Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.3939
Revised: February 5, 2007
Accepted: February 14, 2007
Published online: August 7, 2007
AIM: To investigate whether chronic H pylori infection has the potential to induce pancreatitis in the Mongolian gerbil model, and whether it is dependent on an intact type IV secretion system.
METHODS: Mongolian gerbils were infected with wild type (WT) H pylori typeIstrain B128 or its isogenic mutant B128 ΔcagY (defective type IV secretion). After seven months of infection, H pylori was reisolated from antrum and corpus and H pylori DNA was analyzed by semi-nested polymerase chain reaction (PCR). Inflammation and histological changes were documented in the gastric antrum, corpus, and pancreas by immunohistochemistry. Cytokine mRNA, gastric pH, plasma gastrin, amylase, lipase, and glucose levels were determined.
RESULTS: The H pylori infection rate was 95%. Eight infected animals, but none of the uninfected group, developed transmural inflammation and chronic pancreatitis. Extensive interstitial fibrosis and inflammation of the pancreatic lobe adjacent to the antrum was confirmed by trichrome stain, and immuno-histochemically. Pro-inflammatory cytokine mRNA was significantly increased in the antral mucosa of all infected gerbils. In the corpus, only cytokine levels of WT-infected animals and those developing transmural inflammation and pancreatitis were significantly increased. Levels of lipase, but not glucose or amylase levels, were significantly reduced in the pancreatitis group. H pylori DNA was detected in infected antral and corpus tissue, but not in the pancreas.
CONCLUSION: H pylori infection is able to induce chronic pancreatitis in Mongolian gerbils independently of the type IV secretion system, probably by an indirect mechanism associated with a penetrating ulcer.
-
Citation: Rieder G, Karnholz A, Stoeckelhuber M, Merchant JL, Haas R.
H pylori infection causes chronic pancreatitis in Mongolian gerbils. World J Gastroenterol 2007; 13(29): 3939-3947 - URL: https://www.wjgnet.com/1007-9327/full/v13/i29/3939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.3939
Regular alcohol abuse is known as a risk factor for the development of chronic pancreatitis in humans. Several experimental animal models have been developed for chronic alcoholic pancreatitis, but to date the exact pathological mechanism underlying chronic pancreatitis is still unclear. About 10%-30% of chronic pancreatitis cases can be defined as idiopathic[1]. Despite considerable experimental effort, ethanol feeding alone fails to cause acute or chronic pancreatitis in animals. Ethanol feeding only causes pancreatic injuries if combined with other procedures (e.g. reduced microcirculation, increased pancreatic acinar cell expression of digestive and lysosomal enzymes, limiting pancreatic regeneration, and additional nutritional factors)[1]. Thus, other, yet to be identified genetic or environmental factors possibly play a role. Whether a bacterial infection could be a cause of at least some of these chronic pancreatitis cases remains unclear.
Epidemiologic data suggest that there is an association of H pylori colonization in the stomach with an increased risk of developing pancreatic injury or cancer[2]. A causal role for H pylori in the pathogenesis of peptic ulcer disease has been firmly established[3]. Subsequently, this Gram-negative bacterium was also identified as a risk factor for gastric carcinoma[4] and non-Hodgkins’ lymphoma[5]. H pylori strains are genetically very diverse and certain strains (typeI) carry a type IV secretion system located on the cag pathogenicity island (cag-PAI), which enables the bacteria to translocate an effector protein, the cytotoxin-associated antigen (CagA), into gastric epithelial cells. CagA is phosphorylated on tyrosine residues[6-9] and causes a disruption of eukaryotic signaling cascades[10,11]. Although H pylori has not been found to colonize the pancreas in humans directly[12], epidemiologic evidence suggests that H pylori infection might create an environment favorable for the development of pancreatitis and even pancreatic cancer[12].
Mongolian gerbils are a good animal model for studying H pylori infection and related complications, but it has not been used as a model for pancreatic disease. After several months of infection, Mongolian gerbils show a severe gastritis in the antrum that recapitulates the histological picture of type B-gastritis in humans[13]. We used Mongolian gerbils to examine the effect of gastric colonization on the development of pancreatic injury. In addition to a wild type (typeI) strain, we also generated a knockout mutation in the cag-PAI-specific cagY gene, which renders the strain incompetent for type IV-secretion. We asked the question whether a long-term (7 mo) colonization of H pylori with different virulence potential can induce pancreatitis in vivo without any additional cofactors, such as alcohol, and whether it is a direct or indirect pathogenic mechanism.
The bacterial strains used in this study were H pylori B128, a Mongolian gerbil-adapted type I-strain[14], and its isogenic mutant B128 ΔcagY[15]. Both strains carry a streptomycin resistance gene cassette in the chromosome that allows quantitative recovery of H pylori from the gerbil stomach by antibiotic selection[16].
Quantitative reisolation of H pylori from antral and corpus tissue sections was performed essentially as described before[16]. In brief, the wet mass of antral and corpus tissue sections were determined. Each gastric tissue specimen was homogenized (glass homogenizer, Ochs, Bovenden, Germany) in 1 mL of Brucella broth. Aliquots of appropriate dilutions were spread on selective serum plates (GC agar Difco) supplemented with horse serum (80 mL/L), vancomycin (10 mg/L), trimethoprim (5 mg/L), nystatin (1 mg/L), and streptomycin (250 mg/L) and incubated under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37°C for up to five days. Numbers of colony forming units (CFU) were expressed per gram of gastric tissue. The reisolated and sub-cultivated H pylori from antrum and corpus tissue were tested for urease (urea broth, Oxoid), oxidase (DrySlide, BBL), and catalase (3% hyperperoxide-solution) activity. Furthermore, the reisolates were analyzed for CagY and CagA protein production, as well as tyrosine phosphorylation of CagA by western blot[6]. IL-8 induction was performed in AGS gastric epithelial cells.
Female Mongolian gerbils (n = 26), outbred and grown under specific pathogen free conditions were used in this study. They were housed in SealSafe IVC cages (H-Temp, Tecniplast, Hohenpeissenberg, Germany) in an air-conditioned biohazard room (room temperature, 23 ± 2°C; relative humidity 55% ± 5%; 12/12-h light/dark cycle) with free access to a commercial gerbil diet (ssniff Gerbil, SSNIFF, Soest, Germany) and sterile tap water. Animals at the age of 8-12 wk were challenged orogastrically three-times over five consecutive days with approximately 109 viable H pylori. Age-matched control animals were inoculated with identical volumes of sterile Brucella broth alone. All experiments and procedures carried out were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Regierung von Oberbayern (AZ 211-2531-43/02 and 55.2-1-54-2531-78/05). The animals were sacrificed seven months after inoculation. The stomach was opened along the greater curvature. Antral and corpus tissues were macroscopically separated. Sections for histological analysis were fixed in 10% neutral buffered formalin or shock-frozen for cryo-sections.
Paraffin embedded sections (5 μm) of antrum and corpus were cut longitudinally and hematoxylin-eosin (HE) stained for grading. The intensity of inflammation, metaplasia, and ulcer development (scale 0-5) was analyzed by a pathologist blinded to the treatment according to a published protocol[17]. This grading system was developed to combine the amount of inflammatory cells infiltrating the gastric mucosa (grade of gastritis) with other histo-pathological changes occurring in the inflamed tissue, like metaplasia and ulcerations. The presence of collagen fibers in pancreatitis tissue was confirmed by using a trichrome stain kit (Newcomer supply, Middleton, WI, USA) to paraffin embedded sections.
Longitudinal paraffin sections of antrum and corpus were deparaffinized, then demasked with 0.01 mol/L citrate buffer (pH 6.0), and permeabilized in 3% H2O2. Slides were preincubated with 5% horse or goat normal serum/phosphate-buffered saline and then incubated with a mouse anti-vimentin (Sigma, Taufkirchen, Germany) or rabbit anti-mouse collagen typeIantibody (Linaris, Wertheim-Bettingen, Germany), respectively, followed by a secondary biotinylated anti-mouse or rabbit immunoglobulin G antibody (Linaris). Immune complexes were visualized with avidin-biotin complexes and diaminobenzidine (DAB) (Vectastain Elite ABC Kit, Linaris) as substrate for horseradish peroxidase. Finally, the sections were briefly counterstained with haematoxylin, dehydrated, and mounted (VectaMount, Linaris). Negative controls were performed by omitting the primary antibody.
After opening the stomach and removal of the gastric content with forceps, the pH of the gastric mucosa was measured using color-fixed indicator test sticks (pH-Fix 0.0-6.0, Macherey-Nagel, Dueren, Germany).
After sacrifice, approximately 2 mL of blood was collected by cardiac puncture, aliquoted into lithium-heparinized tubes, and centrifuged at 9000 r/min for 15 min at 4°C. Plasma was collected and immediately stored at -20°C until assayed.
The gastrin radioimmunoassay (RIA) was performed as described previously[18]. Briefly, plasma samples were incubated in duplicate at 4°C with 125I-Met human gastrin amide (G-17) label and antiserum 5135 (CURE, UCLA). G-17 (5 and 50 pmol/L) was used to generate the standard curves. The ID50 was 1 pmol/L and inter- and intra-assay coefficients of variation were less than 2% and 11%, respectively.
Plasma amylase and lipase activity was determined by using commercially available kits (α-Amylase EPS and Lipase colorimetric, Roche, Mannheim, Germany). Glucose concentration was measured using a colorimetric assay (Roche). All three assays were performed in a veterinary laboratory (Vet-Med-Labor, Institute for Clinical Assessment, Ludwigsburg, Germany).
RNA isolation and real-time RT-PCR measurement were applied as described previously[15]. cDNA was synthesized using 1 μg total RNA, random hexamer oligonucleotide primers, and TaqMan Reverse Transcriptase Kit (Roche). Oligonucleotide primer and probes specific for IL-1β, IFN-γ, KC, and the house keeping gene 18S rRNA were applied for real-time RT-PCR (ABI PRISM 7000, Applied Biosystems). RNA samples were used as negative controls.
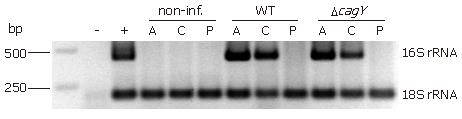
DNA was isolated from 16 cryo-sections (25 μm) by using QIAamp DNA Mini Kit spin columns (Qiagen, Hilden, Germany). DNA was amplified by semi-nested PCR in a thermal cycler T3 (Biometra, Goettingen, Germany) using specific primers for detecting H pylori 16S rRNA. The amplification reaction was carried out with a PCR buffer containing 20 mmol/L Tris-HCl (pH 8.0), 2 mmol/L MgCl2, 100 mmol/L KCl, 200 μmol/L each dNTP, 400 nmol/L each primer, and 0.75 U Takara LA Taq DNA polymerase (Takara Bio Inc., Shiga, Japan). The forward primer for 16S rRNA 1F (5'-CTATGACGGGTATCCGGC-3') and reverse primer 1R (5'-CTCACGACACGAGCTGAC-3') were used in the first step. In the second step, an aliquot of 3 μL of the amplification product of the first step, primer 1F and reverse primer 2R (5'-TCGCCTTCGCAATGAGTATT-3') were used. The PCR amplification profile involved two times 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min starting with a predenaturation at 95°C for 5 min. The 18S rRNA gene was amplified by using the forward primer 3F (5'-CGGCTACCACATCCAAGGAA-3') and reverse primer 3R (5'-GCTGGAATTACCGCGGCT-3'). Detection of PCR-products was done in agarose gels, stained with ethidium bromide, and visualized by UV illumination.
The results were statistically analyzed using the Mann-Whitney U-test for unpaired groups and two-sided Fisher’s exact test. P < 0.05 was considered as significant.
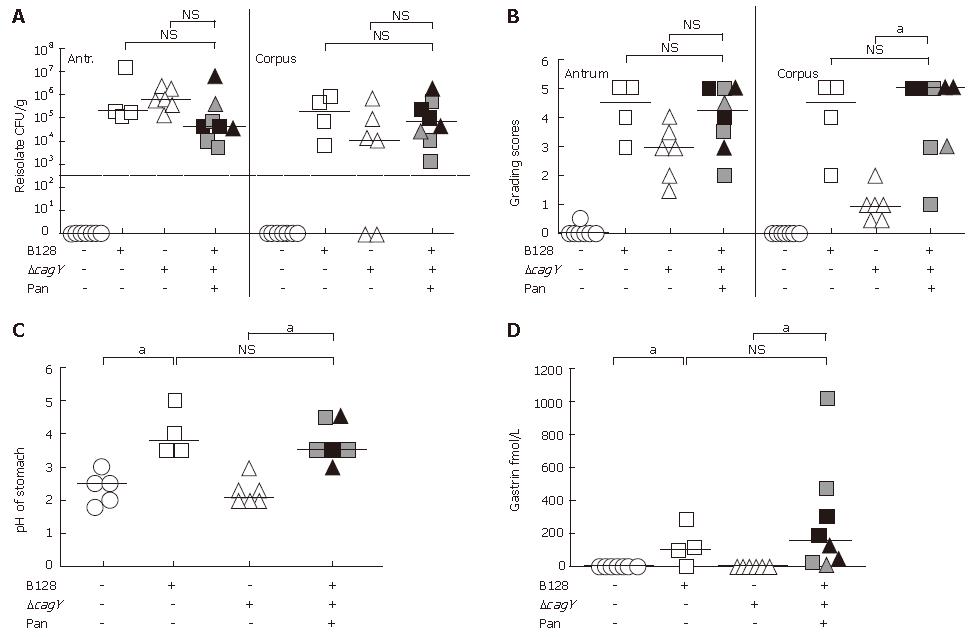
Seven months after oral inoculation of Mongolian gerbils, the successful infection and the H pylori colonization density was determined by reisolation of streptomycin-resistant H pylori from the gastric antrum and corpus region separately. The reisolation rate was similar in both infected animal groups (90%-100%) (Table 1). All Mongolian gerbils infected with H pylori B128 WT strain exhibited a severe inflammation, including large lymphoid aggregates in both the mucosa and submucosa of the stomach. Peptic ulceration of the gastric mucosa and metaplastic changes were observed in 89% and 100% of gerbils infected with the B128 WT strain, respectively (Table 1). Gerbils infected with the cag-PAI defective mutant strain showed a lower number of lymphaggregates and metaplastic changes, and a reduction in the formation of gastric ulcer (Table 1). Thus, the severity of histopathological alterations in the stomach revealed a positive correlation with the presence and activity of the cag-pathogenicity island (cag-PAI).
| Parameter | Non-inf. | B128 | B128ΔcagY |
| No. of animals (n) | 7 | 10 | 9 |
| Reisolation rate | (0/7) 0 | (9/10) 90 | (9/9) 100 |
| Lymphaggregate | (0/7) 0 | (9/9) 100 | (7/9) 78 |
| Gastric ulcer | (0/7) 0 | (8/9) 89 | (3/9) 33 |
| Metaplastic changes | (0/7) 0 | (9/9) 100 | (7/9) 78 |
| Transmural inflammation and pancreatitis1 | (0/7) 0 | (5/9) 56 | (3/9) 33 |
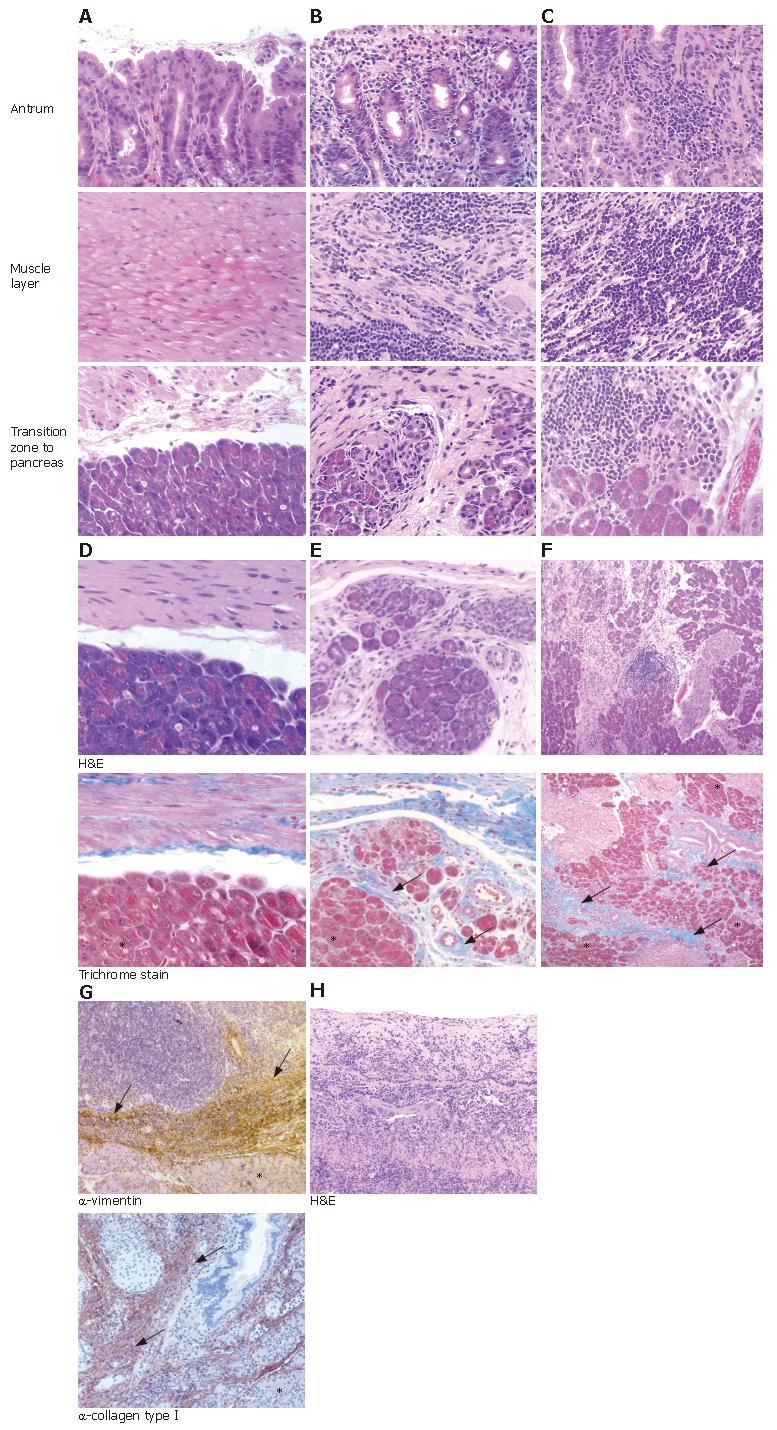
A novel and surprising observation in 5/9 (56%) and 3/9 (33%) animals of the WT- and mutant-infected groups, respectively, but not in the control animals, was strong, transmural inflammation in the antrum of the stomach. This inflammation continued into the adhering pancreatic tissue (Table 1, Figure 1A-C). The often severe inflammation in the antrum extended from the gastric mucosa to the submucosa, resulting in transmural inflammation (compare Figure 1A-C). No histological changes were observed in pancreatic lobes distant from the penetrating ulcer area (data not shown).
The chronic fibrosis in the pancreatic tissue was confirmed using the trichrome stain for collagen tissue, which stains blue (Figure 1D-F). Immunohistochemical analysis of the inflamed pancreatic tissue by anti-collagen typeIand anti-vimentin antibody revealed brown stained fibrous replacement of pancreatic acini as marker for chronic pancreatitis (Figure 1G). In contrast to the atrophy and fibrous replacement of pancreatic acini, the sections revealed relative preservation of the pancreatic ducts and islets of Langerhans (Figure 1E-G). Furthermore, in all animals where pancreatitis was present, severe gastric ulcer lesions and transmural inflammation were observed in the antrum penetrating into the adjacent pancreatic lobes (Figure 1H, Table 1). Thus, the transmural inflammation due to the penetrating gastric ulcer was clearly dependent on the H pylori infection.
Reisolation rates of infected gerbils developing transmural inflammation and pancreatitis did not reveal any significant differences as compared to infected groups without pancreatitis (Figure 2A). The pathological changes in the mucosa were characterized by applying the rodent adapted gastric grading scheme, first described by Garhart et al[17]. As we showed previously[15], the grade of inflam-mation in the corpus of the gerbil strongly depended on the H pylori strain used for infection. The B128 typeIstrain with a functional type IV secretion system, but not the B128 ΔcagY-mutant strain, induced severe inflammation in the corpus, whereas, H pylori-induced inflammation in the antrum was cag-PAI independent. In principle, the same observations were made in this study (Figure 2B). However, those gerbils infected with the B128 ΔcagY-mutant strain that developed pancreatitis behaved differently (Figure 2B). They showed a significant increase in inflammation in the antrum, the corpus, and the adjacent pancreatic tissue (Figures 1 and 2B). Since we observed earlier that chronic inflammation in the corpus is associated with severe atrophy of the parietal cells[15], we found the gastric pH of WT-infected gerbils significantly increased, as compared to the non-infected and mutant infected group without pancreatitis (Figure 2C). Furthermore, in the H pylori-infected group developing pancreatitis the pH of the corpus was significant elevated, as compared to B128 ΔcagY-mutant infected animals without pancreatitis (Figure 2C).
The gastric hormone gastrin was significantly increased in the WT-infected gerbil group, as compared to non-infected and cagY-mutant infected groups without pancreatitis (Figure 2D). In contrast, the B128 ΔcagY-mutant infected gerbils that developed transmucosal inflammation and chronic pancreatitis revealed not only a high pH, but also a significant increase in plasma gastrin levels, as compared to the animals of the same group without pancreatitis (Figure 2C and D).
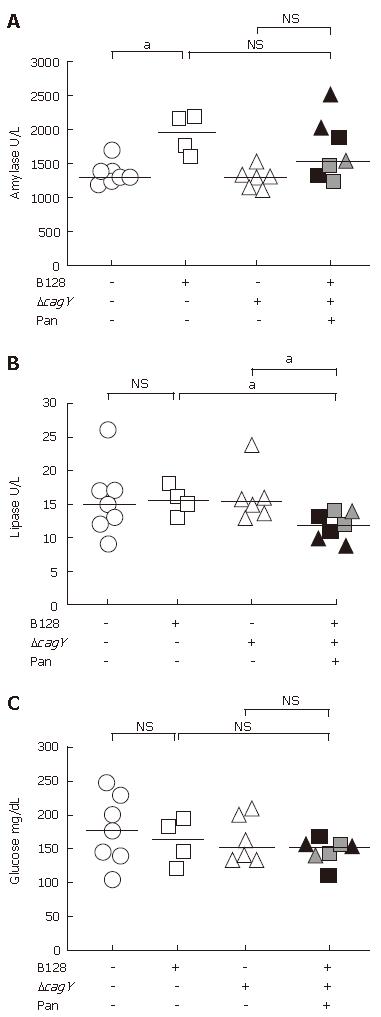
As already observed by trichrome stain (Figure 1E and F) and immunohistochemical analysis (Figure 1G), the penetrating ulcer and transmural inflammation increased fibrosis in the pancreas. To determine if the fibrosis affected the endocrine or exocrine functions, we examined the levels of serum pancreatic enzymes and glucose (Figure 3A). There was no consistent difference in the amylase levels. The inconsistent trend in amylase levels might reflect a mixture of amylase from other sources, e.g., salivary glands. Therefore, lipase levels were assayed, too.
Comparing the plasma lipase level of non-infected with both infected H pylori groups without pancreatitis, the median of all three groups did not differ significantly (Figure 3B). However, WT-infected and mutant-infected animals that developed pancreatitis showed a reduced plasma lipase level (P < 0.05). This confirmed an association with the histopathology observed in the acini of the infected animals where the atrophy of the acini was replaced by fibrous tissue. This represents a trend towards reduced exocrine function as shown in reduced plasma lipase concentrations.
The islet of Langerhans in the pancreas releases hormones, such as insulin, involved in carbohydrate metabolism. Therefore, glucose levels were measured, but the medians of all three animal groups revealed no statistical differences, despite the presence of histological changes in the pancreas (Figure 3C). This result argues for the presence of an intact endocrine pancreas.
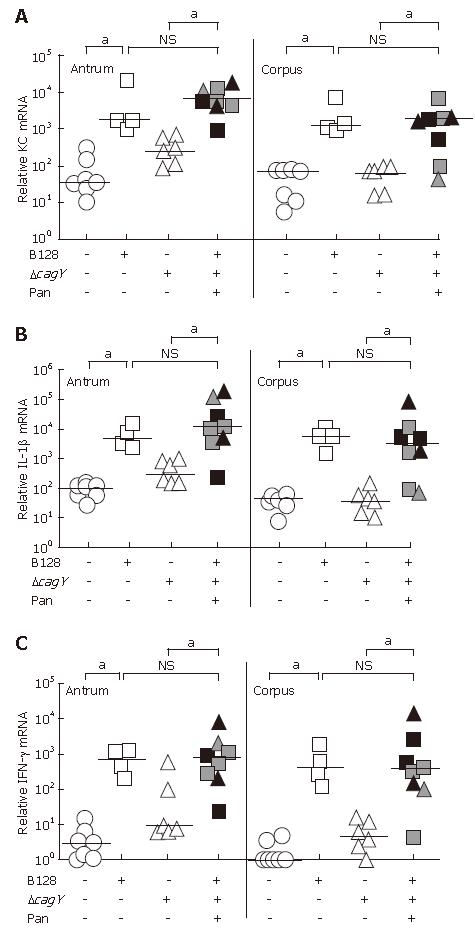
The cytokine mRNA expression was quantified using the real time RT-PCR technique and standardized to 18S rRNA. Gerbil-specific sequences were used to quantify the KC, IL-1β, and IFN-γ mRNA expression levels of antral and corpus mucosa separately. The data in Figure 4A-C demonstrated a significant increase in the proinflammatory cytokine mRNA expression in gerbils infected with the B128 WT strain, as compared to non-infected animals. In the B128 ΔcagY-mutant strain group without pancreatitis, proinflammatory cytokine expression in the corpus was as low as in the non-infected group, consistent with our previous results[15]. Those infected animals showing a transmural inflammation and pancreatitis exhibited significantly higher levels of all three proinflammatory cytokine mRNAs as compared to ΔcagY-mutant infected animals without pancreatitis. These cytokine levels were comparable to the level of B128 WT-infected animals. Thus, the KC, IL-1β, and IFN-γ mRNA levels in animals with severe transmucosal inflammation correlated with the observed inflammatory score (Figure 2B) and histopathologic changes (Figure 1) of those infected gerbils.
An important question to answer was now whether or not H pylori can be detected in the pancreas. Therefore, we applied a semi-nested PCR approach. Total DNA from pancreatic cryo-sections was isolated and the Helicobacter 16S rRNA gene was amplified. All antral and corpus tissue samples of infected Mongolian gerbil revealed an amplified product of 433 bp at the same position as the positive control (DNA of the H pylori B128 strain) (Figure 5). None of the infected animals showed an amplification product of the 16S rRNA from pancreatic DNA sample. All non-infected tissue samples revealed a negative result in the semi-nested PCR analysis. To verify that enough DNA was isolated the eukaryotic housekeeping gene 18S rRNA was amplified from all samples (Figure 5). In addition, we also applied an immunohistochemical approach, using a polyclonal anti-H pylori antibody (AK175) for detection. Paraffin sections of antral and corpus tissue of infected Mongolian gerbils were positively stained for H pylori, but not the pancreatic tissue (data not shown). Unfortunately, this antibody detected other rod-shaped bacteria in the forestomach as well, probably Lactobacillus spp., therefore, we did not apply this technique further. Since absolutely no H pylori-DNA could be detected in pancreatic tissue of the infected animals, this argues for an indirect pathomechanism. The procedure starts with an H pylori-induced antral gastritis, followed by a penetrating gastric ulcer with transmural inflammation into the adjacent lobe of the pancreas.
In this study, we demonstrate that infection of Mongolian gerbils with H pylori for seven months can cause pancreatitis in the pancreatic lobe adjacent to the antral stomach. Although we consider our work as an initial observation, it reports to our knowledge for the first time on a H pylori-induced pancreatitis in an animal model and, therefore, on a possible link between the H pylori infection and chronic pancreatitis due to a penetrating gastric ulcer. Singular case reports of humans and carnivores showed a penetration of the pancreas by a giant gastric ulcer[19,20]. Although, in these rare cases H pylori colonization has not been addressed, the high prevalence of H pylori in ulcer patients suggests an etiologic relation.
Acute pancreatitis, chronic pancreatitis, and pancreatic cancer are the most common pancreatic disorders. Although chronic pancreatitis is generally thought to be affected by ethanol abuse and smoking, there is a large category of patients that have idiopathic causes for their chronic pancreatitis (10%-30%). However, mutations in several genes, such as PRSS1, CFTR or SPINK1, have been shown to be associated with, or may predispose to the development of idiopathic or hereditary chronic pancreatitis[21,22]. Although, we did not analyze such predisposing mutations in the gerbils, our data give a first indication that H pylori induced distal gastritis may be a cause of idiopathic chronic pancreatitis in the animal model.
In future experiments it has to be more rigorously defined whether our observations from the animal model really match the process of chronic pancreatitis in humans. In addition, it would be important to test in the gerbils whether the process is irreversible by performing a bacterial eradication experiment and to test the stability of the pancreatic changes induced by H pylori.
In addition to the typeI H pylori WT strain B128, we also used a genetically modified derivative, unable to use its type IV secretion system (B128 ΔcagY). In general, our data suggest that the capacity of H pylori to induce pancreatitis in Mongolian gerbils is cag-PAI independent. Both strains were able to induce pancreatitis, and therefore were grouped together in the further analysis. In this study we consider a penetrating gastric ulcer with a severe transmucosal inflammation of the gastric mucosa as a pre-pancreatitis stage, since the WT-infected gerbil group without transmural inflammation carried a high potential to develop pancreatitis, and there was no significant difference between WT-infected animals with and without pancreatitis in most parameters analyzed (Figures 2, 3 and 4). This was in contrast to the B128 ΔcagY-infected animals, where only animals with pancreatitis showed significant inflammation and gastric hormone scores (Figures 2, 3 and 4). Therefore, we hypothesize that the infection with a H pylori typeIstrain, carrying a functional type IV secretion system, might represent a higher risk factor to induce chronic pancreatitis in gerbils as compared to a cag-PAI negative strain (type II strain). It will be important to verify and substantiate these initial data with more animals of both groups and different time points of analysis.
A reduction in the CFU of H pylori reisolates was observed comparing infected animals that developed transmural gastric inflammation and chronic pancreatitis with both infected animal groups without pancreatitis (Figure 2A). The reduced bacterial density in the antrum of infected gerbils showing transmucosal inflammation and pancreatitis might be related to the observation that these animals developed severe gastric ulcers. It is well known that H pylori colonizes areas of duodenal ulcer with low density[23], or that a severe inflammatory response might decrease the infection, which might explain the lower numbers of bacteria.
H pylori infection is known to be a risk factor for peptic ulcer disease[24], mucosa-associated-lymphoid tissue lymphoma (MALT)[25], and gastric cancer[26]. A number of studies have addressed the question whether H pylori plays a role in the pathogenesis of chronic pancreatitis, but no direct link has been established. The first epidemiological study performed by Niemann et al[27] demonstrated that H pylori infection contributes, but may not be the only cause of duodenal ulcer in patients with chronic pancreatitis. Their data showed a prevalence of IgG antibodies against H pylori in 60% of patients with chronic pancreatitis complicated by duodenal ulcer, compared to 86% in controls with simple duodenal ulcer. Another study investigated the pathogenesis of duodenal ulcer in chronic alcoholic pancreatitis patients[28] and found a significant increase in the rate of H pylori infection in patients with chronic alcoholic pancreatitis and duodenal ulcer as compared to patients with chronic alcoholic pancreatitis only (87% vs 54%, P = 0.02). These authors suggested that H pylori infection seems to play a significant role in duodenal ulcer disease associated with chronic alcoholic pancreatitis.
There are several human case reports[19,20] that describe a huge gastric ulcer penetrating into the pancreas inducing pancreatic injury. Since we did not detect any H pylori-DNA in the pancreatic tissue of infected Mongolian gerbils that would indicate a direct pancreas colonization of H pylori, we postulate that H pylori induces pancreatitis via an indirect pathogenetic mechanism. Our data support an indirect pathway starting with a H pylori-associated antral gastritis developing into a severe gastric ulcer. The huge lesion of the gastric mucosa occurs together with a severe transmural inflammation that penetrates then in some cases into the adjacent lobe of the pancreas resulting in a chronic pancreatitis with fibrous replacement of pancreatic acini.
Several reports considered changes in gastric hormones (increased gastrin and reduced somatostatin), N-nitroso compounds produced by gastric bacteria that flourish in the hypochlorhydric stomach, and the cagA-status of H pylori[2,29]. These reports are supported by our recent data, showing that an infection with H pylori typeIstrain contributes to severe inflammation in the antral and corpus mucosa over time, leading to corpus-dominant atrophic gastritis[15]. Hypergastrinemia and hypochlorhydria are two typical physiological changes of this process, representing possible risk factors for the development of pancreatic cancer.
Interestingly, H pylori infection has been described as a risk factor for developing pancreatic cancer[29]. Pancreatic cancer is among the most fatal cancers worldwide. In a first published case-control study greater H pylori seropositivity among 92 pancreatic cancer case subjects (65%) was found than among 62 control subjects (45%), resulting in the doubling of the risk for the H pylori-positive group[12]. Furthermore, a first prospective nested case-control study covering a cohort of 29 133 male Finnish smokers described a statistically significant relationship between carriage of H pylori, especially cagA-positive (typeI) strains, and pancreatic cancer[30]. According to recent evidence, chronic pancreatitis has been associated with pancreatic cancer[31]. Therefore, pathologic consequences of H pylori infection similar to those observed in gastric tissue might also be postulated for the pancreas.
In conclusion, our data show for the first time that an H pylori typeIstrain carries a certain potential to induce chronic pancreatitis in the animal model by an indirect mechanism. Whether H pylori-induced pancreatitis progresses to pancreatic cancer remains to be seen and will possibly require re-examining infected gerbils at additional, earlier and later time points. Further studies will be necessary to elucidate conclusively the mechanism of H pylori-induced pancreatic diseases. A major challenge for the future will be to clarify in appropriate human studies whether the H pylori infection per se (without alcohol abuse, smoking or other factors) represents already a risk factor for the development of chronic pancreatitis or even pancreatic cancer in man.
We thank E Hoster, Institute for Biometry and Epide-miology, LMU Munich, for statistical advice. Gastrin radioimmunoassays were performed by the ligand core of the University of Michigan under the grant support of DDRC P30DK34933.
S- Editor Zhu LH L- Editor Rippe RA E- Editor Lu W
| 1. | Schneider A, Whitcomb DC, Singer MV. Animal models in alcoholic pancreatitis--what can we learn? Pancreatology. 2002;2:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Moss S, Calam J. Helicobacter pylori and peptic ulcers: the present position. Gut. 1992;33:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 5. | Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 515] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 967] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 7. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, Naumann M, Meyer TF. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559-14564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 592] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004;12:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Raderer M, Wrba F, Kornek G, Maca T, Koller DY, Weinlaender G, Hejna M, Scheithauer W. Association between Helicobacter pylori infection and pancreatic cancer. Oncology. 1998;55:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Peek RM, Wirth HP, Moss SF, Yang M, Abdalla AM, Tham KT, Zhang T, Tang LH, Modlin IM, Blaser MJ. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest. 2001;107:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Kavermann H, Burns BP, Angermuller K, Odenbreit S, Fischer W, Melchers K, Haas R. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J Exp Med. 2003;197:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Garhart CA, Redline RW, Nedrud JG, Czinn SJ. Clearance of Helicobacter pylori Infection and Resolution of Postimmunization Gastritis in a Kinetic Study of Prophylactically Immunized Mice. Infect Immun. 2002;70:3529-3538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci USA. 2003;100:12944-12949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Zanotti M, Amboldi A, Musazzi M, Gozzini C, Bellone S, Sartirana A. Giant benign gastric ulcer penetrating into the liver, pancreas and mesocolon. Minerva Chir. 1999;54:415-419. [PubMed] |
| 20. | Boysen SR, Tidwell AS, Penninck DG. Ultrasonographic findings in dogs and cats with gastrointestinal perforation. Vet Radiol Ultrasound. 2003;44:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Audrézet MP, Chen JM, Le Maréchal C, Ruszniewski P, Robaszkiewicz M, Raguénès O, Quéré I, Scotet V, Férec C. Determination of the relative contribution of three genes-the cystic fibrosis transmembrane conductance regulator gene, the cationic trypsinogen gene, and the pancreatic secretory trypsin inhibitor gene-to the etiology of idiopathic chronic pancreatitis. Eur J Hum Genet. 2002;10:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Mullins PD, Steer HW. Helicobacter pylori colonization density and gastric acid output in non-ulcer dyspepsia and duodenal ulcer disease. Helicobacter. 1998;3:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615-625. [PubMed] |
| 25. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1386] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 26. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 925] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 27. | Niemann T, Larsen S, Mouritsen EA, Thorsgaard N. Helicobacter pylori infection in patients with chronic pancreatitis and duodenal ulcer. Scand J Gastroenterol. 1997;32:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Chebli JM, de Souza AF, Gaburri PD, Bastos KV, Ribeiro TC, Filho RJ, Chebli LA, Castro Ferreira LE. Prevalence and pathogenesis of duodenal ulcer in chronic alcoholic pancreatitis. J Clin Gastroenterol. 2002;35:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Zalatnai A. Pancreatic cancer - a continuing challenge in oncology. Pathol Oncol Res. 2003;9:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, Perez-Perez G, Taylor PR, Virtamo J, Albanes D. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 31. | Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |