Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4035
Revised: May 3, 2007
Accepted: May 12, 2007
Published online: August 14, 2007
The role of enteric glial cells has somewhat changed from that of mere mechanical support elements, gluing together the various components of the enteric nervous system, to that of active participants in the complex interrelationships of the gut motor and inflammatory events. Due to their multiple functions, spanning from supporting elements in the myenteric plexuses to neurotransmitters, to neuronal homeostasis, to antigen presenting cells, this cell population has probably more intriguing abilities than previously thought. Recently, some evidence has been accumulating that shows how these cells may be involved in the pathophysiological aspects of some diseases. This review will deal with the properties of the enteric glial cells more strictly related to gastrointestinal motor function and the human pathological conditions in which these cells may play a role, suggesting the possibility of enteric neuro-gliopathies.
- Citation: Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: Introducing the neuro-gliopathies. World J Gastroenterol 2007; 13(30): 4035-4041
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4035
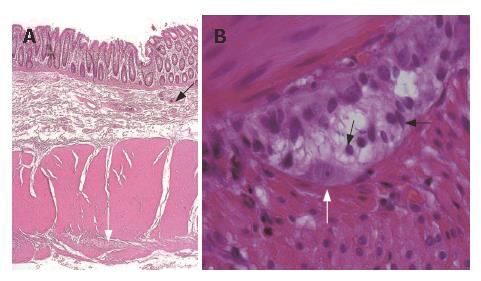
The enteric nervous system (ENS) is organized in a complex structure that controls motility, blood flow, uptake of nutrients, secretion, immunological and inflammatory processes in the gut[1]. Two main cell populations are represented in the ENS, neurons and enteric glial cells (EGC), the latter being much more abundant (up to fourfold) than neurons[2,3] (Figure 1). In humans, the ENS is subdivided into several plexuses (subserous, longitudinal muscle, myenteric, circular muscle, deep muscular, muscularis mucosae, and mucosal)[4]. Ganglionated plexuses are present in the submucosa (Meissner’s and Henle’s plexuses) and in the septum between the circular and longitudinal layers of the muscularis propria (Auerbach’s plexus)[5] (Figure 2A). Most EGC are found within the ganglia, and are also present in the interconnecting nerve strands of the ganglionated and in all non-ganglionated plexuses[6,7].
In the time course, the traditional view of EGC function has changed from simple mechanical support (as their very name, derived from the Greek “glue”, implies) to more articulate and complex ones, extremely important for the homeostasis of the gut, including influence on motility and inflammatory processes[8-10].
In this article we will take into consideration the role of EGC, looking at both experimental animal models and some human diseases for which evidence exists, and in particular their involvement in intestinal motor abnormalities and inflammatory conditions of the gut.
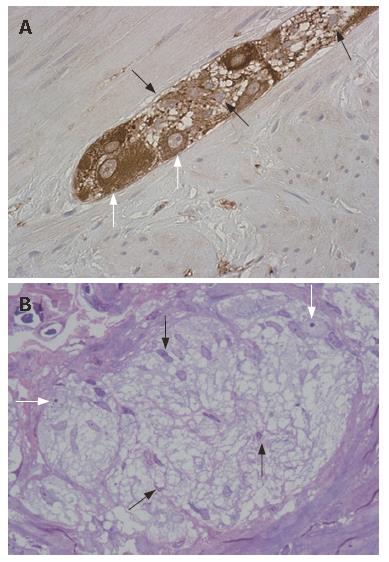
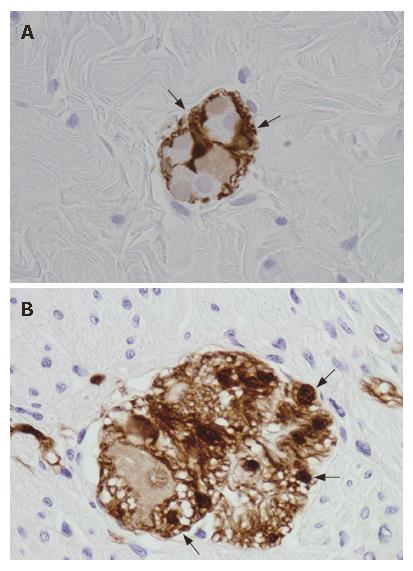
These cells are small, with several projecting processes of various length and shapes, which often confer them a star-like appearance[2,11,12] (Figure 2B). In the ganglia, EGC are very tightly packed around neurons[2,13] (Figure 3) and extend several flat projections which incompletely insulate enteric neurons from extraganglionic cells[2,14,15], whereas in the nerve strands glial processes wrap up several axonal bundles[2,16]. Electron microscopic studies have shown that EGC contain intracellular arrays of 10 nm filaments (mainly constituted by glial fibrillary acidic protein, GFAP[17-19] crisscrossing their bodies, forming axial bundles and anchoring the cells to the ganglionic surfaces[2] (Figure 4).
