once treatment was discontinued. Small, short-term, non-randomized studies suggest that treatment with terlipressin may also improve renal function in patients with type 2 HRS[34]. Terlipressin is not currently licensed for use in North America, but a double-blind, randomized, placebo-controlled trial is now being conducted in the USA and Germany in patients with type 1 HRS. Alpha-1 adrenoreceptor agonists and a somatostatin analogue are readily available in North America and have been studied in type 1 HRS. An observational study compared vasopressin infusion with octeotride infusion in patients with HRS, and found a complete response rate of 41% in the patients treated with vasopressin compared with 0% in the patients treated with octreotide[44]. In type 1 HRS, alpha-1 agonists have only been used in combination with other agents. Few nonrandomized, prospective studies have evaluated treatment with both midodrine and octreotide[45-47]. The study by Angeli[45] included only five patients and showed that after 20 d of treatment, all patients had serum creatinine levels below 2 mg/dL. In the study by Wong et al[46], 10 of 14 patients with HRS treated with midodrine, octreotide, and albumin had their serum creatinine stable at less than 1.5 mg/dL for three days. The use of noradrenalin in combination with intravenous albumin and furosemide was studied in 12 patients[48]. HRS was reversed in 83% of patients, with an adverse event rate of 17%. These small studies suggest a short-term benefit in improving renal function in HRS patients, although larger, randomized studies are required before recommending the routine use of these agents in clinical practice. Other drugs, such as N-acetylcysteine and misoprostol, have been proposed as therapy for HRS, but have not been well-studied.
Transjugular intrahepatic portosystemic shunts (TIPS), by reducing portal hypertension, may be useful in treating HRS (Table 4), although no trials have shown a survival advantage[49-51].
| Author andYear | N (# Type 1,# Type 2) | Study design | Intervention | Outcomemeasures | Meanbaseline SCr | Meanfollow-up SCr | Other results | Comments |
| Brensing et al[50] 2000 | 31 (14/17); an additional 10 were too sick to receive TIPS | Phase II | TIPS | Safety and survival | (Of the 31 patients who received TIPS) 2.3 ± 1.7 mg/dL | Wk 4: 1.5 ± 1.2 mg/dL | Renal function improved within 2 wk after TIPS and subsequently stabilized. | Three-month survival rate was 81% (10% of non- shunted patients survived, but they were felt to be too sick to receive TIPS). There was 1 TIPS-related death. |
| Wong et al[46] 2004 | 14 (14/0) | Prospective | Midodrine, octreotide, albumin, and TIPS | Efficacy (serum creatinine < 135 μmol/L for at least 3 d) | Responders: 233 ± 29 μmol/L Nonresponders: 345 ± 83 μmol/L | Responders: 112 ± 8 μmol/L after medical therapy Nonresponders: 476 ± 139 μmol/L after medical therapy. | Renal function improved in 10 of 14 patients (71%) with medical therapy. Five responders received TIPS; their renal function continued to improve. Mean GFR was 96 ± 20 mL/min by 12 mo post-TIPS. | TIPS was performed in responders who were stable. Two of the five responders who did not receive TIPS underwent liver transplantation, and their SCr remained normal at the time of liver transplantation. |
| Alessandria et al[36] 2002 | 16 (0/11, and an additional 5 with “organic renal disease”) | Prospective, nonrandomized | Terlipressin for 7 d (and TIPS in stable patients) | Efficacy | 2.4 ± 0.9 mg/dL | After terlipressin therapy: 1.8 ± 0.8 mg/dL After TIPS: 1.4 ± 0.3 mg/dL | Terlipressin: 8 of 11 HRS patients had improved renal function (and 7 of the 8 responders had reversal of HRS (SCr < 1.5 mg/dL) Subsequent TIPS: 8 of 9 patients (89%) who underwent TIPS had improved renal function by 1 mo. | Renal function improved significantly after TIPS in all patients who responded to terlipressin. One HRS patient who did not respond to terlipressin underwent TIPS and responded. In the non-HRS group (with “organic renal disease”, only one patient had an improved SCr (from 3.7 to 1.8 mg/dL) with terlipressin treatment. |
| Guevara et al[49] 1998 | 7 (7/0) | Prospective | TIPS | Efficacy | 4.9 ± 0.8 mg/dL | 1 wk after TIPS: 3.7 ± 1.0 mg/dL 1 mo after TIPS: 1.8 ± 0.4 mg/dL | Renal function improved in 6 of 7 patients. | Mean survival was 4.7 ± 2 mo. |
| Witzke et al[53] 2004 | 30 (NS) | Prospective | CVVHD (if mechanically ventilated) vs intermittent HD if not ventilated | Survival | N/A | N/A | 8 of 15 patients who received HD survived. None of the ventilated patients (received CVVHD) survived. | Note that the sickest patients (on a ventilator) all received CVVHD. |
| Keller et al[54] 1995 | 26 (NS); an additional 81 patients had liver disease and renal failure, but were not diagnosed with HRS | Retrospective | HD | Risk factor evaluation and outcomes | N/A | N/A | 7 of 16 patients with HRS who received HD survived, while only 1 out of 16 patients with HRS who did not receive HD survived. | |
| Mitzner et al[55] 2000 | 13 (Type 1) | Prospective, randomized, controlled | MARS and HDF and standard medical therapy vs HDF and medical therapy | Survival | MARS + HDF: 3.8 ± 1.5 mg/dL HDF alone: 4.4 ± 1.3 mg/dL | MARS + HDF: 2.3 ± 1.5 mg/dL HDF alone: 3.8 ± 0.5 mg/dL | At one week: 62.5% mortality in the treatment group, and 100% mortality in the group who did not receive MARS. | None of these patients underwent liver transplantation or received TIPS or vasopressin analogues during the observation period. |
| Jalan et al[66] 2003 | 8 (5/2, and one patient without HRS) | Prospective, nonrandomized | MARS | Safety and efficacy | 162 (51–312) μmol/L | 108 (34–231) μmol/L | 50% survival at 3 mo follow-up | All of the patients had alcoholic hepatitis and were encephalopathic. Renal function improved in all patients. Of the 5 patients with type 1 HRS, 3 remained anuric, but there was normalization of SCr in the other 2 patients. SCr was normalized in both patients with type 2 HRS by the end of treatment. |
| Mitzner et al[55] 2001 | 8 (NS) | Uncontrolled | MARS | Multiple organ function changes | 380 ± 182 μmol/L | 163 ± 119 μmol/L | Improvement in multiple organ functions |
Patients with HRS who progress to severe renal failure can be initiated on renal replacement therapy (RRT), generally given as continuous hemofiltration. Dialysis is usually used as a bridge in patients who are awaiting liver transplantation, and is not recommended for patients who are unlikely to recover from liver failure or are unlikely to receive liver transplantation because of other contraindications. Survival on dialysis is generally dependent on the severity of liver disease[52]. There are only a few small studies evaluating the effects of dialysis in HRS[53,54]. Keller et al[54], in a retrospective study, found that 7 of 16 patients with HRS who received RRT survived, while only 1 out of 16 patients with HRS who did not receive RRT survived. In the prospective study by Witzke et al[53], 30 patients with Child-Pugh C cirrhosis and HRS were treated with continuous veno-venous hemodialysis (CVVHD) if they were on mechanical ventilation, or with intermittent hemodialysis if they were not on mechanical ventilation. No patients on mechanical ventilation survived for more than 30 d, but 8 of 15 patients who were not on mechanical ventilation survived for more than 30 d. The absence of a control group and lack of randomization make it difficult to draw any firm conclusions from this study.
Molecular absorbent recirculating system (MARS) is a form of albumin dialysis, which removes “toxins” such as tumor necrosis factor-alpha, interleukin-6, and nitric oxide via binding to dialysate albumin. A small, randomized trial showed a survival advantage of MARS when compared to standard therapy in HRS patients[55]. To date, there have been no other published trials comparing MARS to standard RRT.
Liver transplantation (LT) is the only effective and permanent treatment for HRS[10,14,56,57] that cures both the liver and renal failure. However, the 5-year survival rate in LT recipients with HRS is significantly less than in LT patients without HRS[56]. Patients who undergo LT may sometimes require postoperative hemodialysis. It is preferable to delay administration of cyclosporine or tacrolimus until renal impairment improves in these patients, as these drugs may further worsen renal function. The issue of whether to transplant a kidney in addition to a liver (LKT, combined liver kidney transplantation) is an important one as well. HRS alone is not considered an indication for a LKT[58]. A renal biopsy may be helpful in some patients to identify the etiology of the renal failure and to determine the presence and extent of glomerular scarring[59]. LKT should be reserved for patients with irreversible renal failure, including HRS patients who are on dialysis for more than 8 wk or patients with progressive primary renal disease[59]. United Network of Organ Sharing (UNOS) data indicate a 5-year survival of LKT recipients of 62% compared with 50% for patients with a serum creatinine > 2.0 mg/dL receiving isolated LT (P = 0.0001). One center's results demonstrated a 5-year patient survival of 48% for LKT patients, 67% for HRS patients receiving isolated LT, and 70% for patients with a serum creatinine > 2.0 mg/dL receiving isolated LT (P not significant comparing all groups)[58]. It is not clear if the advances in management of HRS in recent years have had an impact on post-transplant outcomes. In a case-control study by Restuccia et al[60], patients with HRS treated with vasopressors and albumin prior to LT had similar survival outcomes compared to those patients who underwent OLT without HRS. However, the study had only 9 patients with HRS and as correctly stated by the authors, further confirmation in a larger series of patients is required. Clearly, further prospective studies are needed to guide transplant physicians to determine whether they should transplant the liver and the kidney or the liver alone in patients with liver failure and kidney failure.
Renal failure occurs commonly in patients with severe liver disease and its causes are multifactorial. Patients with type 1 HRS generally have a fatal outcome without expedited liver transplantation. Therapy with terlipressin and albumin looks promising, but there is a paucity of data to make firm conclusions. Use of other vasoconstrictors or TIPS remains experimental. The only proven treatment option is expedited liver transplantation. Dialysis is often used as a bridge to liver transplantation, but there are no controlled studies to support renal replacement therapy in type 1 HRS. Further research is necessary to better elucidate the mechanisms of HRS and to identify treatment strategies to reduce morbidity and mortality in patients with liver disease.
S- Editor Liu Y E- Editor Lu W
| 1. | Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;271:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1019] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 3. | Baldus WP, Feichter RN, Summerskill WH. The Kidney in Cirrhosis. I. Clinical and Biochemical Features of Azotemia in Hepatic Failure. Ann Intern Med. 1964;60:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Baldus WP, Feichter RN, Summerskill WH, Hunt JC, Wakim KG. The Kidney in Cirrhosis. II. Disorders of Renal Function. Ann Intern Med. 1964;60:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Shear L, Kleinerman J, Gabuzda GJ. Renal Failure in Patients with Cirrhosis of the Liver. I. Clinical and Pathologic Characteristics. Am J Med. 1965;39:184-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Iwatsuki S, Popovtzer MM, Corman JL, Ishikawa M, Putnam CW, Katz FH, Starzl TE. Recovery from "hepatorenal syndrome" after orthotopic liver transplantation. N Engl J Med. 1973;289:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [PubMed] |
| 8. | Wilkinson SP, Blendis LM, Williams R. Frequency and type of renal and electrolyte disorders in fulminant hepatic failure. Br Med J. 1974;1:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Platt JF, Ellis JH, Rubin JM, Merion RM, Lucey MR. Renal duplex Doppler ultrasonography: a noninvasive predictor of kidney dysfunction and hepatorenal failure in liver disease. Hepatology. 1994;20:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Dagher L, Moore K. The hepatorenal syndrome. Gut. 2001;49:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Epstein M, Berk DP, Hollenberg NK, Adams DF, Chalmers TC, Abrams HL, Merrill JP. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 281] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Schroeder ET, Shear L, Sancetta SM, Gabuzda GJ. Renal failure in patients with cirrhosis of the liver. 3. Evaluation of intrarenal blood flow by para-aminohippurate extraction and response to angiotensin. Am J Med. 1967;43:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Moore K. The hepatorenal syndrome. Clin Sci (Lond). 1997;92:433-443. [PubMed] |
| 14. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (33)] |
| 16. | Arroyo V, Ginés P, Rimola A, Gaya J. Renal function abnormalities, prostaglandins, and effects of nonsteroidal anti-inflammatory drugs in cirrhosis with ascites. An overview with emphasis on pathogenesis. Am J Med. 1986;81:104-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Arroyo V, Planas R, Gaya J, Deulofeu R, Rimola A, Pérez-Ayuso RM, Rivera F, Rodés J. Sympathetic nervous activity, renin-angiotensin system and renal excretion of prostaglandin E2 in cirrhosis. Relationship to functional renal failure and sodium and water excretion. Eur J Clin Invest. 1983;13:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1021] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 19. | Arroyo V, Clària J, Saló J, Jiménez W. Antidiuretic hormone and the pathogenesis of water retention in cirrhosis with ascites. Semin Liver Dis. 1994;14:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Davidson EW, Dunn MJ. Pathogenesis of the hepatorenal syndrome. Annu Rev Med. 1987;38:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 382] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Cárdenas A. Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol. 2005;100:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Arroyo V. Physiopathology of refractory ascites and the hepatorenal syndrome. Nefrologia. 2002;22 Suppl 5:41-46. [PubMed] |
| 25. | Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodés J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 338] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1000] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 27. | Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493-1502. [PubMed] |
| 28. | Cárdenas A, Ginès P, Uriz J, Bessa X, Salmerón JM, Mas A, Ortega R, Calahorra B, De Las Heras D, Bosch J. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 172] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Maroto A, Ginès A, Saló J, Clària J, Ginès P, Anibarro L, Jiménez W, Arroyo V, Rodés J. Diagnosis of functional kidney failure of cirrhosis with Doppler sonography: prognostic value of resistive index. Hepatology. 1994;20:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 32. | Soper CP, Latif AB, Bending MR. Amelioration of hepatorenal syndrome with selective endothelin-A antagonist. Lancet. 1996;347:1842-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Holt S, Goodier D, Marley R, Patch D, Burroughs A, Fernando B, Harry D, Moore K. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999;353:294-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Moreau R, Lebrec D. The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology. 2006;43:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Guevara M, Ginès P, Fernández-Esparrach G, Sort P, Salmerón JM, Jiménez W, Arroyo V, Rodés J. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Uriz J, Ginès P, Cárdenas A, Sort P, Jiménez W, Salmerón JM, Bataller R, Mas A, Navasa M, Arroyo V. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 241] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichaï P, Abergel A, Halimi C, Pauwels M, Bronowicki JP. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jiménez W, Arroyo V. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | Mulkay JP, Louis H, Donckier V, Bourgeois N, Adler M, Deviere J, Le Moine O. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg. 2001;64:15-19. [PubMed] |
| 41. | Halimi C, Bonnard P, Bernard B, Mathurin P, Mofredj A, di Martino V, Demontis R, Henry-Biabaud E, Fievet P, Opolon P. Effect of terlipressin (Glypressin) on hepatorenal syndrome in cirrhotic patients: results of a multicentre pilot study. Eur J Gastroenterol Hepatol. 2002;14:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Colle I, Durand F, Pessione F, Rassiat E, Bernuau J, Barrière E, Lebrec D, Valla DC, Moreau R. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: a retrospective analysis. J Gastroenterol Hepatol. 2002;17:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 44. | Kiser TH, Fish DN, Obritsch MD, Jung R, MacLaren R, Parikh CR. Vasopressin, not octreotide, may be beneficial in the treatment of hepatorenal syndrome: a retrospective study. Nephrol Dial Transplant. 2005;20:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Angeli P, Volpin R, Piovan D, Bortoluzzi A, Craighero R, Bottaro S, Finucci GF, Casiglia E, Sticca A, De Toni R. Acute effects of the oral administration of midodrine, an alpha-adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites. Hepatology. 1998;28:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 47. | Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, Tsianos EV. The effects of chronic treatment with octreotide versus octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Duvoux C, Zanditenas D, Hézode C, Chauvat A, Monin JL, Roudot-Thoraval F, Mallat A, Dhumeaux D. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Ochs A. Transjugular intrahepatic portosystemic shunt. Dig Dis. 2005;23:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Wilkinson SP, Williams R. Renal failure in cirrhosis: current views and speculations. Adv Nephrol Necker Hosp. 1977;7:15-32. [PubMed] |
| 53. | Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 56. | Gonwa TA, Morris CA, Goldstein RM, Husberg BS, Klintmalm GB. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation. 1991;51:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 193] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Le Moine O. Hepatorenal syndrome--outcome after liver transplantation. Nephrol Dial Transplant. 1998;13:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Jeyarajah DR, Gonwa TA, McBride M, Testa G, Abbasoglu O, Husberg BS, Levy MF, Goldstein RM, Klintmalm GB. Hepatorenal syndrome: combined liver kidney transplants versus isolated liver transplant. Transplantation. 1997;64:1760-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Davis CL. Impact of pretransplant renal failure: when is listing for kidney-liver indicated? Liver Transpl. 2005;11:S35-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 61. | Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, Amodio P, Sticca A, Caregaro L, Maffei-Faccioli A. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 325] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 63. | Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D, Erlinger S, Lebrec D. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Gülberg V, Bilzer M, Gerbes AL. Long-term therapy and retreatment of hepatorenal syndrome type 1 with ornipressin and dopamine. Hepatology. 1999;30:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Kaffy F, Borderie C, Chagneau C, Ripault MP, Larzillière I, Silvain C, Beauchant M. Octreotide in the treatment of the hepatorenal syndrome in cirrhotic patients. J Hepatol. 1999;30:174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |