Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.414
Revised: July 25, 2006
Accepted: December 8, 2006
Published online: January 21, 2007
AIM: To assess the clinical features and prognosis of 151 patients with extrahepatic metastases from primary hepatocellular carcinoma (HCC), and describe the treatment strategy for such patients.
METHODS: After the diagnosis of HCC, all 995 consecutive HCC patients were followed up at regular intervals and 151 (15.2%) patients were found to have extrahepatic metastases at the initial diagnosis of primary HCC or developed such tumors during the follow-up period. We assessed their clinical features, prognosis, and treatment strategies.
RESULTS: The most frequent site of extrahepatic metastases was the lungs (47%), followed by lymph nodes (45%), bones (37%), and adrenal glands (12%). The cumulative survival rates after the initial diagnosis of extrahepatic metastases at 6, 12, 24, and 36 mo were 44.1%, 21.7%, 14.2%, 7.1%, respectively. The median survival time was 4.9 mo (range, 0-37 mo). Fourteen patients (11%) died of extrahepatic HCC, others died of primary HCC or liver failure.
CONCLUSION: The prognosis of HCC patients with extrahepatic metastases is poor. With regard to the cause of death, many patients would die of intrahepatic HCC and few of extrahepatic metastases. Although most of HCC patients with extrahepatic metastases should undergo treatment for the primary HCC mainly, treatment of extrahepatic metastases in selected HCC patients who have good hepatic reserve, intrahepatic tumor stage (T0-T2), and are free of portal venous invasion may improve survival.
- Citation: Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007; 13(3): 414-420
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/414.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.414
Hepatocellular carcinoma (HCC) is a highly malignant tumor with frequent intrahepatic metastasis. The prognosis of HCC patients has improved because of progress in therapeutic procedures, such as surgical resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and transcatheter arterial chemoembolization (TACE)[1-3]. Moreover, progress in diagnostic modalities, such as ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and digital subtraction angiography (AG) has led to a better detection of patients with early and small HCC or asymptomatic extrahepatic metastases.
The above improvements in survival and diagnostic modalities have resulted in increased detection of extrahepatic metastases from primary HCC and further increases are anticipated in the future. Several groups have investigated extrahepatic metastases from HCC, but many of such cases were in autopsy cases, in a small number of cases or case reports[4-15]. At present, the prognosis of patients with extrahepatic metastases from primary HCC is poor[16,17]. In this regard, there is only little information about the causes of death of such patients[18], and there is no consensus on the treatment strategy for extrahepatic metastases from HCC. For example, what treatment strategy should be used to treat intrahepatic HCC or extrahepatic metastases? Among patients with extrahepatic metastases from primary HCC, which patients should be treated? To our knowledge, there are no reports that deal directly with these questions. In this relatively large study, we retrospectively assessed the clinical features and prognosis of 151 patients with extrahepatic metastases from primary HCCs, and described the treatment strategy for such patients.
From June 1990 to December 2005, 995 consecutive patients with HCC were admitted to our hospital. Among these patients, 880 were initially diagnosed with HCC in our hospital while the others were treated previously for HCC in other hospitals. Extrahepatic metastases from primary HCC were detected in 151 (15.2%) of 995 patients. None of the patients was treated for extrahepatic metastases. All the 151 HCC patients with extrahepatic metastases (117 men and 34 women, median age: 64 years, range: 21-82 years) were enrolled in the present study.
Table 1 summarizes the clinical profile of the 151 patients at the initial diagnosis of extrahepatic metastases. These 151 patients were divided into groups A and B. Group A was consisted of 68 patients presented with extrahepatic metastases together with primary HCC at the initial diagnosis of HCC, group B was composed of 83 patients who received treatment for intrahepatic HCC, and developed extrahepatic metastases during the follow-up period. Among them, 37 (25%) patients were treated previously for primary HCC in other hospitals, 90 patients were of performance status (PS) of 0, 43 patients of 1, 9 patients of 2, 6 patients of 3, and 3 patients of 4[19]. The etiology of the background liver disease was hepatitis B virus (HBV) in 33 patients, hepatitis C virus (HCV) in 89 patients, HBV and HCV in 5 patients, and non-B non-C in 24 patients. The hepatic reserve was Child-Pugh grade A in 88 patients, grade B in 48 patients, and grade C in 15 patients. We evaluated the primary tumor stage according to the Liver Cancer Study Group of Japan criteria[20], based on the following three conditions (T factor): solitary, < 2 cm in diameter, and no vessel invasion. T1 was defined as fulfilling the three conditions, T2 as fulfilling two of the three conditions, T3 as fulfilling one of the three conditions, T4 as fulfilling none of the three conditions. The primary HCC tumor stage at the first diagnosis of extrahepatic metastases was T0 (no intrahepatic HCC) in 11 (7%) patients, T1 in 4 (3%) patients, T2 in 13 (9%) patients, T3 in 43 (28%) patients, and T4 in 80 (53%) patients. Twenty seven of 28 patients with intrahepatic tumor stage T0-T2 were treated previously for intrahepatic HCC. The median size of the main intrahepatic primary tumor was 48 mm (range, 0-160 mm). Intrahepatic tumor morphology was nodular type in 83 (55%) patients, non-nodular type in 57 (38%) patients, and no intrahepatic HCC in 11 (7%) patients. Table 1 lists the sites of extrahepatic metastases at enrollment. Among the 151 patients with extrahepatic metastases, the sites of metastases were the lungs in 63 patients, lymph nodes in 60 patients, bones in 51 patients, adrenal glands in 16 patients and other locations (e.g., peritoneum, pancreas and nasal passages). In some patients, two or more distant metastatic tumors were found in one or more organs.
| Age (yr) | 64 (21-82) |
| Sex (male/female) | 117/34 |
| Etiology (HBV/HCV/HBV + HCV/others) | 33/89/5/24 |
| PS (0/1/2/3/4) | 90/43/9/6/3 |
| Intrahepatic tumor stage (T0/1/2/3/4) | 11/4/13/43/80 |
| Intrahepatic main tumor size (mm) | 48 (0-160) |
| Intrahepatic tumor volume (< 50%/≥ 50%) | 103/48 |
| Intrahepatic tumor morphology (nodular type/non nodular type/no intrahepatic HCC) | 83/57/11 |
| Grade of portal vein invasion (Vp 0/1/2/3/4) | 74/0/26/28/23 |
| Child-Pugh grade (A/B/C) | 88/48/15 |
| AFP (ng/mL) | 741.8 (< 5-861 600) |
| DCP (mAU/mL) | 1300 (< 10-391 400) |
| Site of extrahepatic metastases, n (%) | |
| Lung | 63 (42) |
| Lymph nodes | 60 (40) |
| Bone | 51 (34) |
| Adrenal | 16 (11) |
| Peritoneum | 1 (0.7) |
| Pancreas | 1 (0.7) |
| Nasal passages | 1 (0.7) |
A definitive diagnosis of HCC was based on the finding of typical hypervascular radiological features or histopathological examination of needle biopsy specimen. HCC was also assessed by US, CT, and/or AG. Furthermore, CT was obtained during arterial portography and computerized tomographic hepatic arteriography. Further assessment of HCC was conducted by measuring α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP).
Extrahepatic metastases were diagnosed by CT, MRI, bone scintigraphy, X-ray, and/or positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG), or diagnosed by histopathological examination of surgically resected specimen or biopsy. When we suspected extrahepatic metastases with HCC, we always ruled out other malignancies (such as gastric cancer, colon cancer and lung cancer) by several imaging modalities, serological tumor markers and/or pathological examination.
All the 151 HCC patients with extrahepatic metastases were followed up during the observation period and no one was lost to follow-up. The median follow-up period was 4.9 mo (range, 1-37 mo). After the diagnosis of HCC, all patients were screened at regular intervals for the development of intra/extra hepatic metastases by clinical examination, AFP, DCP, and/or various imaging modalities. Serological tumor markers were measured once every month. US, CT or MRI was performed once every three to six months.
Differences between groups were examined for statistical significance using the Mann-Whitney test (U-test) and χ2 test where appropriate. Cumulative survival rate was assessed by the Kaplan-Meier life-table method and the differences were evaluated by the log rank test. The following 15 potential predictors were assessed in this study: PS (0 vs 1-4), age (≤ 65 vs > 65 years), sex (M vs F), Child-Pugh stage (A vs B, C), intrahepatic tumor stage (T0-T2 vs T3, T4), main intrahepatic tumor size (≤ 50 vs > 50 mm), intrahepatic tumor volume (≤ 50% vs > 50%), intrahepatic tumor morphology (nodular type vs non nodular type), portal venous invasion (Vp 0-2 vs > Vp 3, 4), AFP (≤ 400 ng/mL vs > 400 ng/mL), DCP (≤ 1000 mAU/mL vs > 1000 mAU/mL), site of extrahepatic metastases (lung vs others, bone vs others, only lymph node vs others), and treatment for extrahepatic metastases (performed vs not performed). All factors that were at least marginally associated with the survival after diagnosis of extrahepatic metastases (P < 0.05) were entered into a multivariate analysis. The hazard ratio and 95% confidence interval (95% CI) were calculated to assess the relative risk confidence. All analyses described above were performed using the SPSS program (version 11.0, SPSS Inc., Chicago, IL).
The study protocol was approved by the Human Ethics Review Committee of Graduate School of Biomedical Sciences, Hiroshima University and a signed consent form was obtained from each patient.
Table 2 lists the sites of extrahepatic metastases identified throughout the follow-up period. The most frequent site of metastases that were identified throughout the follow-up period was the lung (n = 71 patients, 47%), followed by lymph nodes (n = 68 patients, 45%), bone (n = 56 patients, 37%), and adrenal glands (n = 18 patients, 12%). Brain metastases were identified in 2 (1%) patients. One (0.7%) patient each had metastases in the peritoneum, pancreas, nasal passages, muscle, skin, diaphragm, and colon. Autopsy was performed in 14 cases with metastases. Despite the detection of extrahepatic metastases in these 14 patients before autopsy, additional extrahepatic metastases were detected on postmortem examination (lymph nodes, diaphragm, and colon). At the first diagnosis of extrahepatic metastases, 109 (72%) patients had single-organ metastases, while the others had multiple organ metastases.
| Site | Patients (n = 151), n (%) |
| Lung | 71 (47) |
| Lymph nodes | 68 (45) |
| Bone | 56 (37) |
| Adrenal | 18 (12) |
| Brain | 2 (1) |
| Peritoneum | 1 (0.7) |
| Pancreas | 1 (0.7) |
| Nasal | 1 (0.7) |
| Muscle | 1 (0.7) |
| Skin | 1 (0.7) |
| Diaphragm | 1 (0.7) |
| Colon | 1 (0.7) |
Among the 71 patients with lung metastases, 23 patients had bilateral lung metastases, 14 had additional extrapulmonary site of metastatic disease. The size of pulmonary nodules ranged from 9 to 30 mm at initial diagnosis of extrahepatic HCC. Few patients had symptoms (cough, dyspnea, and pleural effusion) related to lung metastases, and 8 patients who had severe symptoms died subsequently of respiratory failure. The median survival period of these 8 patients was 4.3 mo (range, 2.5-14.4 mo).
Among the 68 patients with lymph node metastases, metastases were identified in 64 regional lymph nodes. The most common site was in the paraaortic nodes (31/64), followed by portohepatic nodes (21/64), periceliac nodes (6/64) and peripancreatic nodes (6/64). The majority of patients with regional lymph nodes metastases were asymptomatic, but few regional lymph nodes (portohepatic nodes) caused obstructive jaundice. Distant nodal metastases were found at 17 sites. The most common site was the mediastinum nodes (10/17), followed by subclavicular nodes (3/17), iliac nodes (2/17), cardiophrenic node (1/17), and retrocrural node (1/17). All distant lymph node metastases were not associated with clinical symptoms in this study.
Fifteen of 56 patients with bone metastases had multiple bone metastases at the initial diagnosis of bone metastases. The total number of bone metastatic sites was 88. The most frequent site was the vertebra (63/88; cervical vertebrae = 9, thoracic vertebrae = 38, and lumbar vertebrae = 16), followed by the ribs (8/88). Bone metastases were diagnosed by CT, MRI, bone scintigraphy, and/or PET with FDG.
Of the 18 patients with adrenal gland metastases, 13 had right adrenal gland metastases, 4 had left adrenal gland metastases and only one patient had bilateral metastases. These metastases were not associated with symptoms.
All patients with Child-Pugh grade other than C or PS other than 2-4 were treated for intrahepatic HCC, and many of them were continuously treated after the diagnosis of extrahepatic metastases. On the other hand, HCC patients with Child-Pugh grade C or PS of 2-4 received supportive care. Forty-nine (32%) of 151 patients were treated for extrahepatic metastases by surgical resection, TACE, systemic chemotherapy, and/or radiotherapy. The 49 patients had extrahepatic metastases that were considered to worsen prognosis.
Surgical resection was performed in three (2%) patients (with regional lymph node, adrenal gland and lung metastases). The survival periods after surgical resection of extrahepatic metastases were 7 mo (in patients with lymph node metastases), 23 mo (in patients adrenal gland metastases), and 37 mo (in patients with lung metastases). These three were all alive without recurrence of extrahepatic metastases during the observation period. In each of these 3 patients, hepatic reserve was Child-Pugh stage A, no intrahepatic HCC was not detected, and PS was 0.
TACE was performed in 8 (5%) patients (7 patients with adrenal gland metastases, and one patient with paraaortic lymph node metastases). Systemic chemotherapy was used in 39 (26%) patients. Chemotherapy included 5-fluorouracil, carboplatin, cisplatin. Twenty-five of the 39 patients had lung metastases, 10 had lymph node metastases, 2 had bone metastases, one had lung and lymph node metastases, and one had lung, adrenal gland and lymph node metastases.
Radiotherapy was performed in 36 (24%) patients. Curative therapy was performed in 10 patients (6 patients with lymph node metastases and 4 patients with adrenal gland metastases). Palliative therapy was performed in the remaining 26 patients who had severe pain due to bone metastases. Furthermore, 9 patients with painful bone metastases were treated with RFA therapy combined with cementoplasty[21]. Nonsteroidal anti-inflammatory drugs or opioids were used in patients with bone metastases due to severe pain.
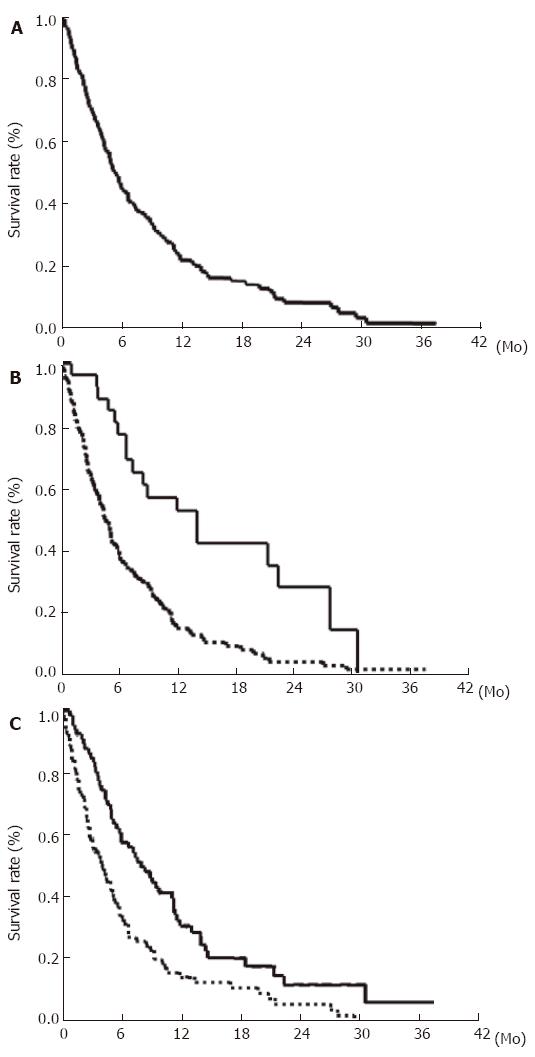
The cumulative survival rates of the 151 HCC patients with extrahepatic metastases after initial diagnosis of extrahepatic metastases at 6, 12, 24, and 36 mo were 44.1%, 21.7%, 14.2%, and 7.1%, respectively (Figure 1A). The median survival period was 4.9 mo (range, 1-37 mo). Survival was compared among patients with intrahepatic tumor stage T0-T2 and T3, T4 (Figure 1B). The rate was significantly higher in the intrahepatic tumor stage T0-T2 groups than in the T3, T4 groups (P < 0.001). We investigated the determinants of survival after initial diagnosis of extrahepatic metastases. Univariate analysis identified the following 9 factors significantly influencing survival: PS, 0 (P < 0.001); Child-Pugh grade, A (P < 0.001); intrahepatic main tumor size, < 50 mm (P < 0.001); intrahepatic tumor volume, < 50% (P < 0.001); portal venous invasion, Vp 0-2 (P < 0.001); use of treatment for extrahepatic metastases (P < 0.001, Figure 1C); bone metastasis (P = 0.012); DCP < 1000 mAU/mL (P = 0.02); and nodular type intrahepatic tumor (P = 0.03) (Table 3). Since the variables could be mutually correlated, multivariate analysis was performed. The analysis identified the following four variables as significant and independent determinants of survival after initial diagnosis of extrahepatic metastases: PS (P < 0.001), portal venous invasion (P < 0.001), treatment of extrahepatic metastases (P = 0.003), and Child-Pugh grade (P = 0.009) (Table 4).
| Variable | Hazard Ratio | 95% CI | P |
| PS (0 vs 1-4) | 2.181 | 1.50-3.17 | < 0.001 |
| Age ( ≤ 65 vs > 65 yr) | 0.988 | 0.97-1.0 | 0.18 |
| Sex (M vs F) | 0.889 | 0.57-1.38 | 0.601 |
| Child Pugh stage (A vs B, C) | 2.323 | 1.73-3.12 | < 0.001 |
| Intrahepatic main tumor size ( ≤ 50 vs > 50 mm) | 2.321 | 1.52-3.54 | < 0.001 |
| Intrahepatic tumor volume ( ≤ 50 vs > 50%) | 2.523 | 1.71-3.72 | < 0.001 |
| Intrahepatic tumor morphology (nodular vs non nodular) | 1.506 | 1.04-2.18 | 0.03 |
| Vp (0-2 vs 3, 4) | 2.247 | 1.53-3.29 | < 0.001 |
| AFP ( ≤ 400 vs > 400 ng/mL) | 1.158 | 0.80-1.68 | 0.439 |
| DCP ( ≤ 1000 vs > 1000 mAU/mL) | 1.584 | 1.08-2.33 | 0.02 |
| Treatment (performed vs not performed)1 | 2.385 | 1.51-3.77 | < 0.001 |
| Site (lung vs others)2 | 1.065 | 0.74-1.52 | 0.731 |
| Site (bone vs others) | 1.61 | 1.11-2.33 | 0.012 |
| Site (only lymph node vs others) | 1.133 | 0.74-1.74 | 0.567 |
| Variable | Hazard ratio | 95% CI | P |
| PS (0 vs 1-4) | 5.576 | 2.431-12.152 | < 0.001 |
| Vp (0-2 vs 3, 4) | 4.792 | 2.137-10.712 | < 0.001 |
| Treatment (performed vs not performed) | 4.134 | 1.539-11.011 | 0.003 |
| Child pugh stage (A vs B, C) | 2.372 | 1.247-4.914 | 0.008 |
Twenty-five patients were still alive at the end of this study while 126 patients died. Of the latter group, intrahepatic tumor stages at the first diagnosis of extrahepatic metastases were T0-2 in 17 patients and T3-4 in 109 patients. One hundred and twelve (89%) patients died of intrahepatic HCC or liver failure. Fourteen (11%) patients died of extrahepatic HCC (Table 5). Eight patients died of respiratory failure due to lung metastases. Four patients died of bone metastases-related disease. Two patients died of obstructive jaundice due to portohepatic node metastasis.
| Case | Presentation | Site | IntrahepaticHCC stage | Sex | Age(yr) | Child-Pugh stage | Etiology |
| 1 | R | Lung | T3 | M | 65 | A | HCV |
| 2 | R | Lung | T4 | M | 35 | CH | HBV |
| 3 | R | Lung | T3 | M | 56 | A | HBV |
| 4 | R | Lung, vertebra | T0 | M | 40 | CH | HBV |
| 5 | R | Lung, vertebra | T1 | M | 69 | A | HBV |
| 6 | R | Lung, LN | T0 | M | 63 | B | HBV |
| 7 | R | Lung, vertebra, nasal | T0 | M | 50 | A | HBV |
| 8 | R | Lung | T3 | M | 73 | A | NBNC |
| 9 | I | Skull | T1 | M | 57 | A | HCV |
| 10 | I | Skull | T2 | F | 72 | C | HCV |
| 11 | I | Skull | T3 | M | 56 | B | HCV |
| 12 | A | Vertebra | T3 | M | 69 | A | HCV |
| 13 | O | Lung, rib, LN | T1 | M | 74 | A | HCV |
| 14 | O | Vertebra, LN | T1 | M | 70 | B | HCV |
Of the 4 patients who died of bone metastases-related disease, 3 died of intracranial hypertension due to skull metastasis. Another patient died of vertebra metastasis-related disease. He was 69-year old at first diagnosis of bone metastases. He suffered from complete spinal cord injury due to vertebral metastasis with gradual worsening of PS. Finally, PS changed to 4 and the patient died of aspiration-related pneumonia. The survival period after first diagnosis of extrahepatic metastases was 11.5 mo.
Among the 14 patients who died of extrahepatic HCC, 3 had chronic hepatitis, 7 had cirrhosis of Child-Pugh grade A, 3 had cirrhosis of Child-Pugh grade B, and 1 had cirrhosis of Child-Pugh grade C. All patients who died of extrahepatic HCC with the exception of that with Child-Pugh grade C had some hepatic reserve until death. Intrahepatic tumor stage at first diagnosis of extrahepatic metastases was T0 (3 patients), T1 (4 patients), T2 (1 patient), T3 (5 patients), and T4 (1 patient). All 8 patients with intrahepatic tumor stage T0-T2 were treated previously for intrahepatic HCC. Eight of 17 (47%) patients with intrahepatic tumor stage T0-T2 died of extrahepatic metastases. On the other hand, 6 of 109 (6%) patients with intrahepatic tumor stages T3 and T4 died of extrahepatic metastases. The mortality rate of patients with intrahepatic tumor stage T0-T2 was significantly higher than that of patients with intrahepatic tumor stages T3 and T4 (P = 0.001) (Table 6).
| Intrahepatic tumor stage | Intrahepatic HCC orliver failure | Extrahepatic HCC |
| T0-2 (n = 17) | 53% (9/17) | 47% (8/17) |
| T3-4 (n = 109) | 94% (103/109) | 6% (6/109) |
The prognosis of HCC patients with extrahepatic metastases is unsatisfactory[16,17] and often not well known[18]. In the present study, we assessed the clinical features and prognosis of 151 consecutive HCC patients with extrahepatic metastases. The incidence of extrahepatic metastases from HCC was 15.2%. The most frequent metastatic sites were the lung, lymph nodes, bone, and adrenal gland. The cumulative survival rates of the 151 patients after the initial diagnosis of extrahepatic metastases at 6, 12, 24, and 36 mo were 44.1%, 21.7%, 14.2%, 7.1%, respectively. The median survival period was 4.9 mo (range, 1-37 mo). The mortality rate due to extrahepatic metastases from HCC was 11% (14/126).
Extrahepatic metastases have been reported to occur in 13.5%-42% of HCC patients[22-24]. In this study, the prevalence of extrahepatic metastases was 15.2%. Though we screened all HCC patients at regular intervals for intra/extra hepatic metastases, not all patients received a full metastatic follow up based on the use of several diagnostic techniques. Since the majority of HCC patients with extrahepatic metastases were asymptomatic, it is possible to miss asymptomatic metastases such as those in the lungs, distant lymph nodes, muscles and rectum.
Based on the initial diagnosis of intrahepatic HCC, Natsuizaka et al[16] reported that patients with advanced HCC develop extrahepatic metastases significantly more frequently than those with less advanced HCC. At the initial diagnosis of extrahepatic metastases, many HCC patients with extrahepatic metastases have been reported to have advanced intrahepatic stage[16,22]. In our study, 123 (81%) patients with extrahepatic metastases had intrahepatic tumor stages T3 (28%) and T4 (53%), at the initial diagnosis of extrahepatic metastases, suggesting that HCC patients with advanced intrahepatic tumor stage (T3, T4) are at risk of developong extrahepatic metastases, and that such patients should be followed up carefully.
On the other hand, our study identified 28 (19%) patients with early intrahepatic tumor stage (T0-T2) at the initial diagnosis of extrahepatic HCC. Eight of the 17 (47%) patients later died of extrahepatic metastases. With regard to previous treatment, 27 of 28 patients with early intrahepatic tumor stage were treated previously for intrahepatic HCC. Considering the possibility of extrahepatic metastases, HCC patients with early intrahepatic tumor stage should be followed up carefully, particurally those who have been treated previously for intrahepatic HCC. This also includes HCC patients who have received complete resection or ablation.
In this study, the most frequent metastatic sites were the lungs, lymph nodes, bones, and adrenal glands. Other studies have reported similar findings[16,22]. HCC is thought to spread mainly via the hematogenous route, thus causing intra/extra hepatic metastases. Most of HCCs are hypervascular tumors. Moreover, HCC tends to invade vessels, such as portal and hepatic veins. Therefore, HCC could spread through the lung and systemic circulation via the hepatic or portal vein. This could explain why the lung is the most frequent site of metastases in HCC. Most of HCC patients with lung metastases are asymptomatic. To detect lung metastases from HCC, chest CT should be performed at regular intervals during routine metastasis follow-up.
Though there is no standard treatment for extrahepatic metastases of primary HCC, several authors have reported the use of various treatment modalities for extrahepatic metastases[5,7,15,23,25-28]. Some reports have described successful treatment of extrahepatic metastases with no or few intrahepatic HCC[5,7,25,26]. However, only few HCC patients can undergo surgical resection of extrahepatic metastases because of hepatic reserve or intraheatic tumor stage. In this study, the prognosis of 3 patients after surgical resection of extrahepatic metastases seemed good. These 3 patients had good hepatic reserve, no intrahepatic HCC (PS = 0) and no intra/extra hepatic HCC and are expected to have good prognosis. The clinical features of HCC patients with extrahepatic metastases varied widely. All patients were not symptomatic and thus not necessary to receive treatment of extrahepatic metastases. Thus, treatment of extrahepatic metastases from primary HCC must be performed carefully taking into consideration the clinical features.
Multivariate analysis in our study identified PS, portal venous invasion, treatment for extrahepatic metastases, and Child-Pugh grade as important determinants of survival after the initial diagnosis of extrahepatic metastases. Ishii et al[17] reported that brain metastases, number of metastatic tumors and primary tumor status are important factors for survival. In our study, only two patients had brain metastases. With regard to the number of metastatic tumors, we might miss asymptomatic metastases. Thus, we did not include brain metastasis and number of metastatic tumors in this multivariate analysis. Treatment of extrahepatic metastases was an important determinant of survival in our study. There might be selection bias of patients treated for extrahepatic metastases because many of them had good hepatic reserve. HCC patients with poor hepatic reserve did not receive treatment for extrahepatic metastases in this study. Regardless of such bias, treatment of extrahepatic metastases might be important for improvement of prognosis.
With regard to the cause of death, many HCC patients with extrahepatic metastases died of intrahepatic HCC or liver failure and few (11%) died of extrahepatic HCC. Of the 14 patients who died of extrahepatic metastases, 10 had good hepatic reserve and 8 had early intrahepatic tumor stage, at the initial diagnosis of extrahepatic metastases. Usually, HCC patients with good hepatic reserve, no or few intrahepatic HCCs, and those without portal venous invasion show relatively good prognosis. According to the univariate analysis of HCC patients with extrahepatic metastases, patients with early intrahepatic tumor stage have a significantly better prognosis than those with advanced intrahepatic tumor stage. In our study, the mortality rate due to extrahepatic metastases with early intrahepatic tumor stage was significantly higher than that due to those with advanced intrahepatic tumor stage. This might be explained by the differences in survival periods between these intrahepatic tumor stage groups. Extrahepatic metastases with early intrahepatic tumor stage can spread during the relatively long survival period, and few patients die of extrahepatic metastases. Extrahepatic metastasis with early intrahepatic tumor stage is a very important cause of death of HCC patients. Successful treatment of extrahepatic metastases in HCC patients with early intrahepatic tumor stage might improve the prognosis.
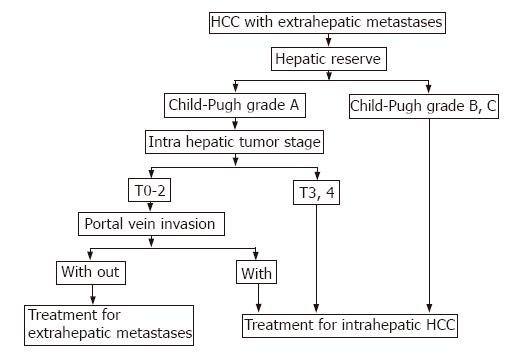
In conclusion, the majority of HCC patients with extrahepatic metastases should undergo treatment for intrahepatic HCC. Selected HCC patients with critical extrahepatic metastases could undergo treatment for extrahepatic metastases. However, these selected patients must have good hepatic reserve, intrahepatic tumor stage: T0-T2, and are free of portal venous invasion (Figure 2). The important sites of critical metastases from primary HCC are the lungs, bones and the portohepatic node. Further studies are needed for the improvement of the prognosis of HCC patients with extrahepatic metastases.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [PubMed] [DOI] [Full Text] |
| 2. | Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol. 2000;6:773-776. [PubMed] |
| 3. | Tang Z. Recent advances in clinical research of hepatocellular carcinoma in China. Chin Med J (Engl). 1995;108:568-570. [PubMed] |
| 4. | Lo CM, Lai EC, Fan ST, Choi TK, Wong J. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br J Surg. 1994;81:1019-1021. [PubMed] [DOI] [Full Text] |
| 5. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. [PubMed] [DOI] [Full Text] |
| 6. | Kuromatsu R, Hirai K, Majima Y, Fujimoto T, Shimauchi Y, Tsukiyama Y, Aoki E, Saitsu H, Nakashima O, Kojiro M. A patient with hepatocellular carcinoma who underwent resection of the primary lesion 10 years ago and resection of a giant adrenal metastasis 8 and a half years later. Gastroenterol Jpn. 1993;28:312-316. [PubMed] |
| 7. | Inagaki Y, Unoura M, Urabe T, Ogino H, Terasaki S, Matsushita E, Kaneko S, Morioka T, Furusawa A, Wakabayashi T. Distant metastasis of hepatocellular carcinoma after successful treatment of the primary lesion. Hepatogastroenterology. 1993;40:316-319. [PubMed] |
| 8. | Okazaki N, Yoshino M, Yoshida T, Hirohashi S, Kishi K, Shimosato Y. Bone metastasis in hepatocellular carcinoma. Cancer. 1985;55:1991-1994. [PubMed] [DOI] [Full Text] |
| 9. | Kay RM, Eckardt JJ, Goldstein LI, Busuttil RW. Metastatic hepatocellular carcinoma to bone in a liver transplant patient. A case report. Clin Orthop Relat Res. 1994;237-241. [PubMed] |
| 10. | Knight TE, Woo AS, Blaisdell JM. Hepatocellular carcinoma invasive to chest wall. Int J Dermatol. 1992;31:273-276. [PubMed] [DOI] [Full Text] |
| 11. | Kim PN, Kim IY, Lee KS. Intraperitoneal seeding from hepatoma. Abdom Imaging. 1994;19:309-312. [PubMed] [DOI] [Full Text] |
| 12. | Barasch E, Frazier OH, Silberman H, Shannon RL, Wilansky S. Left atrial metastasis from hepatocellular carcinoma: a case report. J Am Soc Echocardiogr. 1994;7:547-549. [PubMed] [DOI] [Full Text] |
| 13. | Fujimoto H, Murakami K, Nosaka K, Arimizu N. Splenic metastasis of hepatocellular carcinoma. Accumulation of Tc-99m HDP. Clin Nucl Med. 1992;17:99-100. [PubMed] [DOI] [Full Text] |
| 14. | Kim HS, Shin JW, Kim GY, Kim YM, Cha HJ, Jeong YK, Jeong ID, Bang SJ, Kim do H, Park NH. Metastasis of hepatocellular carcinoma to the small bowel manifested by intussusception. World J Gastroenterol. 2006;12:1969-1971. [PubMed] |
| 15. | Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, Sun HC, Wang BL, Zhang JY, Yu Y. Radiation therapy for adrenal gland metastases from hepatocellular carcinoma. Jpn J Clin Oncol. 2005;35:61-67. [PubMed] [DOI] [Full Text] |
| 16. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [PubMed] [DOI] [Full Text] |
| 17. | Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Nakachi K, Yoshino M. Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Jpn J Clin Oncol. 2004;34:733-739. [PubMed] [DOI] [Full Text] |
| 18. | Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka H, Ikeda K, Yoshimori M. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology. 1997;44:251-257. [PubMed] |
| 19. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] [DOI] [Full Text] |
| 20. | Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer (in Japanese). 4th ed. Tokyo: Kanehara 2000; 19. |
| 21. | Toyota N, Naito A, Kakizawa H, Hieda M, Hirai N, Tachikake T, Kimura T, Fukuda H, Ito K. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28:578-583. [PubMed] [DOI] [Full Text] |
| 22. | Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. [PubMed] [DOI] [Full Text] |
| 23. | Shuto T, Hirohashi K, Kubo S, Tanaka H, Yamamoto T, Higaki I, Takemura S, Kinoshita H. Treatment of adrenal metastases after hepatic resection of a hepatocellular carcinoma. Dig Surg. 2001;18:294-297. [PubMed] [DOI] [Full Text] |
| 24. | Si MS, Amersi F, Golish SR, Ortiz JA, Zaky J, Finklestein D, Busuttil RW, Imagawa DK. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg. 2003;69:879-885. [PubMed] |
| 25. | Nakayama H, Takayama T, Makuuchi M, Yamasaki S, Kosuge T, Shimada K, Yamamoto J. Resection of peritoneal metastases from hepatocellular carcinoma. Hepatogastroenterology. 1999;46:1049-1052. [PubMed] |
| 26. | Kurachi K, Suzuki S, Yokoi Y, Okumura T, Inaba K, Igarashi T, Takehara Y, Konno H, Baba S, Nakamura S. A 5-year survivor after resection of peritoneal metastases from pedunculated-type hepatocellular carcinoma. J Gastroenterol. 2002;37:571-574. [PubMed] [DOI] [Full Text] |
| 27. | Momoi H, Shimahara Y, Terajima H, Iimuro Y, Yamamoto N, Yamamoto Y, Ikai I, Yamaoka Y. Management of adrenal metastasis from hepatocellular carcinoma. Surg Today. 2002;32:1035-1041. [PubMed] [DOI] [Full Text] |
| 28. | Zeng ZC, Tang ZY, Fan J, Qin LX, Ye SL, Zhou J, Sun HC, Wang BL, Wang JH. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005;63:1067-1076. [PubMed] [DOI] [Full Text] |