Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3836
Revised: August 20, 2006
Accepted: August 22, 2006
Published online: July 28, 2007
AIM: To investigate the therapeutic effects of triple therapy combining lafutidine with clarithromycin and amoxicillin on H pylori infection and the resolution of gastroesophageal symptoms after eradication.
METHODS: We conducted a randomized, multicenter, open-label controlled trial to compare the effectiveness of a triple therapy of lafutidine, clarithromycin, and amoxicillin (lafutidine group) with that of a triple therapy of lansoprazole, clarithromycin, and amoxicillin (lansoprazole group) in patients with H pylori infection. The study group comprised 22 patients with gastric ulcers and 18 patients with duodenal ulcers who had H pylori infection.
RESULTS: H pylori eradication rates were similar in the lafutidine group (14/20, 70%) and the lansoprazole group (14/20, 70%). Gastroesophageal reflux and abdominal symptoms improved after eradication therapy in both groups, whereas abdominal discomfort, diarrhea, and constipation were unchanged. H pylori status had no apparent effect on improvement of gastroesophageal reflux or abdominal symptoms after treatment. Adverse events were similar in both groups.
CONCLUSION: The triple therapy including lafutidine is equivalent to triple therapy including lansoprazole in terms of H pylori eradication rates and improvement in gastroesophageal reflux and abdominal symptoms. These results are attributed to the fact that lafutidine has strong, continuous antisecretory activity, unaffected by CYP2C19 polymorphisms.
-
Citation: Hagiwara T, Kato M, Anbo T, Imamura A, Suga T, Uchida T, Fujinaga A, Nakagawa M, Nakagawa S, Shimizu Y, Yamamoto J, Takeda H, Asaka M. Improvement in symptoms after H2-receptor antagonist-based therapy for eradication of
H pylori infection. World J Gastroenterol 2007; 13(28): 3836-3840 - URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3836
Many patients with peptic ulcers present with symptoms of epigastric and abdominal pain. Alleviation of these symptoms is an important goal of treatment for peptic ulcers. H pylori plays a critical role in the pathogenesis of peptic ulceration. Eradication of H pylori infection facilitates ulcer healing and prevents recurrence[1-3]. In Japan, triple therapy combining a proton-pump inhibitor (PPI) with clarithromycin and amoxicillin was approved by the National Health Insurance system in November 2000 and is now widely prescribed clinically. Eradication rates with this regimen range from 80% to 90%[4]. However, decreasing eradication rates due to clarithromycin-resistant strains have recently become a problem.
Lafutidine is an H2-receptor antagonist that conti-nuously suppresses acid secretion even during the daytime, unlike famotidine and cimetidine[5]. After oral administration, lafutidine produces a more rapid rise in intragastric pH than rabeprazole 20 mg in fasting and postprandial H pylori negative male patients, resulting in the early resolution of symptoms[6-9]. Triple therapy with lafutidine, clarithromycin, and amoxicillin yields an 80% eradication rate[10].
We conducted a randomized, multicenter, open-label controlled trial to compare the effectiveness of a triple therapy of lafutidine, clarithromycin, and amoxicillin with that of a triple therapy of lansoprazole, clarithromycin, and amoxicillin, which is widely prescribed in Japan. Our primary goals were to examine the therapeutic effects of triple therapy including lafutidine on the resolution of symptoms after H pylori eradication therapy.
Eligible patients had a diagnosis of active gastric ulcer or duodenal ulcer (mucosal defect of more than 5 mm in size) confirmed by endoscopic examination and were diagnosed from February 2001 through June 2004. Patients who had a history of eradication therapy or who had received antimicrobial agents, anticoagulant drugs, or PPIs within 4 wk before the trial treatment were excluded to eliminate the potential effects of these drugs on H pylori infection. A total of 46 patients, who were confirmed to be positive for H pylori infection at trial entry, were enrolled. Data from 6 patients could not be evaluated because of gastric cancer or a request to be withdrawn from the trial. The remaining 40 patients were included in per-protocol analyses. The trial was performed according to the principles of the Declaration of Helsinki. All patients gave informed consent before trial entry.
At trial entry, H pylori status was assessed on the basis of the results of the rapid urease test (Pyloritec, Sankyo Co. Ltd., Tokyo, Japan) or culture of mucosal-biopsy specimens obtained during endoscopy from both the antrum (within 2 cm of the pyloric ring) and the corpus (along the greater curvature). At diagnosis after eradication, patients were classified as H pylori-negative if the results of the urea breath test and culture were both negative. The cut-off value for the urea breath test was set at 2.5‰; patients were considered H pylori-negative if the value was less than 2.5‰ and H pylori-positive if the value was 2.5‰ or higher.
This randomized, multicenter, open-label controlled trial compared the safety and effectiveness of a combination of lafutidine, clarithromycin, and amoxicillin (lafutidine group) with that of a combination of lansoprazole, clarithromycin, and amoxicillin (lansoprazole group). A primary objective was to compare H pylori eradication rates and the resolution of symptoms.
In the lafutidine group, lafutidine (20 mg), amoxicillin (750 mg), and clarithromycin (200 mg) were given orally twice daily (after breakfast and dinner) for 1 wk. In the lansoprazole group, lansoprazole (30 mg), amoxicillin (750 mg), and clarithromycin (200 mg) were given orally twice daily (after breakfast and dinner) for 1 wk. Eradication of H pylori was evaluated 5 wk after the completion of the 1-wk course of eradication therapy. During these 5 wk of observation, patients received no medication or were given maintenance therapy with lafutidine (10 mg, twice daily). To assess symptoms before and after eradication therapy, each patient completed a written questionnaire based on the Gastrointestinal Symptom Rating Scale-Japanese Version (GSRS-JV). Symptom scores after treatment were compared with the baseline scores to evaluate improvement in symptoms.
Of the 40 patients included in per-protocol analyses, 22 had gastric ulcers and 18 had duodenal ulcers. The demographic characteristics of the patients did not differ significantly between the lafutidine group and the lansoprazole group, except for the distribution of genetic polymorphisms of CYP2C19. Table 1 shows the detailed clinical characteristics of the patients.
| Lafutidinegroup | Lansoprazolegroup | P | ||
| No. of patients | 20 | 20 | - | |
| Sex (male:female) | 13:7 | 14:6 | NS | |
| Mean age (range) | 51.1 (22-66) | 50.6 (20-79) | NS | |
| Site of ulcer (gastric ulcer:duodenal ulcer) | 12:8 | 10:10 | NS | |
| History of ulcer | Initial onset | 3 | 5 | NS |
| Recurrence | 15 | 14 | ||
| Unknown | 2 | 1 | ||
| Pretreatment within | None | 15 | 13 | NS |
| 1 wk before treatment | Done | 3 | 6 | |
| Unknown | 2 | 1 | ||
| Pretreatment with an H2-receptor antagonist | None | 14 | 14 | NS |
| Done | 6 | 6 | ||
| CYP2C19 | 6:9:1:4 | 5:8:6:1 P < 0.05 | ||
| (homoEM : heteroEM:PM:Unknown) | ||||
| Sensitive or resistant to clarithromycin | 16:1:3 | 15:1:4 | NS | |
| Sensitive (MIC < 1) resistant:(MIC ≥ 1):Unknown | ||||
An intention-to-treat analysis of all 46 patients, in whom the response to eradication therapy could be evaluated, showed that H pylori was eradicated in 66.7% (16/24) of the lafutidine group and 72.7% (16/22) of the lansoprazole group. These rates are considered to be similar. Among the 40 patients who were treated according to the protocol (per-protocol analysis), H pylori was eradicated in 70.0% (14/20) of the lafutidine group and 70.0% (14/20) of the lansoprazole group, again showing no difference between the groups (Table 2). When treatment response was analyzed according to CYP2C19 polymorphism type, eradication rates were found to be slightly higher among homozygous-extensive metabolizers in the lafutidine group and poor metabolizers in the lansoprazole group. However, eradication rates did not differ significantly according to the type of CYP2C19 polymorphism in either the lafutidine or lansoprazole group (Table 3).
| Lafutidine group | Lansoprazole group | P | ||
| Intention-to-treat analysis | ||||
| No. of patients | 24 | 22 | - | |
| Eradication rate | 16/24 (66.7%) | 16/22 (72.7%) | NS | |
| Per-protocol analysis | ||||
| No. of patients | 20 | 20 | - | |
| Eradication rate | 14/20 (70.0%) | 14/20 (70.0%) | NS | |
| Lafutidine group | Lansoprazole group | P | |
| Homo EM | 5/6 (83%) | 3/5 (60%) | NS |
| Hetero EM | 6/9 (67%) | 6/8 (75%) | NS |
| PM | 1/1 (100%) | 5/6 (83%) | NS |
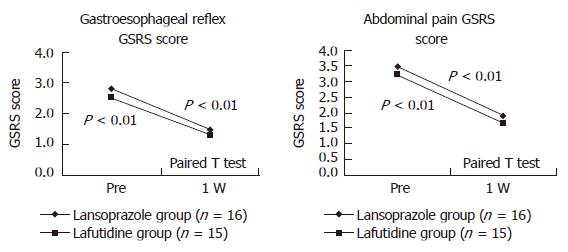
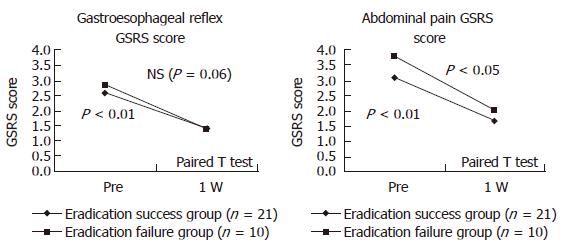
As for upper gastrointestinal symptoms at the time of eradication therapy, the GSRS scores for gastroesophageal reflux and abdominal symptoms improved after eradication therapy in both groups (Figure 1). In contrast, the scores for abdominal discomfort, diarrhea, and constipation were unchanged. The persistence or eradication of H pylori had no apparent effect on the changes in the GSRS scores for gastroesophageal reflux or abdominal symptoms after eradication therapy (Figure 2).
Adverse events occurred in 3 of the 20 patients in the lafutidine group and 3 of the 20 patients in the lansoprazole group. In the lafutidine group, diarrhea, constipation, and drug rash occurred in 1 patient in each group. In the lansoprazole group, stomatitis occurred in 3 patients, and diarrhea, throat pain, and taste disorder occurred in 1 patient in each group. There were no differences in the incidence of adverse events between the groups (Table 4).
| Lafutidine group | Lansoprazole group | P | ||
| Adverse events | Not present | 17 | 17 | NS |
| Present | 3 | 3 | ||
| Incidence of adverse events | 15.0% (3/20) | 15.0% (3/20) | ||
| Adverse events | Stomatitis | 3 | NS | |
| Diarrhea | 1 | 1 | ||
| Constipation | 1 | |||
| Throat pain | 1 | |||
| Drug rash | 1 | |||
| Taste disorder | 1 | |||
Triple-drug therapy combining 2 antimicrobial agents with an antisecretory agent is currently prescribed throughout the world to eradicate H pylori[11,12]. The rationale for combination therapy is that PPIs and H2-receptor antagonists increase intragastric pH by suppressing acid secretion, thereby enhancing the antibacterial activity of antimicrobial agents and increasing the rate of H pylori eradication[13]. PPIs are generally prescribed as antisecretory agents in triple therapy and produce eradication rates of 80% or higher[4]. Several studies have reported good results with regimens including H2-receptor antagonists instead of PPIs, but controlled studies comparing H2-receptor antagonist-based triple therapy with PPI-based triple therapy are scant. Recent studies have shown that triple therapy combining either famotidine or omeprazole with clarithromycin and metronidazole are equally effective for eradication of H pylori infection[14,15]. Famotidine or omeprazole combined with amoxicillin and tinidazole instead of clarithromycin and metronidazole also produce similar results[16]. On the other hand, double-blind trials have shown that triple therapy with omeprazole, amoxicillin, and metronidazole yields a higher rate of H pylori eradication than triple therapy with ranitidine, amoxicillin, and metronidazole[17]. Isomoto et al [10]reported that a 7-d course of triple therapy with lafutidine (20 mg, twice daily), clarithromycin (200 mg, twice daily), and amoxicillin (750 mg, twice daily) produced eradication rates of 85.2% on intention-to-treat analysis and 88.2% on per-protocol analysis. In patients who received lansoprazole (30 mg, twice daily) instead of lafutidine, similar eradication rates were obtained (80.3% on intention-to-treat analysis; 84.5% on per-protocol analysis). We compared similar triple therapies including either lafutidine or lansoprazole. The rates of H pylori eradication were 66.7% (intention-to treat analysis) and 70.0% (per-protocol analysis) in the lafutidine group, as compared with 72.7% (intention-to treat analysis) and 70.0% (per-protocol analysis) in the lansoprazole group. These eradication rates were similar.
In an acidic environment, the antimicrobial activity of metronidazole is preserved, whereas that of clarithromycin is attenuated. A potent inhibitor of acid secretion should therefore be given concurrently when clarithromycin is prescribed for eradication therapy[13]. PPIs generally inhibit acid production for about 24 h. In contrast, H2-receptor antagonists inhibit acid production from late night to early morning, but do not sufficiently suppress acid production during the daytime[14-17]. However, lafutidine, an H2-receptor antagonist that inhibits acid secretion via capsaicin-sensitive afferent nerves and somatostatin, inhibits acid secretion even during the daytime[5,18] and produces a more rapid rise in intragastric pH than does PPI (rabeprazole)[6].
Lansoprazole is mainly metabolized by CYP2C19 drug-metabolizing enzymes in the liver. Metabolism of lansoprazole is therefore influenced by CYP2C19 polymorphisms, resulting in individual differences in antisecretory activity. Isomoto et al[10] reported that a triple therapy including lansoprazole resulted in an eradication rate of 91.7% in poor metabolizers, as compared with only 76.2% in homozygous-extensive metabolizers. In contrast, the eradication rate with a triple therapy including lafutidine was not influenced by genetic polymorphism of CYP2C19 activity. In our study, rates of H pylori eradication were slightly higher in homozygous-extensive metabolizers in the lafutidine group and poor metabolizers in the lansoprazole group. These results are consistent with the finding of Isomoto et al[10]. H pylori eradication rates may be strongly influenced by genetic polymorphism in Japan because 80% to 85% of Japanese people are extensive metabolizers[19].
H pylori eradication rates were similar in the lafutidine and lansoprazole groups. These similar rates are attributed to the potent, continuous antisecretory activity of lafutidine, unaffected by genetic polymorphism of CYP2C19 activity. Recently, rising health care costs due to an aging population have become a matter of concern. Attention has focused on the reduction of health care costs. The cost of lafutidine is about half that of lansoprazole. The use of lafutidine in place of lansoprazole would reduce the cost of H pylori eradication therapy by about 20%. Lafutidine-based triple therapy is thus considered very cost effective.
Peptic ulcer is often accompanied by epigastric pain. Prompt improvement in pain would enhance patient quality of life. Sung et al[20] compared the effect of triple therapy with a 1-wk course of bismuth (120 mg), tetracycline (500 mg), and metronidazole (400 mg), each given 4 times daily, with a 4-wk course of omeprazole (20 mg once daily). Antibacterial treatment alone yielded a 91% eradication rate and an 84% ulcer cure rate, but an antisecretory agent was reported to be necessary for the prompt alleviation of epigastric pain[20]. In our study, the persistence or eradication of H pylori had no influence on improvements in gastroesophageal reflux and abdominal symptoms after eradication therapy in patients with active peptic ulcer. Consequently, the improvements in gastroesophageal reflux and abdominal symptoms were similar in the lafutidine and lansoprazole groups. Lafutidine inhibits acid secretion even during the daytime, unlike other H2-receptor antagonists[5]. The rise in intragastric pH is more rapid with lafutidine than with PPIs, resulting in an earlier resolution of symptoms[6-9]. In contrast, PPIs are prodrugs that require hydrogen ions for activation and suppress acid secretion by acting on proton pumps in gastric parietal cells, which actively secrete acid. However, dormant proton pumps start compensatory production of gastric acid. Inhibition of all gastric proton pumps consequently requires 2 d to 3 d. In our study, similar improvements in gastroesophageal reflux and abdominal symptoms in the lafutidine and lansoprazole groups are most likely ascribed to the fact that the antisecretory activity of lafutidine is comparable to that of lansoprazole during 1 wk of H pylori eradication therapy.
Our study showed that triple therapy including lafutidine is equivalent to triple therapy including lansoprazole in terms of H pylori eradication rates and improvements in gastroesophageal reflux and abdominal symptoms after treatment. These results are attributed to the fact that lafutidine has strong, continuous antisecretory activity, unaffected by CYP2C19 polymorphisms.
S- Editor Liu Y L- Editor Lutze M E- Editor Ma WH
| 1. | Graham DY, Lew GM, Evans DG, Evans DJ, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann Intern Med. 1991;115:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 354] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Tytgat GN. Review article: treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther. 1994;8:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Onodera S, Shibata M, Tanaka M, Inaba N, Arai Y, Aoyama M, Lee B, Yamaura T. Gastroprotective mechanism of lafutidine, a novel anti-ulcer drug with histamine H2-receptor antagonistic activity. Arzneimittelforschung. 1999;49:519-526. [PubMed] |
| 6. | Inamori M, Togawa J, Iwasaki T, Ozawa Y, Kikuchi T, Muramatsu K, Chiguchi G, Matsumoto S, Kawamura H, Abe Y. Early effects of lafutidine or rabeprazole on intragastric acidity: which drug is more suitable for on-demand use? J Gastroenterol. 2005;40:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Miyoshi A, Matsuo Y, Miwa T. Clinical effect of FRG-8813 (lafutidine) on gastritis.pilot study. Rinshouiyaku. 1995;11:97-111. |
| 8. | Miyoshi A, Matsuo Y, Miwa T. Clinical dosage of FRG-8813 (lafutidine) on gastritis. Rinshouiyaku. 1995;11:113-129. |
| 9. | Miyoshi A, Matsuo Y, Miwa T. Clinical evaluation of FRG-8813 (lafutidine) on gastritis. A double-blind comparative study vs. cimetidine. Rinshouiyaku. 1998;14:2121-2138. |
| 10. | Isomoto H, Inoue K, Furusu H, Nishiyama H, Shikuwa S, Omagari K, Mizuta Y, Murase K, Murata I, Kohno S. Lafutidine, a novel histamine H2-receptor antagonist, vs lansoprazole in combination with amoxicillin and clarithromycin for eradication of Helicobacter pylori. Helicobacter. 2003;8:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 843] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 12. | Pounder RE. New developments in Helicobacter pylori eradication therapy. Scand J Gastroenterol Suppl. 1997;223:43-45. [PubMed] |
| 13. | Cederbrant G, Kahlmeter G, Schalén C, Kamme C. Additive effect of clarithromycin combined with 14-hydroxy clarithromycin, erythromycin, amoxycillin, metronidazole or omeprazole against Helicobacter pylori. J Antimicrob Chemother. 1994;34:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gschwantler M, Dragosics B, Wurzer H, Brandstätter G, Weiss W. Eradication of Helicobacter pylori by a 1-week course of famotidine, amoxicillin and clarithromycin. Eur J Gastroenterol Hepatol. 1998;10:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Gschwantler M, Dragosics B, Schütze K, Wurzer H, Hirschl AM, Pasching E, Wimmer M, Klimpfinger M, Oberhuber G, Brandstätter G. Famotidine versus omeprazole in combination with clarithromycin and metronidazole for eradication of Helicobacter pylori--a randomized, controlled trial. Aliment Pharmacol Ther. 1999;13:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hsu CC, Chen JJ, Hu TH, Lu SN, Changchien CS. Famotidine versus omeprazole, in combination with amoxycillin and tinidazole, for eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2001;13:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ell C, Schoerner C, Solbach W, Stolte M, Vieth M, Ridl W, Moser W. The AMOR study: a randomized, double-blinded trial of omeprazole versus ranitidine together with amoxycillin and metronidazole for eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 2001;13:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Fujisawa T, Adachi K, Komazawa Y, Mihara T, Azumi T, Katsube T, Furuta K, Kazumori H, Kinoshita Y. Helicobacter pylori infection prevents the occurrence of the tolerance phenomenon of histamine H2 receptor antagonists. Aliment Pharmacol Ther. 2004;20:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Sung JJ, Chung SC, Ling TK, Yung MY, Leung VK, Ng EK, Li MK, Cheng AF, Li AK. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 183] [Article Influence: 6.1] [Reference Citation Analysis (0)] |