Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.3973
Revised: March 15, 2007
Accepted: April 16, 2007
Published online: August 7, 2007
AIM: To present a case series of MRCP-guided endoscopic biliary stent placement, performed entirely without contrast injection.
METHODS: Contrast-free endoscopic biliary drainage was attempted in 20 patients with malignant obstruction, unsuitable for resection on the basis of tumor extent or medical illness. MRCP images were used to confirm the diagnosis of tumor, to exclude other biliary diseases and to demonstrate the stenoses as well as dilation of proximal liver segments. The procedure was carried out under conscious sedation. Patients were placed in the left lateral decubitus position. The endoscope was inserted, the papilla identified and cannulated by a papillotome. A guide wire was inserted and guided deeply into the biliary tree, above the stenosis, by fluoroscopy. A papillotomy approximately 1 cm. long was performed and the papillotome was exchanged with a guiding-catheter. A 10 Fr, Amsterdam-type plastic stent, 7 to 15 cm long, was finally inserted over the guide wire/guiding catheter by a pusher tube system.
RESULTS: Successful stent insertion was achieved in all patients. There were no major complications. Successful drainage, with substantial reduction in bilirubin levels, was achieved in all patients.
CONCLUSION: This new method of contrast-free endoscopic stenting in malignant biliary obstruction is a safe and effective method of palliation. However, a larger, randomized study comparing this new approach with the standard procedure is needed to confirm the findings of the present study.
- Citation: De Palma GD, Lombardi G, Rega M, Simeoli I, Masone S, Siciliano S, Maione F, Salvatori F, Balzano A, Persico G. Contrast-free endoscopic stent insertion in malignant biliary obstruction. World J Gastroenterol 2007; 13(29): 3973-3976
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/3973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.3973
Endoscopic stent insertion has become the preferred method of biliary drainage for palliation of malignant biliary obstruction. Stent placement can relieve jaundice and pruritus and improve liver function and quality of life[1-4].
Currently, endoscopic stent placement involves the use of contrast media to achieve direct opacification of the biliary duct systems, and to determine the location and the extension of biliary obstruction.
Bacterial cholangitis is the main ERC-related complication in these patients. The risk of cholangitis after contrast injection into the biliary tree in cases where incomplete drainage is achieved is well known[5,6].
Recent reports describe the utility of MRCP or CT imaging to guide selection of the target lobe for subsequent endoscopic stenting. MRCP or CT images are used to confirm the diagnosis of tumor to exclude other biliary diseases and to demonstrate the stenoses as well as dilation of proximal liver performed entirely without contrast injection.
Contrast-free endoscopic biliary drainage was attempted in 20 patients with malignant obstruction unsuitable for resection on the basis of tumor extent or medical illness. Full and informed consent was obtained from all patients.
Baseline clinical and biochemical characteristics of patients are given in Table 1.
| Parameter | n (%) |
| Gender (M/F) | 13/7 |
| Mean age ± SD (yr) | 62.8 ± 8.0 |
| Mean duration of symptoms ± SD (mo) | 1.75 ± 0.72 |
| Jaundice | 20 (100) |
| Pruritus | 20 (100) |
| Fever | 0 (0) |
| Anorexia | 10 (50) |
| Weight loss | 10 (50) |
| Mean serum bilirubin ± SD (mg/dL) | 15.8 ± 9.2 |
| Mean AST ± SD (U/L) | 43.2 ± 9.9 |
| Mean ALT ± SD (U/L) | 41.3 ± 9.9 |
| Mean alkaline phosphatase ± SD (KA units) | 37.3 ± 7.6 |
Three patients (15%) had ampullary carcinoma, 5 (25%) pancreatic cancer, 10 (50%) cholangiocarcinoma of the main biliary duct and 2 (10%) a Klatskin tumor.
The diagnosis was based on ultrasound or computed tomography with ultrasound guided or CT fine needle aspiration cytology. The site of biliary stricture was defined with the help of MRCP. MRCP images were used to confirm the diagnosis of tumor, to exclude other biliary diseases and to demonstrate the length of the stenoses as well as dilation of proximal biliary segments.
The stent placement was carried out as an elective procedure. A side-viewing video-endoscope (Olympus TJF 145R, Olympus TJF 160R; Olympus Europe, Hamburg, Germany) was used.
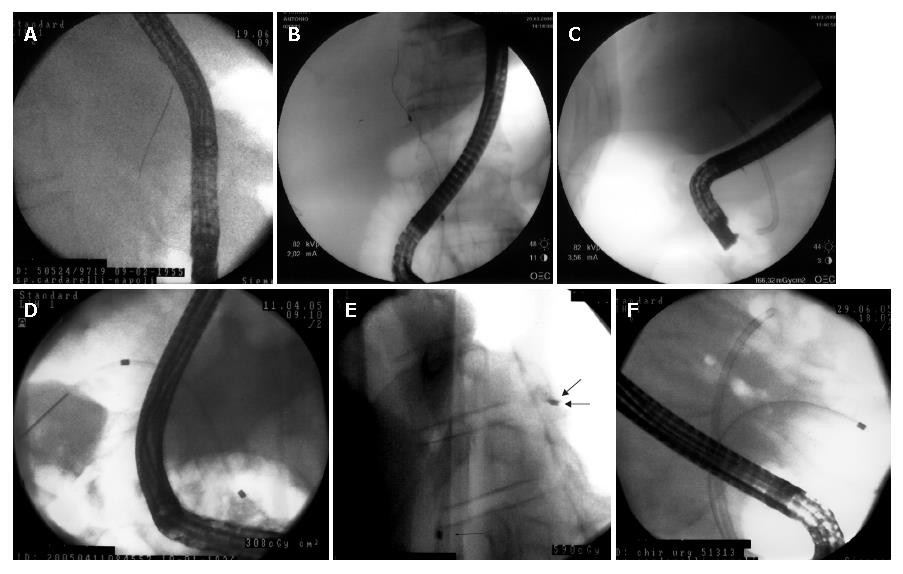
Prophylactic treatment with a broad-spectrum antibiotic was initiated the day of the procedure and continued for 5 d thereafter. The procedure was carried out under conscious sedation. Premedication consisted of 5-10 mg diazepam and 40-60 mg hyoscine butylbromide, in several separate intravenous doses. Patients were placed in the left lateral decubitus position. The endoscope was inserted and the papilla identified and cannulated by a papillotome. A guide wire (Tracer Metro guide-wire, Cook-Endoscopy; Winston-Salem, NC, USA) was inserted and guided deeply into the biliary tree by fluoroscopy. A papillotomy approximately 1 cm. long was performed and the papillotome was exchanged with a guiding-catheter that was introduced and identified, under fluoroscopy, in the common bile duct across the stricture. A 10 Fr, Amsterdam-type plastic stent (Cotton-Leung biliary stent, Cook Endoscopy; Winston-Salem, NC, USA), 7 to 15 cm long, was finally inserted over the guide wire/guiding catheter by a pusher tube system (Figure 1 A-F). The correct length and diameter of the biliary stent is previously determined on the basis of the CPRM image.
Successful stent insertion was defined as passage of the stent across the stricture along with flow of bile through the stent. A decrease in bilirubin (> 0.5 mg/dL per day) and improved cholestatic liver function tests, assessed 7 d later were considered as signs of successful drainage. Complications were defined according to the criteria of Cotton et al[10].
Successful stent insertion was achieved in all patients. There were no major complications. A minor complication (pancreatitis) developed in 1 patient (5%). Successful stent implantation was obtained in all patients and successful drainage, with substantial reduction in bilirubin levels, was achieved in all patients (15.8 ± 9.2 at baseline vs 4.0 ± 2.1 mg/dL at 1 wk). There were no cases of 30-d mortality. The median stent patency was 110.6 ± 17.8 d and the mean survival was 136.8 ± 38.8 d.
Retention of contrast and subsequent segmental cholangitis is a risk associated with endoscopic attempts to treat advanced malignant stenoses of the biliary tract[7].
Risk factors identified as significant in univariate analysis include the use of combined percutaneous-endoscopic procedures, stent placement in malignant strictures, the presence of jaundice, low case volume, and incomplete or failed biliary drainage[11]. A special circumstance is the presence of a hilar obstruction (e.g., “Klatskin tumor”). Post-ERC bacterial cholangitis in patients with Klatskin tumors occurs in 17% to 49%[6,12,13].
Factors that may increase the risk of sepsis in obstruction include disruption of the tight junctions between hepatocytes, impaired Kupffer cell function, and lack of clearance of contaminants which occurs with normal bile flow. In addition the protective action of secretory IgA and biliary mucus which prevent bacterial adherence is lost. In animal studies bacterial reflux from bile to blood is enhanced by increased intrabiliary pressure[14]. This suggests that increased intrabiliary pressure during ERC is the main reason for increased bacterial access to the blood.
Some endoscopists advocate attempts to avoid filling all intrahepatic segments and the importance of draining all intrahepatic segments that are filled with contrast[6,15].
Recent reports describe the utility of MRCP or CT imaging to guide selection of the target lobe for subsequent endoscopic stenting[7-9].
Constrast-free biliary decompression has been performed and previously reported with duodenoscopes, US, and EUS guidance[16-19].
In this present series, stents are placed under fluoroscopic guidance without any injection of contrast.
This method differs somewhat from common practice in a number of ways. The traditional approach consists of obtaining a complete cholangiogram at ERCP with retrograde opacification of obstructed ductal segments and this, particularly for hilar stenoses, may pose a substantial risk for cholangitis and mandate placement of multiple stents. For the MRCP and TC-target biliary stenting approach, contrast injection at ERCP is deliberately limited to the extrahepatic bile duct distal to the tumor while a unilateral or bilateral cholangiogram is completed after the passage of guide-wire and guiding- catheter above the stenosis.
Our approach completely avoids contrast injection. The correct length and diameter of the biliary stent is previously determined on the basis of the CPRM image. Guidance of the guide wire, guiding catheter and stent in the biliary tree is performed under fluoroscopic control allowing safe and effective endoscopic stent insertion.
In this series, all patients had successful drainage. None developed cholangitis or died within 30 d, which may be due to contrast-free stenting.
In conclusion, this new method of contrast-free endoscopic stenting in malignant biliary obstruction is a safe and effective method of palliation. However, larger, randomized studies comparing this new approach with the standard procedure are needed to confirm the findings of the present study.
Currently, endoscopic stent placement involves the use of contrast media to achieve direct opacification of the biliary duct systems, and to determine the location and the extension of biliary obstruction. Bacterial cholangitis is the main ERC-related complication in these patients.
This study shows a new technique for endoscopic biliary stent placement.
This report describes a possible improvement of endoscopic biliary drainage. In this present series, stents are placed under fluoroscopic guidance without any injection of contrast. A sensitive reduction of septic complication is preventable?
Endoscopic drainage of malignant biliary obstruction and particularly drainage of hilar stenoses.
Constrast-free biliary decompression has been performed and previously reported with duodenoscopes, US, and EUS guidance. The current paper gives a prospective analysis of data on endoscopic biliary stent placement, performed entirely without contrast injection. Larger, randomized studies comparing this new approach with the standard procedure are needed to confirm the findings of the present study.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Liu Y
| 1. | Rey JF, Dumas R, Canard JM, Ponchon T, Sautereau D, Helbert T, Escourrou J, Gay G, Giovannini M, Greff M. Guidelines of the French Society of Digestive Endoscopy: biliary stenting. Endoscopy. 2002;34:169-73, 181-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Shah SK, Mutignani M, Costamagna G. Therapeutic biliary endoscopy. Endoscopy. 2002;34:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Baron TH, Mallery JS, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Waring JP, Faigel DO. The role of endoscopy in the evaluation and treatment of patients with pancreaticobiliary malignancy. Gastrointest Endosc. 2003;58:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 285] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273-S282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 271] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, Persico G. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2033] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 11. | Motte S, Deviere J, Dumonceau JM, Serruys E, Thys JP, Cremer M. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology. 1991;101:1374-1381. [PubMed] |
| 12. | Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 200] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Peters RA, Williams SG, Lombard M, Karani J, Westaby D. The management of high-grade hilar strictures by endoscopic insertion of self-expanding metal endoprostheses. Endoscopy. 1997;29:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Bhasin DK, Sharma BC, Dhavan S, Singh K. Another technique for contrast-free deep cannulation of common bile duct. Endoscopy. 1998;30:S53-S54. [PubMed] |
| 17. | Singh V, Singh G, Verma GR, Singh K, Gulati M. Contrast-free unilateral endoscopic palliation in malignant hilar biliary obstruction: new method. J Gastroenterol Hepatol. 2004;19:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | De Palma GD, Puzziello A, Rega M, Mastantuono L, Persico F, Patrone F, Persico G. Ultrasonography-guided endoscopic stent placement for malignant biliary obstruction: a preliminary report of four cases. Endoscopy. 2004;36:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Mosca S, Secondulfo M, Defez M, Calise F. Air contrastography technique for successful urgent ERCP in a high risk allergic patient. Am J Gastroenterol. 2001;96:3458-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |