Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3878
Revised: April 1, 2007
Accepted: April 4, 2007
Published online: July 28, 2007
AIM: To clarify the expression change of Wnt-induced secreted protein-1 (WISP-1) in human rectal cancer and to determine whether it is correlated with invasion and metastasis of human rectal cancer.
METHODS: Eighty-six paired samples of rectal cancer and surgically resected distant normal rectal tissue were collected and allocated into cancer group and control group respectively. WISP-1 mRNA was detected by relative quantitative real-time RT-PCR and WISP-1 protein was examined by immunohistochemical staining.
RESULTS: WISP-1 gene overexpression was found in 65% (56/86) primary rectal cancers, 2-30 times that of the level in normal matched rectal tissues (P = 0.001). The mRNA expression level was correlated with Duke’s staging, histological differentiation grade and lymph node status. The WISP-1 protein expression was in accordance with mRNA expression level. The positive degree of immunohistochemical staining in the cancer group (1.40 ± 0.35) was different from that in control group (1.04 ± 0.08, P < 0.001). Moreover, in cancer group the positive staining degree in high-level mRNA cancers (1.46 ± 0.37, n = 56) was higher than that in low-level mRNA (1.28 ± 0.28, n = 30, P = 0.018).
CONCLUSION: Aberrant levels of WISP-1 expression may play a role in rectal tumorigenesis. WISP-1 may be used as a specific clinical diagnosis and prognosis marker in rectal cancer.
- Citation: Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY, Li L, Luo HZ, Yang L, Zhou B, Gu J. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J Gastroenterol 2007; 13(28): 3878-3882
- URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3878.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3878
Wnt-induced secreted protein-1 (WISP-1) is a member of the connective tissue growth factor that belongs to the Cry61, CTGF and Nov (CCN) superfamily. All members of that family possess a secretory signal peptide at the NH2 terminus, indicating that they are secreted proteins. The biological properties of CCN proteins include stimulation of cell proliferation, migration, adhesion, and extracellular matrix formation. They also regulate more complex biological processes, such as angiogenesis and tumorigenesis[1-3]. WISP-1 is strongly expressed in the fibrovascular stroma of breast tumors developing in Wnt-1 transgenic mice[4]. Moreover, forced overexpression of WISP-1 in normal rat kidney fibroblasts was sufficient to induce their transformation, increase cellular saturation and promoted growth[5].
WISP-1 is a potential downstream target gene of Wnt-1/APC/β-catenin signaling pathway in colorectal cancer[5]. Though the expression of WISP-1 was believed to increase in colon carcinoma[4], it has not been quantitatively detected with a larger number of samples in rectal cancer alone. In this study, mRNA and protein expression of WISP-1 in pair matched rectal samples were measured by real-time RT-PCR and immunohistochemical staining, respectively, and the association between WISP-1 expression level and clinicopathologic features was further investigated. Through this work we aimed to elucidate the role of WISP-1 in the invasion and metastasis of rectal cancer and to provide a basis for its clinical diagnosis and outcome prediction.
Eighty-six paired samples of rectal cancer and distant normal rectal tissues were obtained from inpatients who underwent surgical operation from 2003 to 2004 in West China Hospital of Sichuan University. These patients did not receive any chemotherapy or radiotherapy before operation. Diagnoses and routine histopatholologic examinations of frozen sections were performed at the Department of Pathology, West China Hospital. Immediately after selection of diagnostic tissue samples and dissection of the margins for diagnostic purposes, samples of tumor and tumor-free tissues were collected respectively and stored in liquid nitrogen at -80°C. Samples were fixed by formalin and embedded in paraffin wax. The following information was recorded for each patient: age, sex, Duke’s stage, carcinoembryonic antigen (CEA), lymph node status and histological grade. This study was approved by the Medical Ethical Committee and all patients provided written informed consent to participate in the study.
Total RNA was extracted from each sample using Trizol reagent (Beyozol Co., Ltd) following manufacturer’s instruction. The concentration of RNA was measured by spectrophotomer. Total RNA was reverse-transcripted to cDNA with RT reagents (Takara Biotechnology Co., Ltd) according to the manufacturer’s protocol. Briefly, the RT reaction was carried out in a final volume of 20 μL containing 1 × M-MLV buffer, 20 units of RNase inhibitor, 2.5 μmol/L random hexamers, 100 units of M-MLV RTase, and 5 μL of total RNA. The mixture was incubated at 20°C for 10 min and 42°C for 60 min, and reverse transcriptase was inactivated by heating at 95°C for 10 min.
As shown in Table 1, specific primers and probes for WISP-1 gene and glyceraldehy phosphate dehydrogenase (GAPDH) gene (reference gene) were designed based on sequence data from the ensemble database (http://www.ensembl.org). Primers and probe were placed at the junction between two exons. The primers and probes were purchased from Takara Biotechnology Co. ( Dalian, China).
| Sequence | |
| Target gene/WISP-1 | |
| Forward primer | 5’- CCACCGGGGCCTCTACT-3’ |
| Reverse primer | 5’- CCACACCGACCACCTGT-3’ |
| Probes | 5’-FAM-CTATTGCGTACCTCGGGCGGTC -TAMRA-3’ |
| Reference gene/GAPDH | |
| Forward primer | 5’-CCTCAAGATCATCAGCAAT-3’ |
| Reverse primer | 5’-CCATCCACAGTCTTCTGGGT-3’ |
| Probes | 5’-FAM-ACCACAGTCCATGCCATCAC -TAMRA-3’ |
Conditions for all PCRs were optimized on iCycler iQ(Bio-Rad, USA) and the optimum annealing temperature was 57.5°C. The following iCycler iQ running protocol was used: denaturation (95°C, 5 min), amplification and quantification repeated 50 times (95°C for 20 s, 60.5°C for 30 s, and 72°C for 30 s). In addition, a non-template control (ddH2O control) was analyzed for each template. All samples were amplified simultaneously in triplicate. Equation 1 was applied to calculate the relative expression ratio (R) of WISP-1 gene, which was based on PCR efficiency (E) and the Ct deviation of an unknown sample versus a control. Ratio(R) = (EWISP-1)ΔCtWISP-1 (control-cancer)/ (EGAPDH) ΔCtGAPDH (control-cancer) (Equation 1)[6], ΔCt was the Ct deviation of WISP-1 and GAPDH, PCR efficiency (E) was calculated according to E = 10[–1/slope] [7]. The ratio over 1 represented an up-regulation of mRNA expression of WISP-1 in rectal cancer.
Sections were dewaxed in xylene and rehydrated in alcohol. The endogenous peroxidase activity was suppressed by 3% hydrogen peroxide for 15 min. After rinsing twice in phosphate-buffered saline (PBS), antigen retrieval was performed by immersing the sections in 10 mmol/L sodium citrate buffer (pH = 6.0) and heated for 15 min in a microwave oven. Non-specific binding was blocked by incubation with 3% bovine serum albumin (BSA) for 40 min. The sections were treated for 16 h with rabbit antihuman polyclonal immunoglobulin G antibodies of WISP-1 (Santa Cruz, SC-25441, CA) according to the manufacturer’s recommended concentration (1:50). PBS was used as a negative control. After washed three times in PBS, the sections were treated with biotinylated goat anti-rabbit immunoglobulin (Jin Mei Biotech Co., Ltd) for 40 min and then by horseradish peroxidase-streptavidin complex (Jin Mei Biotech Co., Ltd) for 30 min. The slides were then washed three times in PBS and incubated in DAB for 2 min. The slides were rinsed gently with distilled water and counterstained with haematoxylin for 30 s. The slides were dehydrated in alcohol prior to mounting. Images were collected by Olympus DD70 BX51 (Olympus, Japan) and analyzed by IMAGE-PRO plus 4.1 software (Media Cybernetics, USA). Eight visual fields in each section were randomly selected and the mean value of relative optical density (OD) was measured and calculated by taking the OD of background as 1. The extent of immunohistochemical staining was categorized as positive (1-1.5) and strongly positive (over 1.5).
The relative expression analysis of the target gene was performed using a new software, named REST-XL© (relative expression software tool-XL, available at http://http://www. wzw. tum. de /gene - quantification/) for group-wise comparison in real-time PCR[8]. Using SPSS 12.0 software, a Chi-square test (χ2) test was performed to analyze the correlations of WISP-1 mRNA or protein expression levels with clinical and pathological parameters. P < 0.05 was considered statistically significant.
Patients’ age ranged from 41 to 86 (55.8 ± 13.8; mean ± SD) years, 44 were males and 42 females. Carcinoma cells could be observed in all rectal cancers under a light microscope. Samples at Duke’s Staging A, B, C and D were 15, 32, 25 and 14, respectively. There were 33 papillary adenocarcinomas, 37 tubular adenocarcinomas, 8 mucoid adenocarcinomas, 7 signet-ring cell carcinomas and one squamous carcinoma by histological classification. Histologically, 18, 63 and 5 were poorly, moderately and well differentiated, respectively.
RT-PCR products had the desired length (WISP-1, 80 bp; GAPDH, 141 bp). No primer-dimers were generated during the applied 50 real-time PCR amplification cycles. Real-Time RT-PCR amplification efficiencies were calculated from the given slopes in FTC-2000 software and REST©. Investigated transcripts showed different real-time PCR efficiency in the investigated range with high linearity (Pearson correlation coefficient r = 1). The real-time PCR efficiency of WISP-1 and GAPDH is 1.61 and 1.85, respectively.
The relative ratio (R) is presented as the fold change in gene expression normalized to an endogenous reference gene and relative to the control. Therefore, an R value greater than 1.0 was considered to be an overexpression of WISP-1 gene. Among the 86 rectal cancer RNA samples tested, WISP-1 gene overexpression was found in 65% (56 of 86) primary rectal tumors, being 2-30 times that of the level in normal matched rectal tissues. Major differences in the amount of WISP-1 mRNA were observed: 17 tumors showed an expression level 1-5 times, 6 tumors 5-10 times, and 33 tumors more than 10 times that of the normal rectal tissue RNA. REST© analysis showed mRNA expression of WISP-1 is up-regulated in cancer group in comparison with control group by the factor 7.744, and target gene-cancer group is significantly different from target gene-control group (P = 0.001). Randomization data are shown in Table 2.
| Reference gene (GAPDH) | Target gene (WISP-1) | |
| Efficiency | 1.61 | 1.85 |
| Control group | ||
| n | 86 | 86 |
| Mean | 25.43 | 38.91 |
| Standard deviation | 15.86 | 58.89 |
| CV (%) | 62.35 | 151.36 |
| Sample group | ||
| n | 86 | 86 |
| Mean | 24.74 | 35.04 |
| Standard deviation | 14.3 | 37.1 |
| CV (%) | 57.8 | 105.88 |
A strongly significant association existed between Duke’s staging, lymph node status, and histological grade versus WISP-1 mRNA as shown in Table 3.
| WISP-1 mRNA expression | ||||
| Factors | n | - | + | P |
| Age(yr) | ||||
| < 60 | 48 | 16 | 32 | 0.7351 |
| ≥ 60 | 38 | 14 | 24 | |
| Sex | ||||
| Female | 42 | 11 | 31 | 0.0981 |
| Male | 44 | 19 | 25 | |
| Histological differentiation | ||||
| Poor | 18 | 2 | 16 | |
| Moderate | 63 | 25 | 38 | 0.0392 |
| Well | 5 | 3 | 2 | |
| CEA3 (ng/mL) | ||||
| ≤ 3.4 | 20 | 9 | 11 | 0.1261 |
| > 3.4 | 22 | 5 | 17 | |
| Lymph node status | ||||
| Negative | 51 | 24 | 27 | 0.0041 |
| Positive | 35 | 6 | 29 | |
| Duke’s staging4 | ||||
| A + B | 47 | 22 | 25 | 0.0111 |
| C + D | 39 | 8 | 31 | |
| Histological type | ||||
| Tubular adenocarcinoma | 37 | 17 | 20 | 0.0811 |
| Papillary adenocarcinoma | 33 | 22 | 11 | |
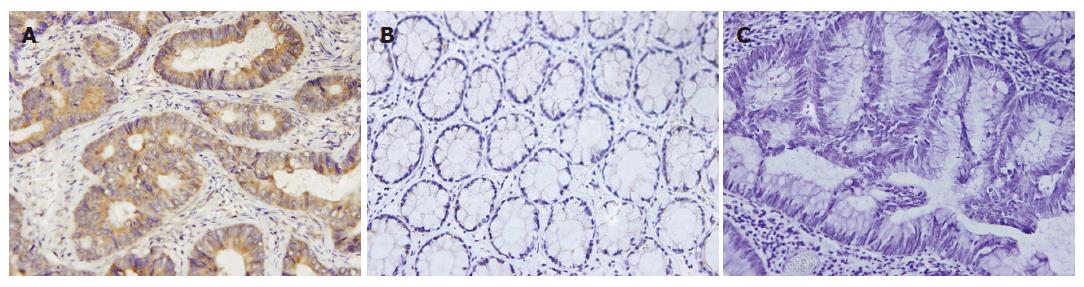
The representative results are shown in Figure 1. The cytoplasm of rectal cancer cells were positively stained, but the stromal components were not. Protein expression of WISP-1 in normal rectal tissues was very weak. There was no detectable immunoreactivity in PBS control slide. The positive degree of immunohistochemical staining in the cancer group (1.40 ± 0.35) was different from that in the normal group (1.04 ± 0.08, P < 0.001). Moreover, in the cancer group, the positive staining degree in high-level mRNA cancers (1.46 ± 0.37, n = 56) was higher than that in low-level mRNA ones (1.28 ± 0.28, n = 30, P = 0.018). WISP-1 protein expression was also correlated with Duke’s staging, lymph node metastasis and histological grade, which is correlated with mRNA expression.
Colorectal carcinoma is the third most common malignancy in the world[9]. Although surgical resection has been shown to be effective for localized disease, current treatment regimens are ineffective for metastatic disease, and thus new treatment strategies are required. A better understanding of the molecular events in colorectal carcinoma is pivotal in the development of novel treatment strategies. WISP-1 is a member of the connective tissue growth factor identified to be a Wnt-1 and β-catenin regulated protein[5]. Overexpression of WISP-1 induced morphological transformation, increased cellular saturation, promoted growth in normal rat kidney fibroblasts, and induced tumor formation in nude mice[5]. WISP-1 was reported to be able to attenuate p53-mediated apoptosis in response to DNA damage through activation of Akt kinase[10]. Although WISP-1 transcript has been overexpressed in 84% of human colon adenocarcinomas and 46% of primary breast tumors[4,11], WISP-1 expression has not been quantitatively detected in a considerable number of rectal cancers till now. In this study, quantitative real-time RT-PCR and immunohistochemistry were used to detect WISP-1 expression in pure rectal cancer and normal rectal tissues.
Although traditional PCR and RT-PCR may also yield relatively precise results, they not only need a rather large amount of template for the analysis of a sample, but also require post-PCR analysis which may create risks in cross-contamination of samples with PCR products[12]. However, real-time RT-PCR can overcome such disadvantages and makes RNA quantifications much more precise and reproducible, because it is based on Ct values established in the early exponential phase of the PCR reaction rather than endpoint measurement of the amount of accumulated PCR product. Real-time RT-PCR has good intra-assay and inter-assay reproducibility and yields statistical confidence values, it therefore, has a high level of interlaboratory standardization and fits interlaboratory comparison[13,14]. In this study, we utilized a new software tool, REST-XL©[8], to test the group difference for significance with a newly developed randomization test[15,16]. The quantities of WISP-1 mRNA derived from ratios and variances can be high, normal distributions could not be expected, and it remains unclear how a parametric test could be best constructed. A randomization test, which makes no assumptions about the distribution of observations in populations, is a useful alternative to more standard parametric tests for analyzing experimental data. It is more flexible than non-parametric tests based on ranks and does not reduce in power compared to parametric test[15]. Therefore, a randomization test with a pair-wise reallocation was seen as the most appropriate approach for this application.
The result suggested that the mRNA expression level of WISP-1 was up-regulated in rectal cancer. WISP-1 mRNA expression was also found to be significantly associated with Duke’s staging, lymph node status, and histological grade, which confirmed the role of WISP-1 in the progression of rectal cancer. The same results were found in previous reports on mRNA expression of WISP-1 in colon cancers and breast cancer[4,11]. As shown in this study, the expression ratio over 1 in the lymph node positive group (82.9%) was higher than that in the lymph node negative group (52.9%), which suggests that WISP-1 may facilitate the lymph metastasis of rectal cancer. We also found that WISP-1 was significantly associated with poor histological differentiation, which was inconsistent with the report in colorectal cancer by Khor et al[17]. This contradiction may be related to sampling errors or insufficient experimental samples. However, our study is the largest study published to date, with 86 human rectal cancers. For Duke's staging, only 25 of 47 (53.2%) cases with stage A or B overexpressed WISP-1 compared with 31 of 39 (79.5%) samples from patients with stage C or D rectal cancers, which is in agreement with the report of Xie et al[11] in breast cancer. The Duke’s staging represented the rectal cancer prognosis. The later the stage, the worse the prognosis was, so the WISP-1 may be selected as a prognosis factor. The above results indicated that overexpression of WISP-1 might play an essential role in invasion and metastasis of rectal cancer. Thus, WISP-1 mRNA expression assay could be used in the evaluation of clinical diagnosis and prognosis of rectal cancer.
WISP-1 protein expression in rectal cancer cells in this study showed moderate to strong diffuse cytoplasmic staining, and weak to non-WISP-1 immunoreactivity in the cytoplasm of apparently normal adjacent rectal tissues, which was usually most pronounced at the invasive front of the colorectal cancer[18]. Our study also found that WISP-1 protein expression in rectal cancer was consistent with its mRNA expression and associated with Duke’s staging, lymph node status and histological grade. For histological differentiation, Khor et al[17] reported that WISP-1 protein expression was associated with well-differentiated colorectal carcinoma tissues. With respect to the mechanisms underlying the difference of WISP-1 expression between rectal cancer and colon cancer, gene mutation may play a key role in different cancer cells and histological specificity after transcription of WISP-1 mRNA. By analyzing the mRNA and protein expression of WISP-1, it tempts us to speculate that WISP-1 plays an important role in rectal carcinogenesis. But the exact mechanisms about the regulation of WISP-1 expression in rectal tumorrigenesis have been unknown. A recent report by Su et al[10] demonstrated that WISP-1 activates the antiapoptotic Akt signaling pathway, inhibits the mitochondrial release of cytochrome c, up-regulates antiapoptotic protein Bcl-XL, and therefore prevents cells from undergoing p53-mediated apoptosis in response to DNA damage. To better understand the regulatory mechanisms of WISP-1 expression, further studies about effects of signaling pathway on WISP-1 expression in rectal cancer are underway.
In summary, we showed for the first time,that the involvement of the WISP-1 overexpression in rectal tumorigenesis may play a role in the invasion and metastasis of human rectal cancer. WISP-1 may be selected as a clinical diagnosis and prognosis index in rectal cancer, which may also serve as a potential therapeutic target for the development of new treatment regimens for rectal caner.
We wish to thank Professors Zhou and Xia for their advice in this study, the members of Pathological Research Institution of West China Hospital and Department of Surgical Laboratory for their useful assistance and colleagues of the Department of Gastrointestinal Surgery for providing us the experimental specimens.
S- Editor Zhu LH L- Editor Ma JY E- Editor Liu Y
| 1. | Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 509] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Perbal B. The CCN family of genes: a brief history. Mol Pathol. 2001;54:103-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717-14722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585-595. [PubMed] |
| 6. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24144] [Cited by in RCA: 25936] [Article Influence: 1080.7] [Reference Citation Analysis (0)] |
| 7. | Bernard PS, Wittwer CT. Real-time PCR technology for cancer diagnostics. Clin Chem. 2002;48:1178-1185. [PubMed] |
| 8. | Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5539] [Cited by in RCA: 5638] [Article Influence: 245.1] [Reference Citation Analysis (0)] |
| 9. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1707] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 10. | Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917-8923. [PubMed] |
| 12. | Bánkfalvi A, Simon R, Brandt B, Bürger H, Vollmer I, Dockhorn-Dworniczak B, Lellé RJ, Boecker W. Comparative methodological analysis of erbB-2/HER-2 gene dosage, chromosomal copy number and protein overexpression in breast carcinoma tissues for diagnostic use. Histopathology. 2000;37:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Bièche I, Onody P, Laurendeau I, Olivi M, Vidaud D, Lidereau R, Vidaud M. Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applications. Clin Chem. 1999;45:1148-1156. [PubMed] |
| 14. | Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 836] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 15. | Horgan GW, Rouault J. Introduction to randomisation tests. Aberdeen, Scotland: Biomathematics and Statistics Scotland; . |
| 16. | Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. Biometrics. 1997;53:1560-1561. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Khor TO, Gul YA, Ithnin H, Seow HF. A comparative study of the expression of Wnt-1, WISP-1, survivin and cyclin-D1 in colorectal carcinoma. Int J Colorectal Dis. 2006;21:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, Imai K. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer. 2001;95:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |