Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.3918
Revised: January 10, 2007
Accepted: January 25, 2007
Published online: August 7, 2007
Hepatic veno-occlusive disease is a clinical syndrome characterized by hepatomegaly, ascites, weight gain and jaundice, due to sinusoidal congestion which can be caused by alkaloid ingestion, but the most frequent cause is haematopoietic stem cell transplantation (STC) and is also seen after solid organ transplantation. The incidence of veno occlusive disease (VOD) after STC ranges from 0 to 70%, but is decreasing. Survival is good when VOD is a mild form, but when it is severe and associated with an increase of hepatic venous pressure gradient > 20 mmHg, and mortality is about 90%. Prevention remains the best therapeutic strategy, by using non-myeloablative conditioning regimens before STC. Prophylactic administration of ursodeoxycholic acid, being an antioxidant and antiapoptotic agent, can have some benefit in reducing overall mortality. Defibrotide, which has pro-fibrinolytic and antithrombotic properties, is the most effective therapy; decompression of the sinusoids by a transjugular intrahepatic portosystemic shunt (TIPS) can be tried, especially to treat VOD after liver transplantation and when multiorgan failure (MOF) is not present. Liver transplantation can be the last option, but can not be considered a standard rescue therapy, because usually the concomitant presence of multiorgan failure contraindicates this procedure.
- Citation: Senzolo M, Germani G, Cholongitas E, Burra P, Burroughs A. Veno occlusive disease: Update on clinical management. World J Gastroenterol 2007; 13(29): 3918-3924
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/3918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.3918
Hepatic veno-occlusive disease is a clinical syndrome characterized by hepatomegaly, ascites, weight gain and jaundice[1-3]. It was first described in a patient who drank an infusion made with pyrrolizidine alkaloids[4,5].
Early histological abnormalities include sinusoidal congestion associated with centrilobular necrosis; later fibrous obliterative lesions in the hepatic venules occur with histological damage located to zone 3 of the acinus[6].
Veno occlusive disease (VOD) is also associated with other toxins such as alcohol, oral contraceptives, toxic oil, terbinafine, or radiation injury[7,8]. The first case associated with haematopoietic stem cell transplantation (STC) was reported in 1979[9].
Since then SCT has become the most important and frequent cause of VOD. In this setting diagnosis is made by the same clinical signs as for other etiologies. Other causes are infrequent but require exclusion. VOD after SCT is a part of the spectrum of multi organ syndromes which include idiopathic pneumonitis, diffuse alveolar haemorrhage, thrombotic microangiopathy, and capillary leak syndrome.
The incidence of VOD after STC ranges from 0 to 70%, dependent on the particular application of specific diagnostic criteria, sample size and risk factors, which are heterogenous among different cohorts[10]. Currently, the incidence and severity of VOD after SCT is decreasing due to earlier timing of SCT in patients with leukaemia, the use of non myeloablative regimens and the decrease of HCV infection amongst transplant candidates[11]. However, the incidence of VOD remains high in patients undergoing aggressive chemotherapy regimens to eradicate cancer.
Veno occlusive disease is also reported after other solid organ transplantation, particularly, kidney transplantation. The largest series report an incidence of 2%-5% (5/200 kidney transplants), mainly related to azathioprine toxicity. In liver transplant recipients the largest series reported an incidence of 1.9% (19/1023 liver transplants) clinical VOD, related to the number and severity of rejection episodes and azathioprine use. In contrast, reversible hepatic venule stenosis including case diagnosed histologically is reported in 43% after liver transplantation and is mainly related to azathioprine[12]. A list of risk factors associated with the development of VOD is shown in Table 1.
| 1 | Pre existing liver disease (hepatitis C, previous fibrosis, hypertransaminasemia) |
| 2 | Previous exposure to a myeloablative regimen |
| 3 | Past history of HSOS |
| 4 | Use of myeloablative regimen |
| 5 | High dose of total body irradiation |
| 6 | Use of cyclophosphamide containing regimes |
| 7 | Administration of cyclophosphamide after busulfan |
| 8 | Fixed dose of Busulfan |
| 9 | Use of oral rather than ev Busulfan |
| 10 | Late timing of SCT in patients with leukaemia |
| 11 | Carriers of haemocromathosis C282Y allele |
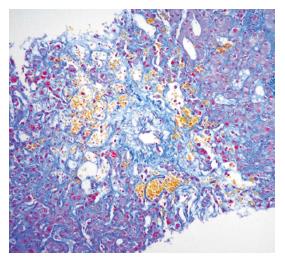
After SCT the high dose cytoreductive therapy used in patients who have a particular susceptibility, produces endothelial injury in both sinusoids and small hepatic venules. This leads to activation of the coagulation cascade, and clot formation. Fibrin-related plugs, intracellular fluid entrapment and cellular debris progressively occlude sinusoids, causing intrahepatic post sinusoidal portal hypertension, responsible for the clinical signs of fluid retention (weight increase), hepatomegaly, ascites and jaundice. Usually fibrosis occurs several weeks after the onset of the disease. Post mortem studies have shown that hepatic venules are partially or completely obliterated by subendothelial collagen fibers (Figure 1). An early deposition of matrix metalloproteinases-2 has also been reported in the sinusoids. Several factors other than cytoreductive therapy contribute to the damage in VOD. Release of cytokines, such as TNF-α, IL1 and 2, due to the endothelial injury, but also due to cyclosporine given to patients with GVHD, have procoagulant activity. Immunological mechanisms have been implicated based on the observation of a lower incidence of VOD following T cell depleted and autologous SCT, and a higher incidence amongst mismatched transplants.
Genetic and secondary susceptibility of the liver also plays a major role. Patients with previous liver disease, previous liver radiation or hepatitis are more prone to develop VOD. All these injuries cause depletion of gluta-thione from hepatocytes, resulting in increased sensitivity to zone 3 damage due to other compounds such as cyclophosphamide, busulfan and BCNU, which can further deplete this antioxidant compound. In parallel with the decline in hepatic venous flow, nitric oxide (NO) levels decrease in the hepatic vein[13]. This change suggests that vasoconstriction related to NO depletion occurs, further incrementing the damage[14].
The classic presentation of VOD is characterized by the triad of weight gain caused by fluid retention, tender hepatomegaly and hyperbilirubinaemia without any known cause[15,16]. Usually this occurs within 10-20 d after SCT when regimens containing cyclophosphamide have been used. With other regimens it occurs later[17]. A chronic picture occurs outside of the SCT setting is the one associated with chronic assumption of pyrrolizidine alkaloids. After liver transplantation VOD occurs over wide intervals, with a mean of 9 wk after transplant. The presence of persistent ascites 6 wk after liver transplantation can be indicative of an outflow obstruction syndrome, therefore, VOD should be excluded[18]. Diagnosis is usually based on signs and symptoms, having ruled out other conditions which can mimic the disease, particularly after STC, such as viral infections and graft versus host disease, cholestatic secondary sepsis, heart failure and tumoral infiltration of the liver[19]. After liver transplantation, fibrosing cholestatic hepatitis due to HBV or HCV should be excluded.
Nowadays, clinical criteria have been formalized in the Baltimore and Seattle criteria (Table 2). The Baltimore criteria are more restrictive and usually patients fulfilling these are diagnosed at a more severe stage of the disease[20].
| Seattle criteria | |
| At least two of the three following criteria: within the first month after stem cell transplantation (STC): | |
| 1 | Jaundice |
| 2 | Hepatomegaly and right upper quadrant pain |
| 3 | Ascites and/or unexplained weight gain |
| Baltimore criteria | |
| Elevated total serum bilirubin (≥ 2 mg/dL) before d 21 after SCT and two of the following three criteria: | |
| 1 | Tender hepatomegaly |
| 2 | Weight gain ≥ 5% from baseline |
| 3 | Ascites |
| Modified Seattle criteria | |
| Occurrence of two of the following events within 20 d of SCT: | |
| 1 | Hyperbilirubinaemia (≥ 2 mg/dL) |
| 2 | Hepatomegaly or right upper quadrant pain of liver origin |
| 3 | Unexplained weight gain (> 2% of baseline bodyweight) because of fluid accumulation |
After SCT, raised serum bilirubin concentration is a sensitive, but not a specific marker for diagnosis; an AST greater than 750 U/L is one marker of poor prognosis[21,22]. Endothelial injury can be suspected even before the appearance of the clinical or other laboratory signs by elevated serum levels of PAI-1, procollagen III, and it’s precursor propeptide (P-III-P)[23,24]. Moreover, elevated serum levels of P-III-P and low levels of protein C before the start of conditioning regimens can predict the patients who will develop VOD[25]. Serum hyaluronic acid, vWF-cleaving protease ADAMTS13 and Ca-125 have also been evaluated as early markers of VOD. In particular, serum elevation of CA-125 appears to be an early and accurate predictive marker in the pediatric population, but the mechanism is unknown[26].
Ultrasound of the liver and abdomen with doppler examination is the first line imaging investigation. Findings include the presence of ascites, hepatomegaly, attenuated hepatic veins and/or biliary dilatation. Ultrasound can also rule out the presence of malignant hepatic infiltration and other biliary diseases. Interestingly, thickness of gallbladder wall has been shown to correlate with HVPG in these patients, (which has prognostic significance - see later). However, none of these signs is specific for diagnosis and must be interpreted within the clinical context[27].
Pulsed Doppler ultrasound can suggest VOD on the basis of a decreased or reversed portal venous flow[28]. Significant elevation of the hepatic artery resistive index in duplex sonography may be a sensitive index of liver damage related to VOD. In infants, a segmental portal flow reversal has also been shown to be strongly associated with early VOD[29]. Magnetic resonance can confirm the ultrasound findings and be suggestive of outflow obstruction if it shows a patchy signal enhancement in the absence of hepatic vein occlusion[30].
The transjugular access is a safe route to perform mea-surement of the hepatic venous pressure gradient (HVPG) and liver biopsy[31-33]. HVPG is the difference between occluded hepatic venous pressure and the non-occluded (free) pressure on the hepatic veins. Normally, the non occluded pressure in hepatic veins is the same as in the IVC. The absence of a significant gradient (< 6 mmHg) along the hepatic veins and in the IVC can exclude anatomical causes of outflow obstruction. Measurement of HVPG can help discriminate between GVHD and VOD because HVPG is greater in the latter. In one study 82% of patients with VOD had HVPG greater than 9 mmHg, but this was found in none of those with GVHD. In another study, an HVPG greater than 10 mmHg was significantly correlated with VOD (91% specificity and 86% positive predictive value)[32].
HVPG can be also helpful to determine prognosis, as patients who will survive with VOD have less severe portal hypertension. A HVPG greater than 20 mmHg is correlated with poor prognosis[34]. The transjugular access allows liver biopsy to be performed safely even if coagulation is impaired (without the need of blood product transfusion) which is often the case. In most series, confirmatory diagnosis of VOD has relied on post mortem histology as transjugular liver biopsy has not been used. Even with this, no single histological feature is pathognomonic of the diagnosis. Another advantage of TJLB is the possibility to perform multiple passes in different sites of the liver which offers a theoretical advantage in diagnosing diseases with patchy distribution like VOD or hepatic venous obstruction syndromes[35].
Bearman et al[34] have developed a model to estimate survival in patients with VOD based on a large cohort of SCT patients. Percentage of weight gained, bilirubin, ascites and peripheral edema were associated with worse survival. Severe VOD was associated with 98% mortality at d 100 after SCT (Table 3). Moreover, HVPG greater than 20 mmHg was confirmed as an independent prognostic marker of mortality.
| Mild | Moderate | Severe | |
| Weight gain (% increase) | 7.0 ± 3.5 | 10.1 ± 5.3 | 15.5 ± 9.2 |
| Maximum bilirubin (mg/dL) | 4.7 ± 2.9 | 7.9 ± 6.6 | 26.6 ± 15.2 |
| Percentage with peripheral edema | 23 | 70 | 85 |
| Percentage with ascites | 5 | 16 | 48 |
| Day 100 mortality (all causes) (%) | 3 | 20 | 98 |
Outcome of VOD after liver transplantation remains unclear, but a poor outcome is reported by Sebagh et al[12] with 63% mortality (12 of 19 patients) in the largest series published. Because of the low incidence of this complication after LT, no specific prognostic factors have been evaluated, but clinical features derived from SCT groups could be used to assess severity of VOD after LT as well. Mortality after liver transplantation is related mainly to liver failure and development of renal insufficiency due to portal hypertensive complications.
Treatment of VOD is primary supportive and spontaneous recovery is reported in 70%-85% of mild forms after stem cell transplantation. However, severe forms do not resolve and given the paucity of effective therapies, prevention is a priority. The use of non myeloablative regimens in patients with risk factors for VOD is now possible[36]. Alternatively monitoring blood concentrations of chemotherapeutic agents, i.e. busulphan[37] has been shown to be less hepatotoxic when administered intravenously rather than orally. The study of genetic polymorphisms of glutathione S-transferase and TNF-α have been evaluated and allow identification of the patients at risk, but further studies are needed[38,39].
Prophylactic administration of ursodeoxycholic acid, being an antioxidant and antiapoptotic agent, has been evaluated in 4 randomized trials[40-43]. Two studies have shown a significant benefit of UDCA 600 mg daily in preventing VOD after SCT (VOD incidence 15% vs 40% and 3% vs 18.5%)[40,41], but in the most recent two (one in combination with heparin) no benefit of UDCA administration per se or when added to heparin was seen. In the trial in which UDCA was given alone at the dose of 12 mg-1 kg-1 daily, there was a decrease overall mortality with a decreased incidence of GVHD[42,43].
Prostaglandin E1 is a vasodilator with protective properties for the endothelium and has antithrombotic activity[44]. One non-randomized trials in which PGE1 was given in combination with heparin or heparin and tPA showed a lower incidence of VOD in the PGE1 group (12.2% vs 25.5%), a finding which was confirmed in a randomised trial published as an abstract[45]. However, the most recent prospective study enrolling 24 patients using PGE1 alone failed to show any advantage and was associated with severe toxicity in all[46].
Supplementation of glutathione to restore hepatic concentrations has been effective in experimental models. In one RCT, 34 patients were randomized to oral supple-mentation of glutamine aiming to maintain GHS levels, or a mixture of amino acids. Glutamine supplementation was able to maintain normal protein C levels after VOD, but clinical outcome was not evaluated[47].
Treatment of the classical syndrome also includes supportive measure. Ascites is treated with sodium restriction, diuretics and paracentesis. Mechanical organ support may be needed when renal or respiratory failure develops. Correction of coagulopathy and prevention of infections in severe forms to avoid bacterial translocation from the gut by intestinal disinfection are often used[48].
Based on the histological presence of microthrombosis and fibrin deposition in the hepatic venules of patients with VOD, the principal specific therapy has been to promote fibrinolysis with or without anticoagulation[49-51]. To date, about 130 patients have been treated with tPA - the response rate is about 30% in the largest series with the addition of concomitant heparin[52]. However, no response was seen amongst patients with MOF, renal or respiratory failure. Moreover, 24% developed severe bleeding. Thus, administration of pro-fibrinolytics and anticoagulants should be avoided in these patients, but conversely should be given early in the course of VOD.
Administration of ATIII and protein C or PGE1 has not been shown to be effective[53]. However, there is some evidence for the use of defibrotide. This is a polydeoxyribonucleic acid derived from porcine and bovine mucosa that has pro-fibrinolytic and antithrombotic properties; it also decreases leukocyte rolling and adherence to the endothelium as well as decreasing thrombin genera-tion and lowering circulating levels of PAI-1.
In the largest series published, treatment with 10 to 60 mg kg-1 d-1 infusion of defibrotide every 6 hours achieved a response rate of 36% and overall survival rate of 35% at 100 d after SCT without adverse events[54,55]. Predictors of survival with therapy, included younger age, autologous SCT and abnormal portal vein flow, while regimens based on busulphan and the presence of encephalopathy predicted worse outcome. Decrease in mean serum creatinine and PAI-1 levels are associated with response to treatment and better survival.
The high mortality rate of VOD after liver trans-plantation leads to the need of specific therapy. In the only two patients treated with defibrotide for VOD after LT, only one survived[56]. No effective medical therapy has been reported.
Decompression of the engorged sinusoids by a tran-sjugular intrahepatic portosystemic shunt can relieve portal hypertension and prevent renal failure in patients with VOD. The recent review on clinical practice guidelines for transjugular intrahepatic portosystemic shunt (TIPS) did not recommend TIPS for veno-occlusive disease (VOD)[57]. This was because TIPS has not been shown to change prognosis in patients with VOD after SCT. However, it was implied that VOD is only seen after SCT, whereas it can be seen in other settings, in which TIPS may offer a potentially useful treatment.
To date 27 VOD patients treated with TIPS have survived, 20% of the total (Table 4). All but three (2 with previous liver and 1 with previous kidney transplantation) had bone marrow transplantation[58]. The most common causes of death usually occurring within 1 to 3 wk follow-ing diagnosis were multi organ failure (MOF), sepsis and haemorrhage, due to underlying haematological disease. The remainder were due to portal hypertension and renal insufficiency complicating VOD. An issue not touched upon in the review is that the reported failure of TIPS to change prognosis may be due to a misdiagnosis of VOD. Sometimes it is difficult to distinguish it from cholangitis lenta or acute GVHD, and so in some patients, specific therapy for VOD may be delayed.
| Autho | Patients | Etiology | Severity VOD | Improvement portal hypertension | Early mortality (< 1 m) | Late mortality (> 1 m) |
| Azoulay (2000) | 10 | BMT | Severe | 10/10 | 5/10 | 4/5 |
| Fried (1996) | 6 | BMT | Severe | 6/6 | 4/6 | 1/2 |
| Annarolo (2004) | 1 | BMT | Severe | yes | Alive 3 yr f-up | |
| Zenz (2001) | 3 | BMT | Severe/modetate | 3/3 | 3/3 | - |
| Azoulay (1998) | 1 | KTx | Severe | yes | Alive 36 mo f-up | |
| Shen (1996) | 1 | Irradiation pelvis | Moderate | yes | Alive 5 mo f-up | |
| Leny (1996) | 1 | BMT | Severe | yes | yes | - |
| De la Rubia (1996) | 1 | BMT | Moderate | yes | Alive 9 mo f-up | |
| Smith (1996) | 1 | BMT | Severe | yes | yes | - |
| Meacher (1999) | 1 | BMT | Severe | - | yes | yes |
| Lerut (1999) | 1 among series | LT | - | yes | - | - |
| Sebagh (1999) | 1 among series | LT | - | - | re-OLT | |
The interval between the onset of VOD and TIPS placement is usually not mentioned and only a few reports specifically comment on this. The interval could influence the success of TIPS. If MOF is already present, patients are probably being treated too late. Conversely, in the only patient who survived in one series, the TIPS was placed 180 d after VOD diagnosis, probably recovery would have occurred in any case. Earlier intervention may be worthwhile, but it needs formal assessment. A further issue is that when VOD is complicated by direct parenchymal injury, e.g. due to cyclophosphamide, TIPS may also be contraindicated, as liver dysfunction would not be expected to improve with decompression of the hepatic outflow.
Indeed, the causes of death in patients with VOD are usually renal and cardiopulmonary failure and sepsis, rather than liver failure per se. In liver transplantation 4 patients with VOD have been treated with TIPS, one died, one was retransplanted, but information in these two patients about the course or the cause of liver failure or death was not reported. We have recently described two patients who developed severe veno-occlusive disease post liver transplantation, failed defibrotide therapy and were treated by TIPS. One has had a full recovery with histological amelioration and one resolved the portal hypertension, but without amelioration in histology at 16 mo[59]. Similarly, histological changes after TIPS were reported by Fried et al[60] in 6 patients, 3 (50%) showing amelioration of sinusoidal congestion and haemorrhagic necrosis.
Thus, although TIPS is not recommended for patients with severe VOD, this may apply only to SCT patients in whom MOF conditions the prognosis. If a severe VOD is diagnosed in a liver transplanted patient and if medical therapy fails, transjugular porto systemic shunt should be considered.
Liver transplantation has been reported anedoctically as a rescue therapy in patients with VOD after SCT not responding to medical therapy. The presence of malignancies and multiple organ failure (if VOD is advanced) contraindicates OLT. When VOD develops after liver transplantation itself, retransplantation can be performed as a rescue therapy as the liver is the only organ damaged. Previous placement of TIPS allows more time to the clinician to re-list the patients for OLT and it does not jeopardize subsequent OLT.
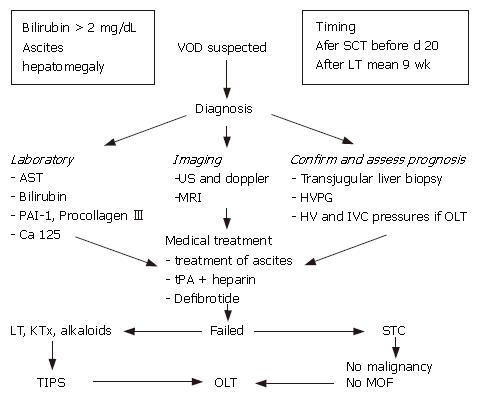
A flow-chart for the suggested diagnosis and manage-ment of VOD is reported in Figure 2.
S- Editor Liu Y L- Editor Rippe RA E- Editor Liu Y
| 1. | McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 582] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005-3020. [PubMed] |
| 3. | Richardson P, Guinan E. The pathology, diagnosis, and treatment of hepatic veno-occlusive disease: current status and novel approaches. Br J Haematol. 1999;107:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Bras G, Jaliffe DB, Stuart KL. Veno-occlusive disease of liver with nonportal type of cirrhosis, occurring in Jamaica. AMA Arch Pathol. 1954;57:285-300. [PubMed] |
| 5. | Zuckerman M, Steenkamp V, Stewart MJ. Hepatic veno-occlusive disease as a result of a traditional remedy: confirmation of toxic pyrrolizidine alkaloids as the cause, using an in vitro technique. J Clin Pathol. 2002;55:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 427] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 7. | Alpert LI. Veno-occlusive disease of the liver associated with oral contraceptives: case report and review of literature. Hum Pathol. 1976;7:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Fajardo LF, Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med. 1980;104:584-588. [PubMed] |
| 9. | Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc. 2003;78:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, Bandini G, Esperou H, Russell J, de la Rubia J. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599-3604. [PubMed] |
| 11. | Lee JH, Choi SJ, Lee JH, Kim SE, Park CJ, Chi HS, Lee MS, Lee JS, Kim WK, Lee KH. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Sebagh M, Debette M, Samuel D, Emile JF, Falissard B, Cailliez V, Shouval D, Bismuth H, Reynès M. "Silent" presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection. Hepatology. 1999;30:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | DeLeve LD, Wang X. Decresce in nitric oxide production contributes to hepatic venoocclusive disease. Hepatology. 1999;30:218A. |
| 14. | DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, Tokes ZA, Tsai J, McCuskey RS. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 667] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 827] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Toh HC, McAfee SL, Sackstein R, Cox BF, Colby C, Spitzer TR. Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant. 1999;24:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ben-Ari Z. Ascites after transplantation--a mystery. Liver Transpl. 2004;10:1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Costa F, Choy CG, Seiter K, Hann L, Thung SN, Michaeli J. Hepatic outflow obstruction and liver failure due to leukemic cell infiltration in chronic lymphocytic leukemia. Leuk Lymphoma. 1998;30:403-410. [PubMed] |
| 20. | Blostein MD, Paltiel OB, Thibault A, Rybka WB. A comparison of clinical criteria for the diagnosis of veno-occlusive disease of the liver after bone marrow transplantation. Bone Marrow Transplant. 1992;10:439-443. [PubMed] |
| 21. | Shulman HM, McDonald GB, Matthews D, Doney KC, Kopecky KJ, Gauvreau JM, Thomas ED. An analysis of hepatic venocclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79:1178-1191. [PubMed] |
| 22. | Strasser SI, Mc Donald SJ, Schoch HG, Severe hepatocellular injury after hematopoietic cell transplant: incidence and etiology in 2136 consecutive patients. Hepatology. 2000;32:299A. |
| 23. | Eltumi M, Trivedi P, Hobbs JR, Portmann B, Cheeseman P, Downie C, Risteli J, Risteli L, Mowat AP. Monitoring of veno-occlusive disease after bone marrow transplantation by serum aminopropeptide of type III procollagen. Lancet. 1993;342:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Rio B, Bauduer F, Arrago JP, Zittoun R. N-terminal peptide of type III procollagen: a marker for the development of hepatic veno-occlusive disease after BMT and a basis for determining the timing of prophylactic heparin. Bone Marrow Transplant. 1993;11:471-472. [PubMed] |
| 25. | Tanikawa S, Mori S, Ohhashi K, Akiyama H, Sasaki T, Kaku H, Hiruma K, Matsunaga T, Morita T, Sakamaki H. Predictive markers for hepatic veno-occlusive disease after hematopoietic stem cell transplantation in adults: a prospective single center study. Bone Marrow Transplant. 2000;26:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Petäjä J, Pitkänen S, Vettenranta K, Fasth A, Heikinheimo M. Serum tumor marker CA 125 is an early and sensitive indicator of veno-occlusive disease in children undergoing bone marrow transplantation. Clin Cancer Res. 2000;6:531-535. [PubMed] |
| 27. | Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Lassau N, Auperin A, Leclere J, Bennaceur A, Valteau-Couanet D, Hartmann O. Prognostic value of doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation. 2002;74:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Ghersin E, Brook OR, Gaitini D, Engel A. Color Doppler demonstration of segmental portal flow reversal: an early sign of hepatic veno-occlusive disease in an infant. J Ultrasound Med. 2003;22:1103-1106. [PubMed] |
| 30. | Dumont Ch, Lambert M, Van Beers BE. MR imaging findings in a patient with hepatic veno-occlusive disease. Acta Gastroenterol Belg. 2004;67:236-238. [PubMed] |
| 31. | Carreras E, García-Pagán JC, Bosch J, Rozman C. Transvenous liver biopsies in marrow transplant recipients. Transplantation. 1995;60:1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Shulman HM, Gooley T, Dudley MD, Kofler T, Feldman R, Dwyer D, McDonald GB. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation. 1995;59:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Senzolo M, Burra P, Cholongitas E, Lodato F, Marelli L, Manousou P, Patch D, Sturniolo GC, Burroughs AK. The transjugular route: the key hole to the liver world. Dig Liver Dis. 2007;39:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729-1736. [PubMed] |
| 35. | Cholongitas E, Quaglia A, Samonakis D, Senzolo M, Triantos C, Patch D, Leandro G, Dhillon AP, Burroughs AK. Transjugular liver biopsy: how good is it for accurate histological interpretation? Gut. 2006;55:1789-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1060] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 37. | McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | The WT, Beyer W, Pendleton JD. Genetic polymorphisms in glutathione s-transferase and plasminogen activator inhibitor and risk of veno-occlusive disease (VOD). Blood. 2000;96:390A. |
| 39. | Poonkuzhali S, Vidya S, Shaji RV. Glutathione S-transferase gene polymorphism and risk of major undergoing allogeneic bone marrow transplantation. Blood. 2001;98:852A. |
| 40. | Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, Snyder M, Lewis SK, Allerton JP, Thompson JM. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, Okamoto S, Aotsuka N, Saito K, Nishimura M. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, Jung CW, Im YH, Yoon SS, Kang WK. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Giles F, Garcia-Manero G, Cortes J, Thomas D, Kantarjian H, Estey E. Ursodiol does not prevent hepatic venoocclusive disease associated with Mylotarg therapy. Haematologica. 2002;87:1114-1116. [PubMed] |
| 44. | Vaughan DE, Plavin SR, Schafer AI, Loscalzo J. PGE1 accelerates thrombolysis by tissue plasminogen activator. Blood. 1989;73:1213-1217. [PubMed] |
| 45. | Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H, Lehn P, Faure P, Drouet L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. 1990;74:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Bearman SI, Shen DD, Hinds MS, Hill HA, McDonald GB. A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol. 1993;84:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Goringe AP, Brown S, O'Callaghan U, Rees J, Jebb S, Elia M, Poynton CH. Glutamine and vitamin E in the treatment of hepatic veno-occlusive disease following high-dose chemotherapy. Bone Marrow Transplant. 1998;21:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Strasser SI, McDonald GB. Gastrointestinal and hepatic complications. Hematopoietic Cell Transplantation. 2nd ed. Oxford: Blackwell Publishing 1998; . |
| 49. | Lee JH, Lee KH, Choi JS, Zang DY, Kim SB, Kim SW, Suh C, Lee JS, Kim WK, Lee YS. Veno-occlusive disease (VOD) of the liver in Korean patients following allogeneic bone marrow transplantation (BMT): efficacy of recombinant human tissue plasminogen activator (rt-PA) treatment. J Korean Med Sci. 1996;11:118-126. [PubMed] |
| 50. | Bearman SI, Shuhart MC, Hinds MS, McDonald GB. Recombinant human tissue plasminogen activator for the treatment of established severe venocclusive disease of the liver after bone marrow transplantation. Blood. 1992;80:2458-2462. [PubMed] |
| 51. | Leahey AM, Bunin NJ. Recombinant human tissue plasminogen activator for the treatment of severe hepatic veno-occlusive disease in pediatric bone marrow transplant patients. Bone Marrow Transplant. 1996;17:1101-1104. [PubMed] |
| 52. | Bearman SI, Lee JL, Barón AE, McDonald GB. Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant patients. Blood. 1997;89:1501-1506. [PubMed] |
| 53. | Morris JD, Harris RE, Hashmi R, Sambrano JE, Gruppo RA, Becker AT, Morris CL. Antithrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1997;20:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D, Kinchla NM, Neuberg D, Waller EK, Antin JH. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737-744. [PubMed] |
| 55. | Chopra R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C, Neumeister P, Finazzi G, Iacobelli M, Bowyer K. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Mor E, Pappo O, Bar-Nathan N, Shaharabani E, Shapira Z, Tur-Kaspa R, Ben-Ari Z. Defibrotide for the treatment of veno-occlusive disease after liver transplantation. Transplantation. 2001;72:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 58. | Senzolo M, Cholongitas E, Patch D, Burroughs AK. TIPS for veno-occlusive disease: is the contraindication real? Hepatology. 2005;42:240-241; author reply 241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Senzolo M, Patch D, Cholongitas E, Triantos C, Marelli L, Stigliano R, Dhillon A, Burroughs A. Severe venoocclusive disease after liver transplantation treated with transjugular intrahepatic portosystemic shunt. Transplantation. 2006;82:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Fried MW, Connaghan DG, Sharma S, Martin LG, Devine S, Holland K, Zuckerman A, Kaufman S, Wingard J, Boyer TD. Transjugular intrahepatic portosystemic shunt for the management of severe venoocclusive disease following bone marrow transplantation. Hepatology. 1996;24:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |