Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.4015
Revised: March 18, 2007
Accepted: March 31, 2007
Published online: August 7, 2007
AIM: To study the effect of glucose on sodium butyrate-induced proliferation inhibition and apoptosis in HT-29 cell line, and explored its possible mechanisms.
METHODS: HT-29 cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum, and were allowed to adhere for 24 h, and then replaced with experimental medium. Cell survival rates were detected by MTT assay. Apoptosis was detected by TUNEL assay. Glucose transport protein 1 (GLUT1) and monocarboxylate transporter 1 (MCT1) mRNA expression was detected by RT-PCR.
RESULTS: Low concentration of glucose induced apoptosis and regulated proliferation in HT-29 cell line, and glucose can obviously inhibit the effect of proliferation inhibition and apoptosis induced by sodium butyrate. Glucose also down-regulated the expression of MCT1mRNA (0.28 ± 0.07 vs 0.19 ± 0.10, P < 0.05), and decreased the expression of GLUT1mRNA slightly (0.18 ± 0.04 vs 0.13 ± 0.03, P < 0.05).
CONCLUSION: Glucose can regulate the effect of proliferation inhibition and apoptosis induced by sodium butyrate and this influence may be associated with the intracellular concentration of glucose and sodium butyrate.
- Citation: He L, Li X, Luo HS, Rong H, Cai J. Possible mechanism for the regulation of glucose on proliferation, inhibition and apoptosis of colon cancer cells induced by sodium butyrate. World J Gastroenterol 2007; 13(29): 4015-4018
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/4015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.4015
Butyrate is a naturally occurring product of colonic microbial fermentation of dietary carbohydrates. It is important for maintaining homeostasis of normal colonic mucosa, but it also possesses anti-proliferative and pro-differentiating properties and can induce apoptosis in many cancer cell lines[1,2].
Butyrate is the principal energy source for colonic epithelial cells. But in colorectal cancer cell line Caco-2, butyrate was not significantly metabolized within 10 min. It may not be the main energy source in colon cancer cells. Previous studies showed that malignant tissues had increased glucose uptake and utilization. Colon cancer cells may burn 30 to 40 times more glucose for energy than normal cells. All these suggest that glucose is an important energy source for colon cancer cells.
Glucose transport is the rate-limiting step in glucose metabolism and is controlled through glucose transport proteins (GluTs) with GluT1 being the important isoform in human colonic cancer cells[3,4]. Nagachi et al assessed GluT1 expressing in all the colonic tumor samples while none of the samples of normal epithelium expressed GluT1. The expression of GluT1 is associated with the uptake of glucose and the intracellular glucose concentration and the metabolic rates in tissues. At the colonic luminal pH, butyrate (pKa 4.7) dissociates almost entirely to the butyrate anion. This charged species cannot cross the luminal membrane by free diffusion and therefore requires a specific transport system. MCT1 is a member of the family of monocarboxylate transporters (MCTs), which can transport butyrate across the membrane[5]. The expression of MCT1 is associated with the uptake and the intracellular concentration of butyrate. The ability of butyrate to exert its effects may depend upon its intracellular concentration. The expression of MCT1 may be associated with the effect of butyrate.
Both glucose and sodium butyrate are the energy source for colonic cancer cells. The regulation of glucose on the uptake of itself and sodium butyrate in colonic cancer cell was not clear. In this study, we examined the regulation of glucose on effect of proliferation inhibition and apoptosis induced by sodium butyrate, and the regulation of glucose on the GluT1 and MCT1mRNA in the presence of sodium butyrate.
HT-29 human colon cancer cells were grown as a monolayer in RPMI-1640 medium (Sigma) supplemented with 10% fetal calf serum (GIBCO), cultured in T-75 cm2 culture flasks, maintained at 37°C in 5% CO2, humidified atmosphere. At the beginning of the experiment mycoplasma-free cells in the exponential growth phase were removed from the flask with 0.25% trysin and 0.02% EDTA solution and seeded in T-75 cm2 flask in RPMI-1640 medium with 10% fetal calf serum. The cells were allowed to adhere for 24 h, and then replaced with experimental medium.
Cell were seeded onto 96-well plates at the density of 2 × 104 per well for HT-29 colonic cell line and incubated in a humidified atmosphere with 5% CO2 for 48 h, then 0, 100, 200, 500, 1000, 2000, 4000 mg/L glucose in RPMI 1640 with 10% fetal calf serum and 5 mmol/L butyrate was added or not. For the control, the complete medium (RPMI 1640 containing 2000 mg/L glucose) was added without sodium butyrate. After incubated for 48 h, MTT 20 μL (5 g/L) was added. The plates were incubated for 4 h and 100 μL DMSO was added. The absorbance was recorded using an ELISA reader and the survival rate was calculated.
Cells were kept for in the medium containing (0, 500, 1000 or 2000 mg/L glucose in RPMI-1640) supplemented with 5 mmol/L sodium butyrate. After harvesting of the cells, total RNA was extracted by means of Trizol (Invitrogen) according to the manufactures instruction. The concentration of RNA was quantitated by absorbance 260 and 280 nm. Total RNA was suspended in DEPC-treated water and stored at -80°C.
Single-stranded cDNA was prepared using 2 μg of total RNA, 200U MMLV Reverse transciptase (Promega), MMLV buffer (Promega) 5 μL, 10 mmol/L dNTP (Promega) 1.25 μL, oligod T (Promega) 1 μg, RNasin (Biostar) 25 U in a 25 μL solution and incubated for 90 min at 42°C. An amplification of the resulting cDNA sequence was carried out using polymerase chain reaction (PCR). cDNA 1 μL was combined with 1 pmol oligonucleotide primers specific for human GluT1, MCT1, β-actin and Tap DNA Polymerase (Biostar) 0.5 U, Tap buffer (Biostar) 2.5 μL, 10 mmol/L dNTP 0.5 μL in a 25 μL solution. The conditions for the reaction were the following: 5 min at 95°C (predenaturation), followed by 28 cycles of 1 in at 95°C (denaturation), 45 s at 60°C(annealing), and 1 min at 72°C (extension), and 7 min at 72°C (final extension). Then 5 μL samples of amplified products were resolved by electrophoresis in 2% agarose gel, stained with ethidium bromide. The level of each PCR product was semiquantitatively evaluated using a digital camera and an image analysis system (Viber lourmat, France), and normalized to β-actin. Following are the primers for:
β-actin: sense: 5'CGAGCGGGAAATCGTGCGTGACATTAAGGAGA3', antisense: 5'CGTCATACTCCTGCTTGCTGATCCACATCTGC3'; GLUT1: sense: 5'CGGGCCAAGAGTGTGCTAAA3', antisense: 5'TGACGATACCGGAGCCAATG3'; MCT1: sense: 5'CACCACCAGCGAAGTGTC3', antisense: 5'AGAAAGAAGCTGCAATCAAG3'.
Terminal dUTP Nick End labeling (Tunel) assay was used to detect apoptosis in HT-29 human colonic cell line.
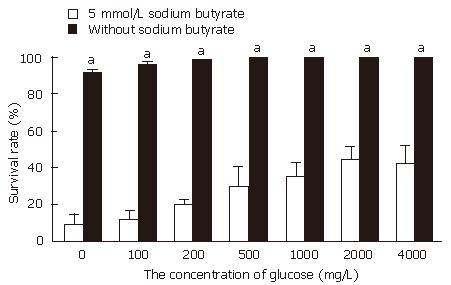
Without treatment with sodium butyrate, when the concentration of glucose was 0 or 100 mg/L, the growth of cancer cell was slightly slower than that in control (2000 mg/L glucose in RPMI 1640). But when the concentration of glucose was more than 200 mg/L, the proliferation of cancer cells was similar to the control (P > 0.05). When with 5 mmol/L sodium butyrate was added, with the concentration of glucose increase from 0 to 2000 mg/L, the survival rate increase obviously. When the concentration of glucose is 0 mg/L, the survival rate is 9.5% ± 5.3%, and when the concentration of glucose reached to 2000 mg/L, the survival rate is 42.7% ± 9.4%. However when the concentration of glucose increased from 2000 to 4000 mg/L, the effect of survival rate did not continue to increase (Figure 1).
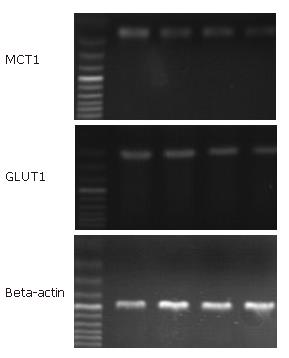
After treatment with 5 mmol/L sodium butyrate, the expression of GluT1 and MCT1 mRNA was regulated by the concentration of glucose. When the concentration of glucose increased from 0 to 2000 mg/L, there was a slightly decrease of GLUT1/β-actin from 0.18 ± 0.04 to 0.13 ± 0.03. And MCT1 mRNA was also slightly reduced from 0.28 ± 0.07 to 0.19 ± 0.10 (P < 0.05) (Figure 2).
Without treatment with sodium butyrate, low concentration of glucose (0, 100, 200 mg/L) can induce apoptosis in HT-29. When the concentration of glucose was 0 mg/L, the apoptosis rate was 3.5% ± 1.4%. But when the concentration of glucose was more than 200 mg/L, there is no significant change of apoptosis rate compared with the control (2000 mg/L in RPMI 1640). When 5 mmol/L sodium butyrate was added, with the concentration of glucose increasing from 0 mg/L to 2000 mg/L, the apoptosis decreased obviously (P < 0.05). The concentration of glucose was 0 mg/L, the apoptosis rate was 28.7% ± 9.3%, and when the concentration of glucose reached to 1000 mg/L, The apoptosis decreased to 7.5%± 3.2% (P < 0.05). But when the concentration of glucose reached to 4000 mg/L from 2000 mg/L, the apoptosis did not decrease continuously in HT-29 cell line (Figure 3). This result was consistent with that we have found in previous study by using flow cytometric assay.
Short-chain fatty acid (SCFA), which occurs in millimolar amounts, is rapidly absorbed in the colon, providing important energy supplies for the colorectal epithelium. Out of three SCFA, butyrate is considered to be the main energy source and it accounts for 70% of total energy in rat colonocytes. But tumors display a high metabolism rate of glucose and glycolysis. Glucose may be a more important energy for colon cancer cell. Sodium butyrate was transported by MCT1. Glucose is controlled by GluTs with GluT1 being the most important isoform in human colonic cancer cells[6,7].
In the experiment, low concentration of glucose or glucose deprivation without sodium butyrate induced apoptosis and inhibited proliferation in HT-29 cell line. Many researches have the similar results. Low intracellular concentration of glucose or glucose deprivation may induce ATP depletion and stimulation of mitochondrial death pathway cascade, and may induce oxidative stress and tigger bax-associated events including the JNK/MAPK signal pathway[8,9]. But it was interesting that when the concentration of glucose increased more than 200 mg/L, glucose seemed to have no regulation on proliferation and apoptosis on HT-29 cell line.
When cells were treated with sodium butyrate, with the increase of the concentration of glucose from 0 to 200 mg/L, the survival rate increased obviously and the apoptosis of HT-29 colonic cancer cell reduced obviously. And when the concentration of glucose continued to increase more than 200 mg/L (in this condition, glucose had no significant influence on apoptosis and proliferation), the effect of sodium butyrate inhibited by glucose was still observed. This suggested that glucose could inhibit the effect of sodium butyrate to a certain extent.
Increased the concentration of glucose not only inhibited the effect of proliferation inhibition and apoptosis induced by sodium butyrate, but also downregulated the expression of MCT1mRNA. MCT1 is a member of family of monocarboxylate transporters, which mediate the transport of sodium butyrate. The increase of expression and activity of MCT1 may serve as a mechanism to maximize intracellular availability of sodium butyrate. A more rapid accumulation of intracellular sodium butyrate may promote the effect of itself. Many of the cellular effects of butyrate are concentration-dependent, and time-dependent. The ability of sodium butyrate to exert its effects may depend upon its intracellular concentration[10,11]. The increased concentrations of glucose down-regulated the expression of MCT1. The decreased expression of MCT1 mRNA may reduce the intracellular concentration of sodium butyrate, which may weaken the effect of sodium butyrate. So, the change of intracellular concentration of sodium butyrate may play a role in inhibition of glucose on the effect of sodium butyrate.
When treatment with sodium butyrate, increased of concentration of glucose slightly decreased the Glut1 mRNA expression. Because glucose passes through membrane by facilitated diffusion, with the concentration of glucose increasing obviously, the intracellular glucose may still increase. The increase of intracellular glucose may protect cell[12-14]. In contrast, the decrease of intracellular glucose sensitized tumor cells to apoptosis[15,16]. So, the intracellular concentration of glucose may also be closely associated with the inhibition of glucose on the effect of sodium butyrate.
The results showed that low concentrations of glucose induced apoptosis and reduced the growth of HT-29 cancer cell. And glucose can inhibit the effect of proliferation inhibition and apoptosis induced by sodium butyrate which may be associated with the intracellular concentration of glucose and sodium butyrate. We have been found that sodium butyrate may affect apoptosis by regulating intracellular concentration of glucose in the past. Now, in the present study, we found that intracellular concentration of glucose and sodium butyrate may contribute to the inhibition of glucose on proliferation inhibition and apoptosis of colon cancer cells induced by sodium butyrate. All these may help further study the mechanism of sodium butyrate, and provided a novel possible way to enhance the effect of sodium butyrate.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Lu W
| 1. | Miki K, Unno N, Nagata T, Uchijima M, Konno H, Koide Y, Nakamura S. Butyrate suppresses hypoxia-inducible factor-1 activity in intestinal epithelial cells under hypoxic conditions. Shock. 2004;22:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Joseph J, Mudduluru G, Antony S, Vashistha S, Ajitkumar P, Somasundaram K. Expression profiling of sodium butyrate (NaB)-treated cells: identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene. 2004;23:6304-6315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Matsuzu K, Segade F, Matsuzu U, Carter A, Bowden DW, Perrier ND. Differential expression of glucose transporters in normal and pathologic thyroid tissue. Thyroid. 2004;14:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Mellanen P, Minn H, Grénman R, Härkönen P. Expression of glucose transporters in head-and-neck tumors. Int J Cancer. 1994;56:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol. 2000;279:G775-G780. [PubMed] |
| 6. | Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151-1154. [PubMed] |
| 7. | Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer. 2002;86:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Kansara M, Berridge MV. Oncogenes modulate cell sensitivity to apoptosis induced by glucose deprivation. Anticancer Res. 2004;24:2503-2510. [PubMed] |
| 9. | Muñoz-Pinedo C, Ruiz-Ruiz C, Ruiz de Almodóvar C, Palacios C, López-Rivas A. Inhibition of glucose metabolism sensitizes tumor cells to death receptor-triggered apoptosis through enhancement of death-inducing signaling complex formation and apical procaspase-8 processing. J Biol Chem. 2003;278:12759-12768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol. 2002;539:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Singh B, Halestrap AP, Paraskeva C. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogenesis. 1997;18:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Lee WN, Guo P, Lim S, Bassilian S, Lee ST, Boren J, Cascante M, Go VL, Boros LG. Metabolic sensitivity of pancreatic tumour cell apoptosis to glycogen phosphorylase inhibitor treatment. Br J Cancer. 2004;91:2094-2100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab. 2002;283:E784-E793. [PubMed] |
| 14. | Li H, Télémaque S, Miller RE, Marsh JD. High glucose inhibits apoptosis induced by serum deprivation in vascular smooth muscle cells via upregulation of Bcl-2 and Bcl-xl. Diabetes. 2005;54:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252-40257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899-5912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 422] [Article Influence: 17.6] [Reference Citation Analysis (0)] |