Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.378
Revised: October 25, 2006
Accepted: November 6, 2006
Published online: January 21, 2007
AIM: To establish the rats model of chronic fibrosing pancreatitis and to prove the anti-fibrotic effect of emodin in chronic pancreatitis with fibrosis.
METHODS: Fifty rats were randomly divided into five groups, 10 rats in each group. Trinitrobenzene sulfonic acid (TNBS) was infused into the pancreatic duct to induce chronic pancreatitis in rats (except for normal group). Emodin-treated rats were fed with different doses of emodin (20, 40 and 80 mg/kg body weight) for 28 d, while normal group and control group received 0.9% sodium chloride solution. Serum levels of hyaluronic acid (HA) and laminin (LN) were determined by radioimmunoassay. Histopathological alterations were studied by optical microscopy. Expression of collagen was also examined while transforming growth factor-beta-1 (TGF-β1) was localized by immunochemistry.
RESULTS: In emodin-treated rats, the serum levels of HA and LN were decreased significantly (HA, 62.2 ± 19.3 μg/L vs 112.7 ± 26.5 µg/L, P < 0.05; LN 44.3 ± 10.4 μg/L vs 86.2 ± 16.5 µg/L, P < 0.05); the degree of fibrosis was ameliorated observably; the expression of collagen in pancreatic tissue was reduced especially in high-dose emodin-treated group (36% ± 5% vs 42% ± 6%, P < 0.05); with the increased doses of emodin, the expression of TGF-β1 was declined, compared with those in control group.
CONCLUSION: Emodin has an anti-fibrotic effect on pancreatic fibrosis in rats. Because of its anti-fibrotic effect, it could be a potential herb for the treatment of chronic pancreatitis.
- Citation: Wang CH, Gao ZQ, Ye B, Cai JT, Xie CG, Qian KD, Du Q. Effect of emodin on pancreatic fibrosis in rats. World J Gastroenterol 2007; 13(3): 378-382
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/378.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.378
Chronic pancreatitis is characterized morphologically by a progressive pancreatic fibrotic process[1]. Extracellular matrix (ECM) components are distributed in both healthy and diseased pancreas, and there is an increased deposition of disorganized ECM components in chronic pancreatitis[2]. However, the mechanism of the fibrotic process is not fully understood. Progressive fibrosis is a characteristic feature of chronic pancreatitis of various etiologies, thus anti-fibrosis is the key to the therapy of chronic pancreatitis. However, no ideal treatment is available yet. Could traditional Chinese herbs be effective? Emodin (1, 3, 8-trihydroxy-6-methylanthraquinone) isolated from a great deal of herbs is an effective constituent with many effects. Lots of pharmaceutical studies have demonstrated that emodin has many biological effects[3-5], such as anticancer, antimicrobial, and anti-inflammatory effects, etc. Moreover, several studies[6,7] have revealed that emodin is efficacious in the management of acute pancreatitis and hepato-cirrhosis, but studies on chronic pancreatitis are rare. We designed this experiment to investigate the effect of emodin on pancreatic fibrosis in rats, and elucidate its potential therapeutic mechnisms.
Male Sprague-Dawley rats weighing 200-250 g were provided by the Animal Center of Zhejiang University. Trinitrobeneze sulfonic acid (TNBS) was obtained from Sigma Chemical Company. Radioimmunoassay kits of rat hyaluronic acid (HA) and laminin (LN) were purchased from Shanghai Tiancheng Science and Technology Ltd. Emodin was offered by Hunan Huaguang Chemical Company. TGF-β1 polyclonal antiboby was provided by Santa Cruz Company. Immunochemistry kits (Envision TM) were offered by DAKO, Denmark.
Fifty male Sprague-Dawley rats were randomly divided into five groups, 10 rats in each group. A chronic pancreatitis model was induced in rats by infusion of TNBS into the pancreatic duct with a modification of the method described by Puig-Divi et al[8] (except for normal group). After fasted for 3 d, the rats in group 1 (normal group, nonoperation) and group 2 (control group, nontreating) received oral 0.9% sodium chloride solution. Emodin-treated rats in groups 3-5 were fed with different doses of emodin (20, 40 and 80 mg/kg body mass). Twenty-eight days after treatment, all rats were killed. Pancreatic tissues and blood samples were collected and kept for further study.
Blood samples were obtained 28 d after treatment, and serum levels of HA and LN were determined by radioimmunoassay.
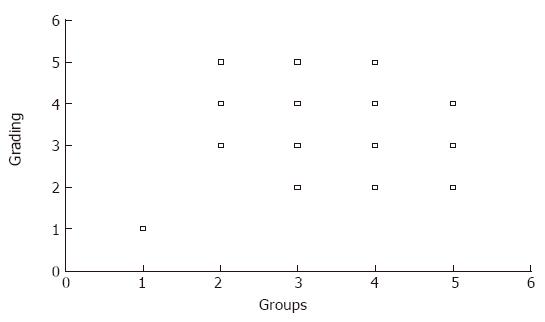
Paraffin sections of rat pancreatic tissue were stained with hematoxylin and eosin. We evaluated the morphologic changes by light-microscopy. Grading of pancreatic fibrosis was carried out as previously described[9] (Table 1).
| Grade | Ductdilation | Periductalfibrosis | Intralobularfibrosis | Glandularatrophy |
| F0 | - | - | - | - |
| F1 | + | - | - | - |
| F2 | + | + | - | - |
| F3 | + | + | + | - |
| F4 | + | + | + | + |
Pancreatic fibrosis was observed by the Van Gieson staining, semi-quantitative analysis of collagen fibres was carried out by the KS400 imaging analysis system (KS400 ver. 2.0).
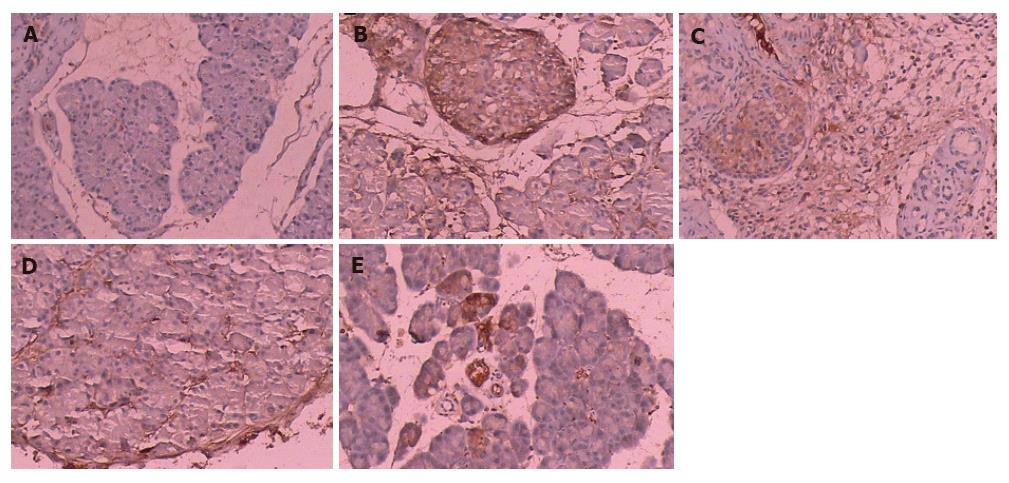
Paraffin-embedded tissue sections were subjected to immunostaining using the DAKO EnVisin two-step procedure. Tissue sections were submerged for 10 min in Tris buffered saline (TBS) (10 mmol/L Tris-HCl, 0.85% NaCl, pH 7.4) containing 3% hydrogen peroxide in order to quench the endogenous peroxidase activity. Following washing with distilled water, the sections were incubated in TBS with polyclonal antibodies against TGF-β1 diluted at 1:200 for 30 min. After 10-min washing with TBS, the sections were incubated with Envision TM for another 30 min. After washing with TBS for 10 min, the sections were incubated with diaminobenzidine (DAB) as a substrate and counterstained. The results were evaluated by microscopy. All samples were classified according to the percentage of TGF-β1 positive cells (brown colour reaction). We defined 0 as no, + as 0%-25%, +2 as 25%-50%, +3 as 50%-75%, +4 as > 75%.
Serum levels of HA and LN and pancreatic collagen content were expressed as mean ± SE. Student’s t test was used for statistical analysis. Chi square test was used for the ranked data statistical analysis. If the expected cell count was less than or equal to 5, Fisher’s exact test was used. SPSS 11.0 was used for statistical analysis, and P < 0.05 was considered statistically significant.
The survival rate was 83.3% in emodin-treated groups (25/30) and 80.0% (8/10) in control group. However, no significant difference was found between these groups.
The mean serum level of HA and LN was the lowest in normal group (group 1, P < 0.05), and the levels of HA and LN were significantly lower in emodin-treated groups (groups 3-5) than in control group (group 2, P < 0.05). With the increased doses of emodin, the serum levels of HA and LN were decreased in emodin-treated groups, but there was no statistically significant difference between them (Table 2).
Pancreatic fibrosis was evaluated according to the criteria described above (Table 1), and the results are shown in Figure 1. The extent of fibrosis was much milder in emodin-treated groups than in control group. Structural changes such as periductal fibrosis, intralobular fibrosis and glandular atrophy were ameliorated. With the increased doses of emodin, the changes were significantly improved.
Pancreatic collagen was detected by Van Gieson staining. An extensive accumulation of collagen and glandular atrophy were found in control group, while the accumulation of collagen was decreased and no glandular atrophy was found in emodin-treated groups. We obtained the positive proportions of Van Gieson staining by the imaging analysis system. The positive proportions in each group are shown in Table 3. With the increased doses of emodin, the changes were improved obviously. Only a significant difference was found between group 5 and group 2 (P < 0.05).
Four groups (except for normal group) showed TGF-β1 expression (brown colour reaction) (Figure 2). The results are shown in Table 4. With the increased doses of emodin, the changes were improved obviously, similar to the above results. For the sake of statistical analysis, we divied all samples into two large groups: low expression of TGF-β1 (0, +1, +2) and high expression of TGF-β1 (+3, +4). Fisher’s exact test was used for statistical analysis. Compared with group 2 (0 rat in low expression group and 8 rats in high expression group), a significant difference was found between group 4 (7 rats in low expression group and 1 rat in high expression group) and group 5 (9 rats in low expression group and 0 rat in high expression group) (P < 0.05). Compared with group 3 (4 rats in low expression group and 4 rats in high expression group), only group 5 had a significant difference (P < 0.05). There was no statistically significant difference between any other two groups.
| Group | n | 0 | + | +2 | +3 | +4 |
| 1 (Normal) | 10 | 9 | 1 | 0 | 0 | 0 |
| 2 (Control) | 8 | 0 | 0 | 0 | 4 | 4 |
| 3 (emodin, 20 mg/kg) | 8 | 0 | 0 | 4 | 4 | 0 |
| 4 (emodin, 40 mg/kg) | 8 | 0 | 4 | 3 | 1 | 0 |
| 5 (emodin, 80 mg/kg) | 9 | 4 | 5 | 0 | 0 | 0 |
The incidence of chronic pancreatitis is increasing, and the most important etiological factor is alcohol. The pathogenesis of chronic pancreatitis has not been completely understood. Nevertheless, it was reported that various factors such as TGF-β1, ECM, oxidative stress etc, are involved in the progression of pancreatic fibrosis[10,11]. Since pancreatic fibrosis is the characteristic feature of chronic pancreatitis, preventing and inverting pancreatic fibrosis is the key to therapy of chronic pancreatitis and amelioration of its prognosis.
Drugs which could prevent or invert pancreatic fibrosis might be developed and interventioned for the treatment of chronic pancreatitis with fibrosis. Emodin isolated from a great deal of herbs is an effective constituent with many effects[3-5]. It was reported that emodin could inhibit the expression of α-smooth muscle actin (α-SMA) and reduce the transformation and synthesis of TGF-β1[6]. Some researchers observed that emodin could inhibit transformation of hepatic stellate cells (HSCs) from G1 phase to S phase and S phase to G2 phase that could prevent HSC proliferation. It also has been reported that emodin could inhibit lipid peroxidation[12]. However, previous studies of emodin mainly focused on how to inhibit liver fibrosis, while studies on pancreatic fibrosis are rare. The aim of this research was to study the effect of emodin on pancreatic fibrosis, and the results of our study indicate that emodin has an anti-fibrotic effect on pancreatic fibrosis in rats.
Cells similar to HSCs have been identified and isolated from the pancreas, known as pancreatic stellate cells (PSCs)[13,14]. It was reported that PSCs are activated in both experimental and human pancreatic fibrosis and these activated PSCs are the main cellular source of collagen in chronic pancreatitis[15], suggesting that pathological alteration of pancteatic fibrosis is similar to liver fibrosis, and may share the same mechanism of fibrosis and anti-fibrosis.
The potential mechanisms of emodin include the following aspects. The first is cell protection. As we know, tissue injury is the first step of fibrosis. Apte et al[16] showed that activated PSCs around the pancreatic micrangium express α-SMA, which could aggravate fibrosis of the pancreas, because of vasoconstriction and pancreatic ischemia caused by α-SMA. Emodin could protect pancreatic cells and organs from impairment. In our study, the extent of fibrosis was much milder in emodin-treated groups than in control group. This may be the first mechanism of emodin’s anti-fibrotic effect. The second is the effect of emodin on cytokines. PSCs produce ECM after stimulation with TGF-β1, platelet-derived growth factor (PDGF), etc[17]. It was reported that TGF is the key regulating factor of fibrosis. Modulation of TGF-β signaling may be the therapeutic target for the prevention of chronic pancreatitis[18]. It was reported that emodin could inhibit the expression of TGF-β1 in liver fibrosis model of rats[6]. Imanishi et al[19] found that emodin could modulate the effect of PDGF, which could slow down the progression of fibrosis. In our study, emodin-treated groups showed low expression of TGF-β1, especially in high-dose emodin-treated group. Emodin could counteract cytokines and their receptors, which are relevant to the progression of fibrosis, and then brings its anti-fibrotic effect into play. The third is the regulatory effect of emodin on ECM metabolism. Disbalance in ECM metabolism is the common way of fibrosis. Chronic pancreatitis is due to the cumulative intrapancreatic ECM deposition. ECM is consisted of collagens and noncollagenous glycoproteins (fibronectin, laminin). It was reported that serum HA and LN are two important markers responsible for the degrees of fibrosis[20]. Our study revealed that the serum levels of HA and LN were significantly lower in emodin-treated groups than in control group, suggesting that emodin can affect the metabolism of ECM. However, the exact mechanism by which emodin regulates the content of ECM still needs further investigation. The fourth is the effect of emodin on ECM-producing cells. PSCs rapidly proliferate following injury and are the major source of pancreatic ECM component during pancreatic fibrogenesis, and accumulating evidence has placed PSCs at the center of pancreatic fibrosis[15]. Emodin not only inhibits proliferation and activation of PSCs, but also induces their apoptosis. This is another mechanism underlying the anti-fibrotic effect of emodin. The fifth is the antioxidizing effect of emodin. Yoo and co-workers[11] reported that oxidative stress is principally involved in the pathogenesis of chronic pancreatitis with fibrosis and antioxidants could prevent and ameliorate the extent of pancreatic fibrosis, suggesting that antioxidants can be considered in preventing and treating chronic pancreatitis. Eomdin could inhibit lipid peroxidation[12], and antioxidization maybe another mechanism underlying the anti-fibrotic effect of emodin.
Chronic pancreatitis is a progressive pancreatic fibrotic process, and anti-fibrosis is the key therapy for liver fibrosis. However, previous studies on the effect of emodin on chronic pancreatitis in rats are not available. Our study showed that emodin had an effect on pancreatic fibrosis in rats.
The results of this study indicate that emodin can significantly affect the development of pancreatic fibrosis by reducing the serum levels of HA and LN, ameliorating the degrees of fibrosis, diminishing the content of collagen, inhibiting TGF-β1 expression. Therefore emodin has an excellent prospect in the development of some new praeparatum for the management of chronic pancreatits. However, there are still some questions we need to answer, such as whether emodin can be used in patients with chronic pancreatitis, whether it is safe, since few previous data are available so far. Further detailed experiments and clinical trails are required to prove the potential clinical application. As a traditional herb, emodin also has a long long way to go.
Chronic pancreatitis is characterized by a progressive pancreatic fibrotic process. However, the mechanism of fibrotic process is not fully understood. Thus anti-fibrosis is the key to the therapy of chronic pancreatitis.
Emodin has many biological effects, but studies about its application in chronic pancreatitis are rare.
There are few studies on the application of emodin in chronic pancreatitis. We designed this experiment to investigate the effect of emodin on pancreatic fibrosis.
No ideal therapy is available so far for the treatment of chronic pancreatitis. Emodin may be efficacious in the management of chronic pancreatitis in rats.
Emodin (1, 3, 8-trihydroxy-6-methylanthraquinone), isolated from a great deal of herbs, is an effective constituent with many effects, such as anticancer, antimicrobial, and anti-inflammatory effects, etc.
The topic is of interest because up to now no antifibrotic treatment option is available in patients with chronic pancreatitis. The positive effect of emodin on development of pancreatic fibrosis described in this paper shows that emodin may be a potential treatment option.
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
| 1. | Keith RG. Definition and classification of chronic pancreatitis. World J Surg. 2003;27:1172-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Kennedy RH, Bockman DE, Uscanga L, Choux R, Grimaud JA, Sarles H. Pancreatic extracellular matrix alterations in chronic pancreatitis. Pancreas. 1987;2:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Chang CJ, Bacus SS, Hung MC. Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res. 1995;55:3890-3896. [PubMed] |
| 4. | Chang CH, Lin CC, Yang JJ, Namba T, Hattori M. Anti-inflammatory effects of emodin from ventilago leiocarpa. Am J Chin Med. 1996;24:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001;50:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Zhan YT, Liu B, Li DG, Bi CS. Mechanism of emodin for anti-fibrosis of liver. Zhonghua Ganzangbing Zazhi. 2004;12:245-246. [PubMed] |
| 7. | Lou KX, Gong ZH, Yuan YZ. Study on effect of emodin on TGF beta 1 expression in pancreatic tissue of rats suffering from acute pancreatitis. Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:433-436. [PubMed] |
| 8. | Puig-Diví V, Molero X, Salas A, Guarner F, Guarner L, Malagelada JR. Induction of chronic pancreatic disease by trinitrobenzene sulfonic acid infusion into rat pancreatic ducts. Pancreas. 1996;13:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chinese Medical Association. Prevention and treatment of Viral Hepatitis. Zhonghua Neike Zazhi. 2001;40:62-68. |
| 10. | Yoshikawa H, Kihara Y, Taguchi M, Yamaguchi T, Nakamura H, Otsuki M. Role of TGF-beta1 in the development of pancreatic fibrosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G549-G558. [PubMed] |
| 11. | Yoo BM, Oh TY, Kim YB, Yeo M, Lee JS, Surh YJ, Ahn BO, Kim WH, Sohn S, Kim JH. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology. 2005;5:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Huang SS, Yeh SF, Hong CY. Effect of anthraquinone derivatives on lipid peroxidation in rat heart mitochondria: structure-activity relationship. J Nat Prod. 1995;58:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 712] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 14. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 796] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 480] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grünert A, Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2001;281:C532-C543. [PubMed] |
| 18. | Yoo BM, Yeo M, Oh TY, Choi JH, Kim WW, Kim JH, Cho SW, Kim SJ, Hahm KB. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71-e79. [PubMed] |
| 19. | Imanishi Y, Maeda N, Otogawa K, Seki S, Matsui H, Kawada N, Arakawa T. Herb medicine Inchin-ko-to (TJ-135) regulates PDGF-BB-dependent signaling pathways of hepatic stellate cells in primary culture and attenuates development of liver fibrosis induced by thioacetamide administration in rats. J Hepatol. 2004;41:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Zheng M, Cai W, Weng H, Liu R. Determination of serum fibrosis indexes in patients with chronic hepatitis and its significance. Chin Med J (Engl). 2003;116:346-349. [PubMed] |