Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.444
Revised: June 20, 2006
Accepted: December 11, 2006
Published online: January 21, 2007
AIM: To assess the efficacy of premedicaton with pronase or N-acetylcysteine (NAC) at 20 min before upper gastrointestinal (UGI) endoscopy and to determine whether pronase or NAC pretreatment influences the reliability of the rapid urease test.
METHODS: A total of 146 patients were prospectively and randomly assigned into the study groups according to different premedications before endoscopy. One endoscopist assessed mucosal visibility (MV) with scores ranged from 1 to 4 at four sites in the stomach. The sum of the MV scores from these four locations was defined as the total mucosal visibility (TMV) score. Identification of H pylori was performed using CLO test, histology, and serology.
RESULTS: The Group with pronase premedication had a significantly lower TMV score than did the groups with gascon and gascon water (p < 0.001 and p < 0.01, respectively). The group with NAC had a significantly lower TMV score than the group with gascon (p < 0.01) and a trend of a lower MV score than the group with gascon water (p = 0.06). The TMV score did not significantly differ between the group with pronase and the group with NAC (p = 0.39 and p = 0.14, respectively). The sensitivity and specificity of the CLO test were 92.5% and 93.9%, respectively, in groups premedicated with pronase and NAC together.
CONCLUSION: Premedication with pronase or NAC at 20 min before UGI endoscopy improves the mucosal visibility of the stomach. Neither pronase nor NAC produces any obvious interference with the CLO test for the identification of H pylori infection.
- Citation: Chang CC, Chen SH, Lin CP, Hsieh CR, Lou HY, Suk FM, Pan S, Wu MS, Chen JN, Chen YF. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: An endoscopist-blinded, prospective, randomized study. World J Gastroenterol 2007; 13(3): 444-447
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/444.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.444
The 5-year survival rate for early gastric cancer exceeds 90%[1,2]. Western endoscopists are less successful than Japanese endoscopists in the detection of gastric cancers at an early stage. This is not because the Japanese endoscopists detect the cancers by screening the asymptomatic population. Even in Japan, most cases are incidental findings of opportunistic screening at endoscopy, and only 10% of gastric cancers are picked up by screening program[3,4]. More than 50% of all gastric cancers in Japan were found in an early stage but probably fewer than 5% are in the West[5,6]. A single-center study in the UK reported that 13.5% patients (11 of 81 patients presenting with advanced gastric cancer) supposed to be missed of early gastric cancer those who had undergone endoscopy within the previous 2 years[7]. A diagnosis of early gastric cancer can be improved by effective premedication with a defoaming agent during upper gastrointestinal endoscopy. Dimethylpolysiloxane (DMPS) is commonly used at most endoscopic centers to eliminate bubbles and foam[8-10]. However, a lot of mucus and bubbles can still be encountered with premedication of DMPS during gastroendoscopy. Pronase is a proteolytic enzyme isolated in 1962 from the culture filtrate of Septomyces griseus which has already been used as a raw material to prepare anti-inflammatory and digestive enzymes. In 1964, Koga and Arakawa used this enzyme as a premedication for roentgenographic examinations to remove gastric mucus[11]. Since then, Ida et al[12] applied this enzyme to gastroendoscopy. Fujii et al[13] also concluded that premedication with pronase improved endoscopic visualization during conventional endoscopy and chromoendoscopy. They recommended routinely use of pronase for endoscopy is helpful. N-acetylcysteine (NAC) is both a mucolytic agent and a thiol-containing antioxidant. NAC, unlike some other bronchial mucolytics such as carbocysteine and bromhexine, has been shown in in vitro studies to change the viscoelastic properties of gastric mucin[14]. In this study, we investigated the effectiveness of premedications with DMPS only, DMPS with water, DMPS with pronase, and DMPS with NAC on the visibility during UGI endoscopy.
Pronase and NAC are both mycolytic agents. There are two studies discussing about H pylori detection after treatment with pronase and NAC[15,16]. Rapid urease testing is most commonly used to identify H pylori during the examination of UGI endoscopy. Therefore, we also attempted to determine whether pronase or NAC pretreatment influences the reliability of identifying H pylori infection by the rapid urease test.
From January to July 2005, 146 consecutive patients were referred to our department for upper gastrointestinal (UGI) endoscopy. We excluded those patients with previous gastric surgery, gastric malignancy, corrosive gastric injury, or gastrointestinal bleeding.
UGI endoscopic procedures were performed by a single experienced endoscopist (Chang CC) between 9:00 am and 1:00 pm in the endoscopic room of Taipei Medical University Hospital. Patients who consented to participate in the study were randomly assigned to four different oral liquid solutions for premedication before endoscopy. All oral solutions were given around 20 min before UGI endoscopy. The endoscope used was a GIF-Q240X video endoscope (Olympus, Tokyo, Japan).
The patients were randomly divided into four groups according to the treatment with oral liquid solutions. Group A: 100 mg, 5 mL of DMPS (Gascon, Kisssi Corp., Matsumoto, Japan); Group B: 100 mg, 5 mL of DMPS plus water up to 100 mL; Group C: 20 000 U pronase (Pronase MS, Kaken Corp., Tokyo, Japan), 1.2 g sodium bicarbonate, 100 mg, 5 mL of DMPS plus water up 100 mL water; and Group D: 400 mg N-acetylcysteine (Acetin, Synmosa Corp., Taipei, Taiwan), 5 mL of DMPS plus water up 100 mL.
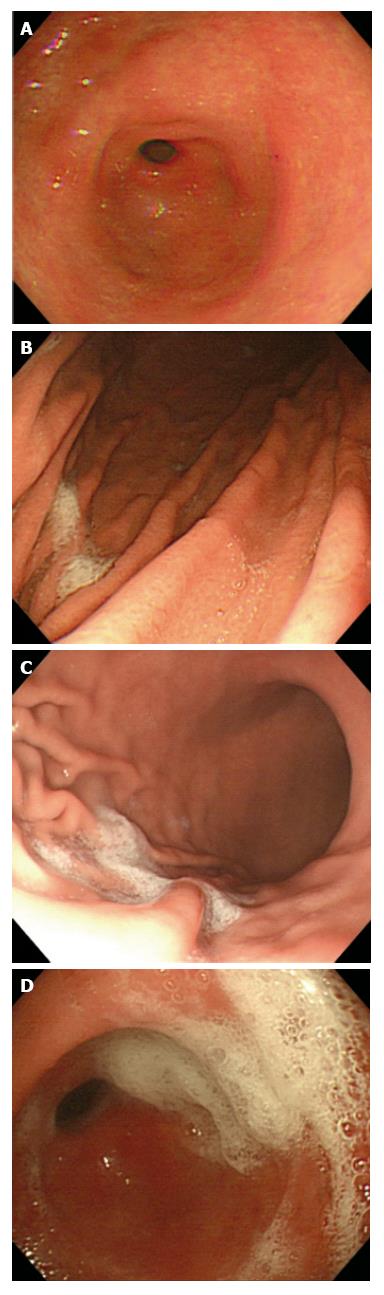
The endoscopist was unaware of the kind of oral liquid solution used for premedication for each patient before UGI endoscopy. UGI endoscopy was performed to check the mucosal visibility of the gastric antrum, the greater curvature of the gastric lower body, the greater curvature of the gastric upper body, and the gastric fundus. The scores of mucosal visibility were classified from 1 to 4 and this score was modified from Kuo et al[15] (Figure 1A-D).
Score 1: No adherent mucus on the gastric mucosa; Score 2: little amount of mucus on the gastric mucosa but no obscuring vision; Score 3: large amount of mucus on the gastric mucosa, with less than 50 mL of water to clear; Score 4: large amount of mucus on the gastric mucosa, with more than 50 mL of water to clear. The sum of the scores from the four locations was defined as the total mucosal visibility score (TMVS).
The rapid urease test using the Campylobacter-like organism test (CLO test; Delta West, Perth, Australia), the histologic examination for H pylori using hematoxylin-eosin staining during the UGI endoscopy and serum anti-H pylori IgG were carried out to determine the presence of infection by H pylori. H pylori infection was considered when two of the three tests were positive.
The demographic characteristics were assessed using a chi-square test or one-way analysis of variance. The visibility scores for the four groups were assessed using one-way analysis of variance with Tukey’s multiple comparisons. The correlation of the results of the CLO test was analyzed using a chi-square test. The results were expressed as mean ± SD. P < 0.05 was considered statistically significant.
Of 147 patients (70 men and 77 women), 39, 35, 34, and 39 patients were randomly placed in groups A, B, C, and D, respectively. The demographic data of patients are shown in Table 1. The mean (± SD) ages of groups A, B, C, and D were 44.4 ± 14.2, 44.1 ± 14.4, 47.8 ± 13.9, and 48.7± 16.5 years, respectively. The ratios of males to females among groups A, B, C, and D were 1:1.1, 1:0.9, 0.9:1 and 1:1.1, respectively. There was no significant statistical difference between any pair of groups for age or the gender ratio.
| Category | Group A | Group B | Group C | Group D |
| Number (n) | 39 | 35 | 34 | 39 |
| Age (yr) | 44.4 ± 14.2 | 44.1 ± 14.4 | 47.8 ± 13.9 | 48.7 ± 16.5 |
| Gender (M:F) | 18:21 | 18:17 | 16:18 | 18:21 |
| Indication | ||||
| Cancer screening | 9 | 9 | 7 | 10 |
| Dyspepsia | 20 | 17 | 18 | 22 |
| Acid regurgitation | 10 | 9 | 9 | 7 |
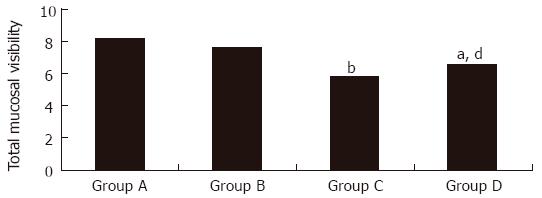
The means of the TMV score among groups A, B, C, and D were 8.2 ± 3.1, 7.6 ± 2.6, 5.8 ± 2.3, and 6.5 ± 2.2, respectively. Group C had a significantly lower TMV score than groups A and B (P < 0.001 and P < 0.01, respectively). Group D also had a significantly lower TMV score than group A (P < 0.01) and a trend of a lower TMV score than group B (P = 0.06) (Figure 2). The TMV score did not significantly differ between groups A and B or between groups C and D (P = 0.39 and P = 0.14, respectively). The visibility score of each location of the stomach and TMV score for each group are shown in Table 2.
| Antrum | Greater curvatureof the lowergastric body | Greater curvatureof the uppergastric body | Fundus | |
| Group A | 1.74 ± 0.91eg | 2.18 ± 1.14eg | 2.92 ± 1.06ceg | 1.38 ± 0.67ceg |
| Group B | 1.49 ± 0.82eg | 1.89 ± 0.87eg | 2.49 ± 0.82ae | 1.80 ± 0.80ae |
| Group C | 1.18 ± 0.63ac | 1.38 ± 0.78ac | 2.06 ± 0.89ac | 1.21 ± 0.41ac |
| Group D | 1.21 ± 0.52ac | 1.77 ± 0.90ac | 2.33 ± 0.79a | 1.28 ± 0.67ac |
| Total | 1.41 ± 0.77 | 1.82 ± 0.95 | 2.46 ± 0.94 | 1.41 ± 0.69 |
The scores of mucosal visibility at different locations in all patients were 1.4 ± 0.7 at the gastric fundus, 2.5 ± 0.9 at the greater curvature of the upper gastric body, 1.8 ± 0.9 at the greater curvature of the lower gastric body, and 1.4 ± 0.7 at the gastric antrum. A significantly poorer visibility score of the greater curvature of the upper gastric body compared to that of the gastric fundus, the greater curvature of the lower gastric body, and the gastric antrum was noted (all P < 0.05). In addition, the greater curvature of the lower gastric body had a significantly poor visibility score than did the gastric fundus and the gastric antrum (both P < 0.05). There was no significant difference of mucosal visibility score between the gastric antrum and the gastric fundus.
According to the effect of pronase and NAC on the reliability of the CLO test for identifying H pylori infection (Table 3), 68 of 73 patients had matching results between the CLO test and H pylori infection (P < 0.05). The sensitivity and specificity were 92.5 % and 93.9%, respectively.
| Category | H pylori-positive | H pylori-negative |
| Positive CLO test | 37 | 2 |
| Negative CLO test | 3 | 31 |
For better visualization of the gastric mucosa, decreasing the amount of mucus and bubbles is very important during UGI endoscopy. Adequate premedication can eliminate the need to carry out flushing during the procedure. In Japan, pronase is widely used as a mucolytic agent before gastrointestinal endoscopy. A randomized study by Fujii et al[13] showed that premedication with pronase significantly improved visibility before and after methylene blue spraying and also significantly shortened the time for the chromoendoscopic examination. Kuo et al[15] also concluded that premedicaiton with 2000 U pronase, 1.2 g of sodium bicarbonate, 100 mg of DMPS plus up to 100 mL of warm water provided the clearest endoscopic visibility. Without the application of DMPS, pronase alone could not improve endoscopic visibility[15]. Similarly, in our study, we found that group C (premedication with 20 000 U pronase, 1.2 g of sodium bicarbonate, 100 mg of DMPS plus up to 100 mL of warm water) had better TMV scores than those in groups A and B. In comparison to the study by Kuo et al[15], we used 20 000 U pronase rather than 2000 U pronase. In addition to the different doses of pronase, we gave all premedications around 20 min before UGI endoscopy, and we did not ask patients to change position before UGI endoscopy. Because the fluid ingested by those patients flowed into the gastric fundus, then gradually into the gastric antrum by the way of the gastric body, we thought it was not necessary to change the position before UGI endoscopy. Moreover, there can still be a lot of bubbles in the stomach 10 min after administering DMPS prior to UGI endoscopy in our previous experience.
NAC is a mucolytic agent which is commonly used for digestion of the esophageal mucus for the detection of Barrett’s esophageal cancer prior to chromoendoscopy with methylene blue[17,18]. Its effectiveness in improving the mucosal visibility of the stomach during UGI endoscopy is not known. In our study, NAC also provided better TMV scores in the stomach as did pronase. Premedication with NAC can achieve good mucosal visibility during gastrointestinal endoscopy if pronase is not available.
In our study, we found the greater curvature of the upper gastric body had the poorest mucosal visibility among all locations evaluated, suggesting that this area needs to be observed with caution during UGI endoscopy.
Pronase and NAC can disrupt the gastric mucus by a mucolytic effect. It is not well known whether pronase or NAC interferes with the accuracy of the CLO test for identifying H pylori infection. Our study showed greater than 90% sensitivity and specificity for the CLO test with premedication with pronase or NAC, which is consistent with the study by Kuo et al[15] based on the urea breath test.
In conclusion, in order to improve the mucosal visibility of the stomach, premedication with pronase or NAC at 20 min before UGI endoscopy is feasible. It is not necessary to change the position of the patient from supine, left or right lateral to prone before UGI endoscopy. The greater curvature of the upper gastric body needs to be cautiously observed, for it had the poorest mucosal visibility among all locations evaluated. Neither pronase nor NAC produce any obvious interference with the CLO test for identifying H pylori infection.
S- Editor Liu Y L- Editor Kumar M E- Editor Lu W
| 1. | Everett SM, Axon AT. Early gastric cancer: disease or pseudo-disease? Lancet. 1998;351:1350-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 2. | Sue-Ling HM, Martin I, Griffith J, Ward DC, Quirke P, Dixon MF, Axon AT, McMahon MJ, Johnston D. Early gastric cancer: 46 cases treated in one surgical department. Gut. 1992;33:1318-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Medical Examinations for Digestive Cancer in 1995 Group. National Report. J Gastroenterol Mass Surv. 1998;130:251-269. |
| 4. | Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Shimizu S, Tada M, Kawai K. Endoscopic ultrasonography in inflammatory bowel diseases. Gastrointest Endosc Clin N Am. 1995;5:851-859. [PubMed] |
| 6. | Ballantyne KC, Morris DL, Jones JA, Gregson RH, Hardcastle JD. Accuracy of identification of early gastric cancer. Br J Surg. 1987;74:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Suvakovic Z, Bramble MG, Jones R, Wilson C, Idle N, Ryott J. Improving the detection rate of early gastric cancer requires more than open access gastroscopy: a five year study. Gut. 1997;41:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Banerjee B, Parker J, Waits W, Davis B. Effectiveness of preprocedure simethicone drink in improving visibility during esophagogastroduodenoscopy: a double-blind, randomized study. J Clin Gastroenterol. 1992;15:264-265. [PubMed] |
| 9. | McDonald GB, O'Leary R, Stratton C. Pre-endoscopic use of oral simethicone. Gastrointest Endosc. 1978;24:283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Waye JD, Pitman E, Weiss A, Krueger K. The bubble problem in endoscopy. An evaluation of a new aid in endoscopy. A double blind study. Gastrointest Endosc. 1967;14:34-35. [PubMed] |
| 11. | Koga M, Arakawa K. On the application of enzymatic mucinolysis in x-ray diagnosis of the stomach. Nihon Igaku Hoshasen Gakkai Zasshi. 1964;24:1011-1031. [PubMed] |
| 12. | Ida K, Okuda J, Nakazawa S, Yoshino J. Clinical evaluation of premedication with KPD (pronase) in gastroendoscopy-placbo-contolled double blind study in dye scattering endoscopy. Clin Rep. 1991;25:1793-1804. |
| 13. | Fujii T, Iishi H, Tatsuta M, Hirasawa R, Uedo N, Hifumi K, Omori M. Effectiveness of premedication with pronase for improving visibility during gastroendoscopy: a randomized controlled trial. Gastrointest Endosc. 1998;47:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Misawa M, Imamura N. In vitro evaluation of mucolytic activities of some expectorants using porcine gastric mucin. Nihon Yakurigaku Zasshi. 1988;92:263-270. [PubMed] [DOI] [Full Text] |
| 15. | Kuo CH, Sheu BS, Kao AW, Wu CH, Chuang CH. A defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy. 2002;34:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Huynh HQ, Couper RT, Tran CD, Moore L, Kelso R, Butler RN. N-acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig Dis Sci. 2004;49:1853-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Wo JM, Ray MB, Mayfield-Stokes S, Al-Sabbagh G, Gebrail F, Slone SP, Wilson MA. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a preliminary study. Gastrointest Endosc. 2001;54:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Dave U, Shousha S, Westaby D. Methylene blue staining: is it really useful in Barrett's esophagus? Gastrointest Endosc. 2001;53:333-335. [PubMed] |