Moreover, some studies have suggested that EGC in the various plexuses layers of the ENS may be constituted by functionally heterogeneous populations[9,20-22].
The EGC were first described in 1899 with methylene blue staining on full thickness preparations[23]; today, immunohistochemical methods are most frequently employed for their identification. Mature EGC strongly express vimentin[24] (also expressed in myofibroblasts)[25] and GFAP, considered a specific gut glial marker[17] even though its cellular functions are still obscure[26]. Another frequently used EGC marker is the S100 protein, which is thought to yield the best results in identifying these cells[27]. This protein regulates cytoskeletal structure and function and calcium homeostasis in the cytoplasm of glial cells[28]; in the ENS S100 is thought to be exclusively localized in these cells[29]. Other putative antibodies for EGC, such as glutamine synthetase (GS)[30] and the glial cell surface antigen Ran-2[31] have not been widely employed.
It is also important to underline the fact that EGC, being of neuroectodermal origin[32], are not related to microglia in the central nervous system, which has a monocyte-macrophage lineage[33]. Instead, EGC share more similarities with astrocytes, the predominant glial cells of the central nervous system, that regulate synaptic transmission and neurovascular coupling[34], in addition to be of paramount importance for the formation and function of the blood-neural barrier[35]. However, it must be stressed that although EGC display some similarities with astrocytes, there are differences between these two cell populations, such as the dependence on neuregulins in EGC[9,36] and the functional properties[9,25]. Finally, the EGC are structurally and functionally different from the Schwann cells of the peripheral nerves (including those within the intestinal wall)[37,38]; Schwann cells of intramural nerves are S-100-positive but GFAP-negative[39].
Experimental evidence suggests that EGC are essential for maintaining the homeostasis of enteric neurons. Studies in animal models demonstrated that the loss of enteric glia causes neuronal degeneration[40,41] and/or alterations of the neurochemical coding of enteric neurons[42]. It is thought that the structural and functional integrity of enteric neurons may be due to the glial synthesis of some still unknown trophic factor[43]. Some studies, for instance, have shown that mature EGC produce glial-derived neurotrophic factor and neurotrophin-3[44-46], even though no neuronal populations depending on these substances have so far been described in the ENS of mammals. Recent investigations have shown that EGC may have a dipeptide transport function, contributing to the clearance of neuropeptides in the ENS[47].
EGC are anchored to the surface of enteric ganglia and nerve strands by means of GFAP bundles[48], and respond to mechanical stimulation increasing the expression of the immediate-early c-fos gene[49], raising intracellular Ca2+ levels and spreading intercellular Ca2+ waves[50]. Thus, it is thought that these cells support and stabilize the ENS through continuous adaptations to the structural and metabolic impairments of the gut wall[9]. Moreover, EGC express voltage-activated inward and outward K+-channels[51], suggesting a possible role in preventing extracellular accumulation of K+, which can impair synaptic transmission and ion channel kinetics in the ENS.
EGC might also be involved in the enteric neurotrans-mission. In fact, due to the exclusive expression of GS by these cells[24,30], and the presence of glutamate immuno-reactivity in human EGC[52], enteric glia could have a role in glutamatergic signaling[9] and represents a source of glutamine for neuronal glutamate and gamma-aminobutyric acid (GABA) resynthesis[53]. This is further supported by the demonstration that immunoreactivity to the high-affinity GABA transporter GAT2 mostly occurs in EGC[54], suggesting that the latter might rapidly remove GABA from the extracellular space. Moreover, since EGC but not enteric neurons display immunoreactivity to L-arginine (an essential precursor for nitric oxide)[55,56], a role in nitrergic neurotransmission might also be possible.
Owing to the fact that EGC propagate intercellular Ca2+ waves, an orchestrated intestinal glial activity has been postulated[50] that would act through a functional network[57,58]. This network is suggested by the demonstration of cell-to-cell coupling between EGC (probably through gap junctions)[12,50,51,58] and the expression of the P2Y4 receptor on these cells[59]. EGC may also transfer information to the neurons by means of nucleotide signaling[60-62]. Numerous molecules (serotonin[60], histamine[60], endothelin[63], protease-activated receptors[64]) can also activate EGC, which increase intracellular Ca2+ concentrations[65] or express the c-fos gene, a marker of early cell activation. It has also been shown that EGC express purinoreceptors[60,66] and that multiple lipid-activated signalling mechanisms exist in these cells[67,68].
Experimental animal studies have demonstrated that EGC may have a role in intestinal inflammatory processes[9], and that initiation and/or progression of inflammatory bowel disease (especially Crohn’s disease) might be ascribed to an immune-mediated damage to enteric glia[69]. The fact that EGC functionally interact with lymphocytes[70-73], respond actively to inflammation, and become activated as antigen-presenting cells[74] attracting immune cells to the ENS[9,75], suggests that this cell population is likely involved in inflammatory processes of the gut. Moreover, the immune cells are usually nearby, since the intestine physiologically contains such a cell population that provides a series of pattern recognition receptors interacting with bacterial molecular patterns, and helps to modulate intestinal innate immunity and an appropriate adaptive immune response[76-78].
Thus, it is not difficult to imagine these cells as active participants in the pathogenesis of the so-called “functional” gastrointestinal disorders. These are usually thought to occur in the absence of anatomical or biochemical abnormalities[79]. However, this definition now seems outdated, because structural and molecular abnormalities have begun to be recognized in subsets of patients[80]. For instance, studies in patients with irritable bowel syndrome disclosed the presence of inflammatory infiltrates closely associated with the enteric plexuses and mucosal activation of the immune system[81,82], and some patients with intestinal dysmotility and megacolon have a lymphoplasmacellular infiltrate within the myenteric plexus that likely accounts for their symptoms[83].
The role of EGC has been investigated in only a few human diseases, even though there is still no pathological condition entirely ascribable to EGC dysfunction. For instance, patients with colonic diverticular disease have a significant decrease of EGC and of interstitial cells of Cajal (ICC) in the enteric plexuses[84]. Owing to the fact that in colonic diverticulosis the smooth muscle hypertrophy acts as a partially obstructive mechanism, the EGC population loss might be partly due to this mechanism, similar to that documented for ICC in analogous experimental animal models[85].
The number of EGC, together with that of enteric neurons and ICC, is also considerably decreased in patients with severe constipation (slow-transit type) undergoing surgery for intractable symptoms[86]. Interestingly, the loss of EGC, but not of ICC and enteric neurons, was also documented in the terminal ileum of these patients[87]; this implies that this cell population may be involved in the small bowel dysmotility repeatedly described in these patients[88,89]. A significant decrease of EGC, but not of other elements of the ENS, was then described in the myenteric and submucosal plexuses of patients with severe constipation due to obstructed defecation refractory to medical treatment and biofeedback training[90]. These findings are intriguing, and consistent with the recent hypothesis, based on abnormal colonic manometric findings, that at least one subpopulation of patients with obstructed defecation might result from defective colonic, rather than anorectal, function[91]. Of practical importance, our results could give an explanation for the lack of response to treatments, especially to biofeedback, in these patients.
More recently, we have reported a significant decrease of EGC in patients with chagasic and idiopathic megacolon compared to controls[92]; it is worth noting that all the above human pathological conditions in which EGC have been found to be decreased share a common denominator, i.e. constipation.
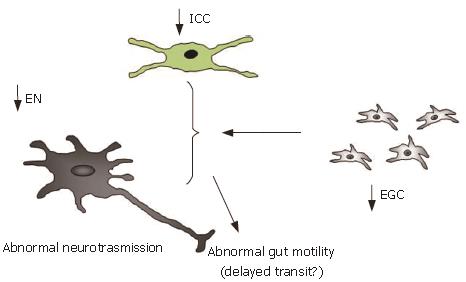
We could hypothesize that the reduced number of EGC, together with the decrease/loss of other cell populations essential for gastrointestinal motility, may play a role in these diseases. For instance, the ICC decrease impairs the pacemaker enteric signals and might add to an abnormal neurotransmission secondary to the decreased number of enteric neurons, and be further worsened by an impairment of EGC. The summation of the loss of the properties of these cell populations might thus lead to dysmotilities of the involved viscera, by means of several mechanisms (Figure 5): impairment of the mechanical properties of the plexuses, decreased gut neurotransmission, and reduced homeostatic support to the enteric neurons, leading to neurodegeneration and/or phenotypic shift, even in the absence of inflammation[9,93]. Experimental animal models also support this hypothesis, suggesting that EGC play a major role in the modulation of enteric neural circuits that regulate intestinal motility[94].
Why EGC, ICC, and enteric neurons are decreased in such patients is still unknown. Evidences in experimental animal models suggest that the number of EGC reduces with aging[95], but this has not been evaluated in human beings[96]. Other mechanisms, such as the damaging effect of antraquinone laxatives on the ENS, have not been confirmed with modern immunohistochemical methods[97].
Probably, the EGC should be looked at differently, since evidence is mounting concerning an ever more active role in the complex organization of the gastrointestinal tract, including enteric neuroplasticity[98]. The (limited) data so far accumulated suggest that these cells are probably somewhat involved in some motor dysfunction of the gastrointestinal tract, mainly those characterized by constipation. Thus, it is likely that in the future other “functional” disorders of the gut, in addition to the irritable bowel syndrome, may be reclassified. For instance, we have recently proposed consideration of at least some subtypes of constipation such as enteric neuropathies[99], although seen in the light of the data on EGC we should probably reformulate this definition in terms of neuro-gliopathies. However, more evidence are needed to establish a more precise role for this fascinating cell population, especially considering new perspectives, such as the possibilities of neural stem cell transplantation[100-102] for the treatment of disorders of the peripheral and central nervous system. Hopefully, studies on EGC will possibly be useful to establish new therapeutic approaches to some gut disorders.
We are warmly grateful to Professor Giorgio Gabella, Department of Anatomy and Developmental Biology, University College London, London, UK, for kindly providing useful bibliographic references.
Enteric glial cells (EGC) are to date thought to be more than simple support structures for the enteric nervous system (ENS). Recent developments in their biological properties led to the belief that this cell population may have pathophysiological importance in inflammatory and dysmotility conditions of the gut.
The EGC have also, in addition to mechanical support function in the ENS, homeostatic functions (are essential for enteric neuronal vitality), neurotransmitter functions, immunological functions (may act as antigen-presenting cells) and appear critically involved in the pathophysiology of inflammatory bowel diseases, especially Crohn’s disease.
Recent research in human beings showed that the EGC are likely involved also in the pathophysiological mechanisms of some diseases presenting with abnormal gastrointestinal motility, and especially those characterized by constipation. In fact, significant decreases of EGC have been reported in diverticular diseases, slow transit constipation, some subsets of obstructed defecation, Chagasic and idiopathic megacolon.
The study of EGC, in addition to that of other components of the ENS (neurons, interstitial cells of Cajal), might result in a better understanding of the pathophysiological grounds of conditions characterized by abnormal gut activity, and perhaps lead to a more targeted therapeutic approach to these disorders.
This is an interesting review dealing with the properties of the enteric glial cells, more strictly related to gastrointestinal motor function and the human pathological conditions in which these cells may play a role, suggesting the possibility of enteric neuro-gliopathies.
S- Editor Zhu LH L- Editor Zhu LH E- Editor Liu Y
| 1. | Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 469] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kühnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 1:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Driessen A, Creemers J, Geboes K. Anti-Leu-19 is a marker for nervous tissue in the mucosa of the human rectum. Acta Anat (Basel). 1995;153:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Rühl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | von Boyen G, Steinkamp M. The enteric glia and neurotrophic factors. Z Gastroenterol. 2006;44:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Björklund H, Dahl D, Seiger A. Neurofilament and glial fibrillary acid protein-related immunoreactivity in rodent enteric nervous system. Neuroscience. 1984;12:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hanani M, Reichenbach A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 1994;278:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Komuro T, Bałuk P, Burnstock G. An ultrastructural study of neurons and non-neuronal cells in the myenteric plexus of the rabbit colon. Neuroscience. 1982;7:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol. 1978;180:467-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Komuro T. Direct contacts between Auerbach's ganglia and smooth muscle cells in the small intestine of the rat. Neurosci Lett. 1988;92:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Cook RD, Burnstock G. The ultrastructure of Auerbach's plexus in the guinea-pig. II. Non-neuronal elements. J Neurocytol. 1976;5:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 292] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Jessen KR, Thorpe R, Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J Neurocytol. 1984;13:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Bishop AE, Carlei F, Lee V, Trojanowski J, Marangos PJ, Dahl D, Polak JM. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry. 1985;82:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Rothman TP, Tennyson VM, Gershon MD. Colonization of the bowel by the precursors of enteric glia: studies of normal and congenitally aganglionic mutant mice. J Comp Neurol. 1986;252:493-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Tjwa ET, Bradley JM, Keenan CM, Kroese AB, Sharkey KA. Interleukin-1beta activates specific populations of enteric neurons and enteric glia in the guinea pig ileum and colon. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1268-G1276. [PubMed] |
| 22. | von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Dogiel AS. Uber den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Saugethiere. Z Naturforsch B. 1899;5:130-158. |
| 24. | Jessen KR, Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983;3:2206-2218. [PubMed] |
| 25. | Rühl A, Trotter J, Stremmel W. Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat. Neurogastroenterol Motil. 2001;13:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res. 2000;25:1439-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1028] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 27. | Krammer HJ, Karahan ST, Sigge W, Kühnel W. Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg. 1994;4:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002;172:69-80. [PubMed] |
| 29. | Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Kato H, Yamamoto T, Yamamoto H, Ohi R, So N, Iwasaki Y. Immunocytochemical characterization of supporting cells in the enteric nervous system in Hirschsprung's disease. J Pediatr Surg. 1990;25:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Jessen KR, Mirsky R. Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J Neuroimmunol. 1985;8:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Young HM, Bergner AJ, Müller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Aloisi F. Immune function of microglia. Glia. 2001;36:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 928] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 34. | Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 922] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 35. | Kim JH, Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 384] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 37. | Eccleston PA, Jessen KR, Mirsky R. Control of peripheral glial cell proliferation: a comparison of the division rates of enteric glia and Schwann cells and their response to mitogens. Dev Biol. 1987;124:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Gabella G. Structure of muscles and nerves in the gastrointestinal tract. Physiology of the gastrointestinal tract, 3rd edition. New York: Raven Press 1994; 751-793. |
| 39. | Nada O, Kawana T. Immunohistochemical identification of supportive cell types in the enteric nervous system of the rat colon and rectum. Cell Tissue Res. 1988;251:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 41. | Bush TG. Enteric glial cells. An upstream target for induction of necrotizing enterocolitis and Crohn's disease? Bioessays. 2002;24:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Aubé AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Rühl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004;16 Suppl 1:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Hoehner JC, Wester T, Påhlman S, Olsen L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology. 1996;110:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Bär KJ, Facer P, Williams NS, Tam PK, Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung's disease. Gastroenterology. 1997;112:1381-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Kondyli M, Varakis J, Assimakopoulou M. Expression of p75NTR and Trk neurotrophin receptors in the enteric nervous system of human adults. Anat Sci Int. 2005;80:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Rühl A, Hoppe S, Frey I, Daniel H, Schemann M. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J Comp Neurol. 2005;490:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Gabella G. On the plasticity of form and structure of enteric ganglia. J Auton Nerv Syst. 1990;30 Suppl:S59-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Sharkey KA, Parr EJ, Keenan CM. Immediate-early gene expression in the inferior mesenteric ganglion and colonic myenteric plexus of the guinea pig. J Neurosci. 1999;19:2755-2764. [PubMed] |
| 50. | Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia. 2003;42:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Hanani M, Francke M, Härtig W, Grosche J, Reichenbach A, Pannicke T. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2000;278:G644-G651. [PubMed] |
| 52. | Giaroni C, Zanetti E, Chiaravalli AM, Albarello L, Dominioni L, Capella C, Lecchini S, Frigo G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur J Pharmacol. 2003;476:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Fletcher EL, Clark MJ, Furness JB. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res. 2002;308:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Aoki E, Semba R, Kashiwamata S. Evidence for the presence of L-arginine in the glial components of the peripheral nervous system. Brain Res. 1991;559:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Nagahama M, Semba R, Tsuzuki M, Aoki E. L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. Biol Signals Recept. 2001;10:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Hanani M, Zamir O, Baluk P. Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Res. 1989;497:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Maudlej N, Hanani M. Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 1992;578:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Van Nassauw L, Costagliola A, Van Op den Bosch J, Cecio A, Vanderwinden JM, Burnstock G, Timmermans JP. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci. 2006;126-127:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem. 1996;66:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Sarosi GA, Barnhart DC, Turner DJ, Mulholland MW. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am J Physiol. 1998;275:G550-G555. [PubMed] |
| 62. | Braun N, Sévigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Zhang W, Sarosi GA, Barnhart DC, Mulholland MW. Endothelin-stimulated capacitative calcium entry in enteric glial cells: synergistic effects of protein kinase C activity and nitric oxide. J Neurochem. 1998;71:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Garrido R, Segura B, Zhang W, Mulholland M. Presence of functionally active protease-activated receptors 1 and 2 in myenteric glia. J Neurochem. 2002;83:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Zhang W, Sarosi G, Barnhart D, Yule DI, Mulholland MW. Endothelin-activated calcium signaling in enteric glia derived from neonatal guinea pig. Am J Physiol. 1997;272:G1175-G1185. [PubMed] |
| 66. | Vanderwinden JM, Timmermans JP, Schiffmann SN. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003;312:149-154. [PubMed] |
| 67. | Segura BJ, Zhang W, Cowles RA, Xiao L, Lin TR, Logsdon C, Mulholland MW. Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience. 2004;123:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Segura BJ, Zhang W, Xiao L, Turner D, Cowles RA, Logsdon C, Mulholland MW. Sphingosine-1-phosphate mediates calcium signaling in guinea pig enteroglial cells. J Surg Res. 2004;116:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA. 2001;98:13306-13311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO. Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Koretz K, Momburg F, Otto HF, Möller P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am J Pathol. 1987;129:493-502. [PubMed] |
| 72. | Geboes K, Rutgeerts P, Ectors N, Mebis J, Penninckx F, Vantrappen G, Desmet VJ. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992;103:439-447. [PubMed] |
| 73. | Rühl A, Franzke S, Collins SM, Stremmel W. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1163-G1171. [PubMed] |
| 74. | Hirata I, Berrebi G, Austin LL, Keren DF, Dobbins WO. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Wittig BM, Zeitz M. The gut as an organ of immunology. Int J Colorectal Dis. 2003;18:181-187. [PubMed] |
| 77. | Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 Suppl 1:S41-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:613-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 80. | Talley NJ. A unifying hypothesis for the functional gastrointestinal disorders: really multiple diseases or one irritable gut? Rev Gastroenterol Disord. 2006;6:72-78. [PubMed] |
| 81. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 576] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 82. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 83. | De Giorgio R, Barbara G, Stanghellini V, De Ponti F, Salvioli B, Tonini M, Velio P, Bassotti G, Corinaldesi R. Clinical and morphofunctional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: a study of three cases. Am J Gastroenterol. 2002;97:2454-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Bassotti G, Battaglia E, Bellone G, Dughera L, Fisogni S, Zambelli C, Morelli A, Mioli P, Emanuelli G, Villanacci V. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol. 2005;58:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Bassotti G, Villanacci V, Cathomas G, Maurer CA, Fisogni S, Cadei M, Baron L, Morelli A, Valloncini E, Salerni B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum Pathol. 2006;37:1252-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Panagamuwa B, Kumar D, Ortiz J, Keighley MR. Motor abnormalities in the terminal ileum of patients with chronic idiopathic constipation. Br J Surg. 1994;81:1685-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Bassotti G, Stanghellini V, Chiarioni G, Germani U, De Giorgio R, Vantini I, Morelli A, Corinaldesi R. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci. 1996;41:1999-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Bassotti G, Villanacci V, Nascimbeni R, Asteria CR, Fisogni S, Nesi G, Legrenzi L, Mariano M, Tonelli F, Morelli A. Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod Pathol. 2007;20:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, Cook IJ. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004;127:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Iantorno G, Bassotti G, Kogan Z, Lumi CM, Cabanne AM, Fisogni S, Varrica LM, Bilder CR, Munoz JP, Liserre B. The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol. 2007;31:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Rühl A. Glial regulation of neuronal plasticity in the gut: implications for clinicians. Gut. 2006;55:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G912-G927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl). 2004;209:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Villanacci V, Bassotti G, Cathomas G, Maurer CA, Di Fabio F, Fisogni S, Cadei M, Mazzocchi A, Salerni B. Is pseudomelanosis coli a marker of colonic neuropathy in severely constipated patients? Histopathology. 2006;49:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126-127:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 99. | Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609-4613. [PubMed] |
| 100. | Rauch U, Hänsgen A, Hagl C, Holland-Cunz S, Schäfer KH. Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis. 2006;21:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |